Renal and Extra Renal Manifestations in Adult Zebrafish Model of Cystinosis
Abstract
:1. Introduction
2. Results
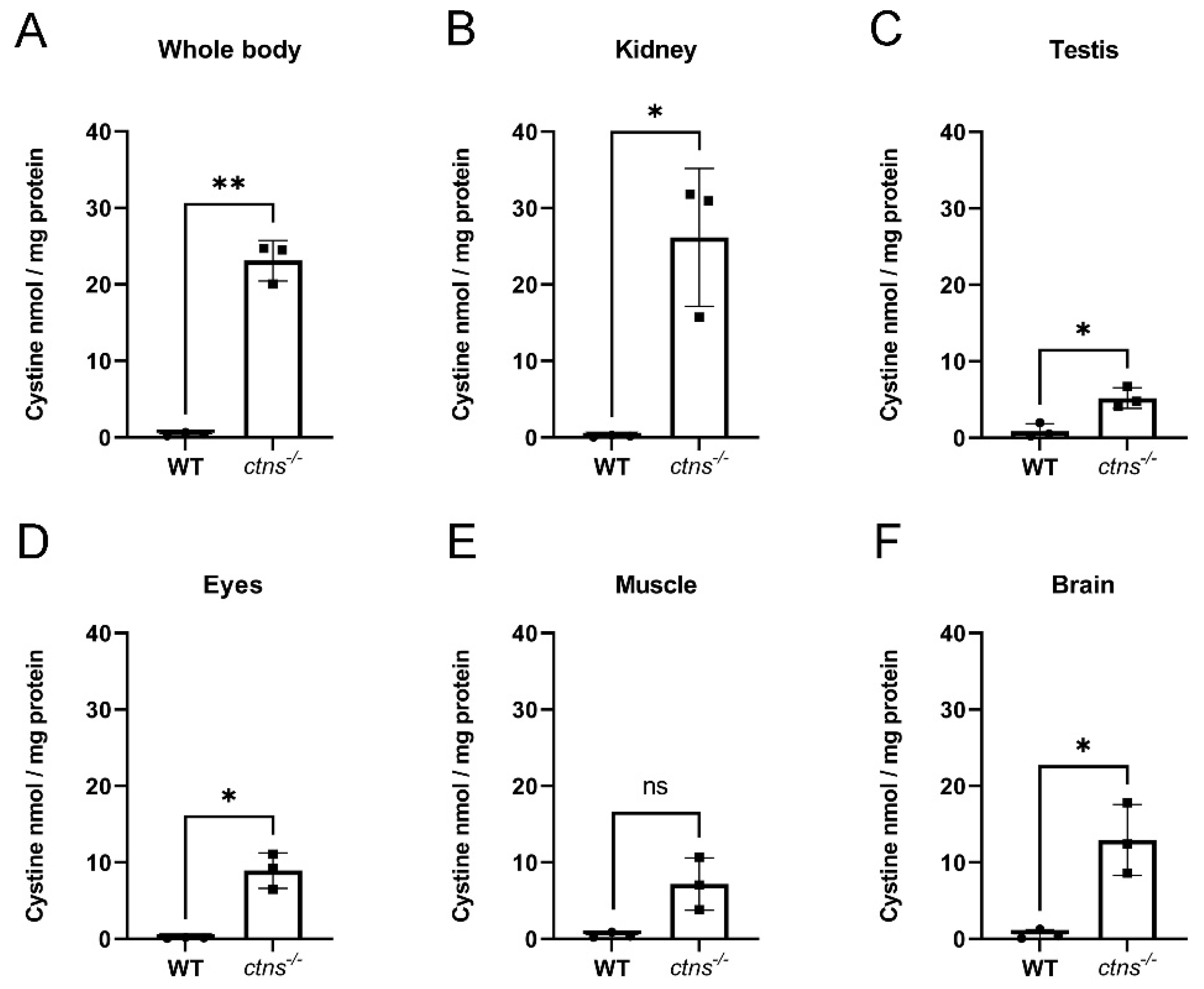
2.1. Cystine Accumulates in Ctns−/− Zebrafish
2.2. Renal Manifestation
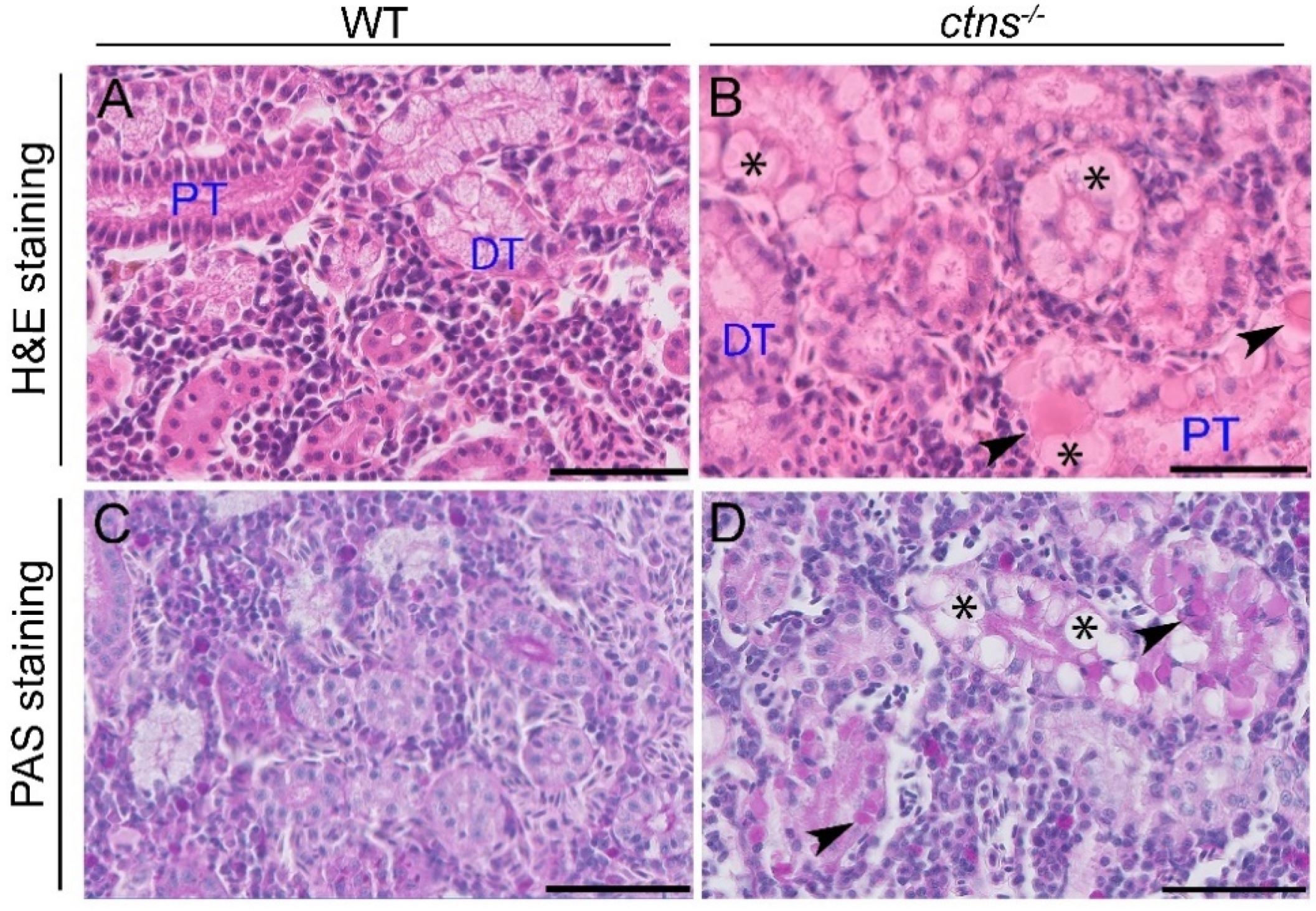
2.2.1. Ctns−/− Zebrafish Present PTEC Damage
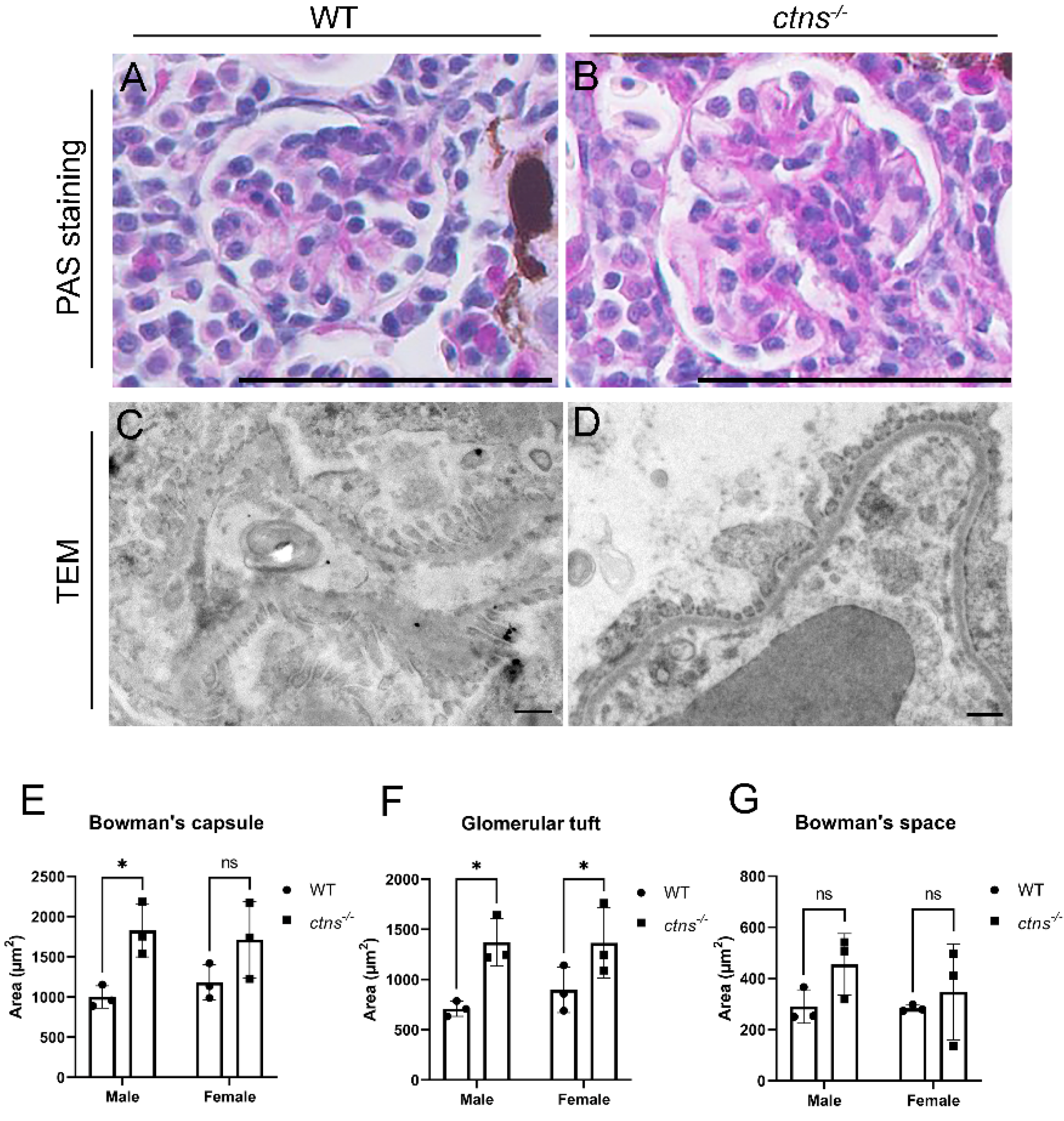
2.2.2. Ctns−/− Zebrafish Show Glomerular Hypertrophy
2.2.3. Ctns−/− Zebrafish Display Increased Apoptosis at The PTEC Level
2.3. Extra Renal Manifestations
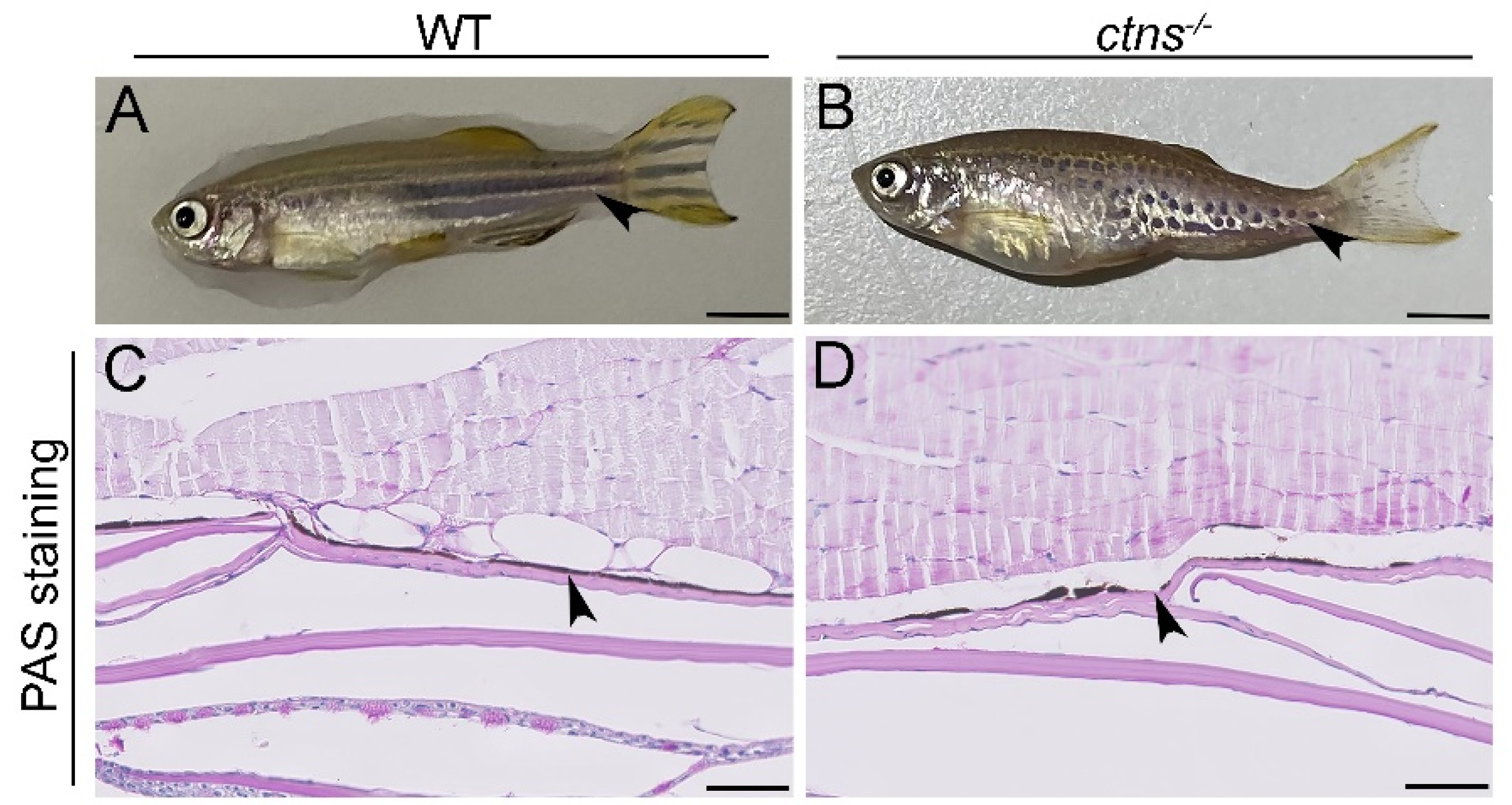
2.3.1. Ctns−/− Zebrafish Show a Characteristic Hypopigmented Spotted Skin Pattern
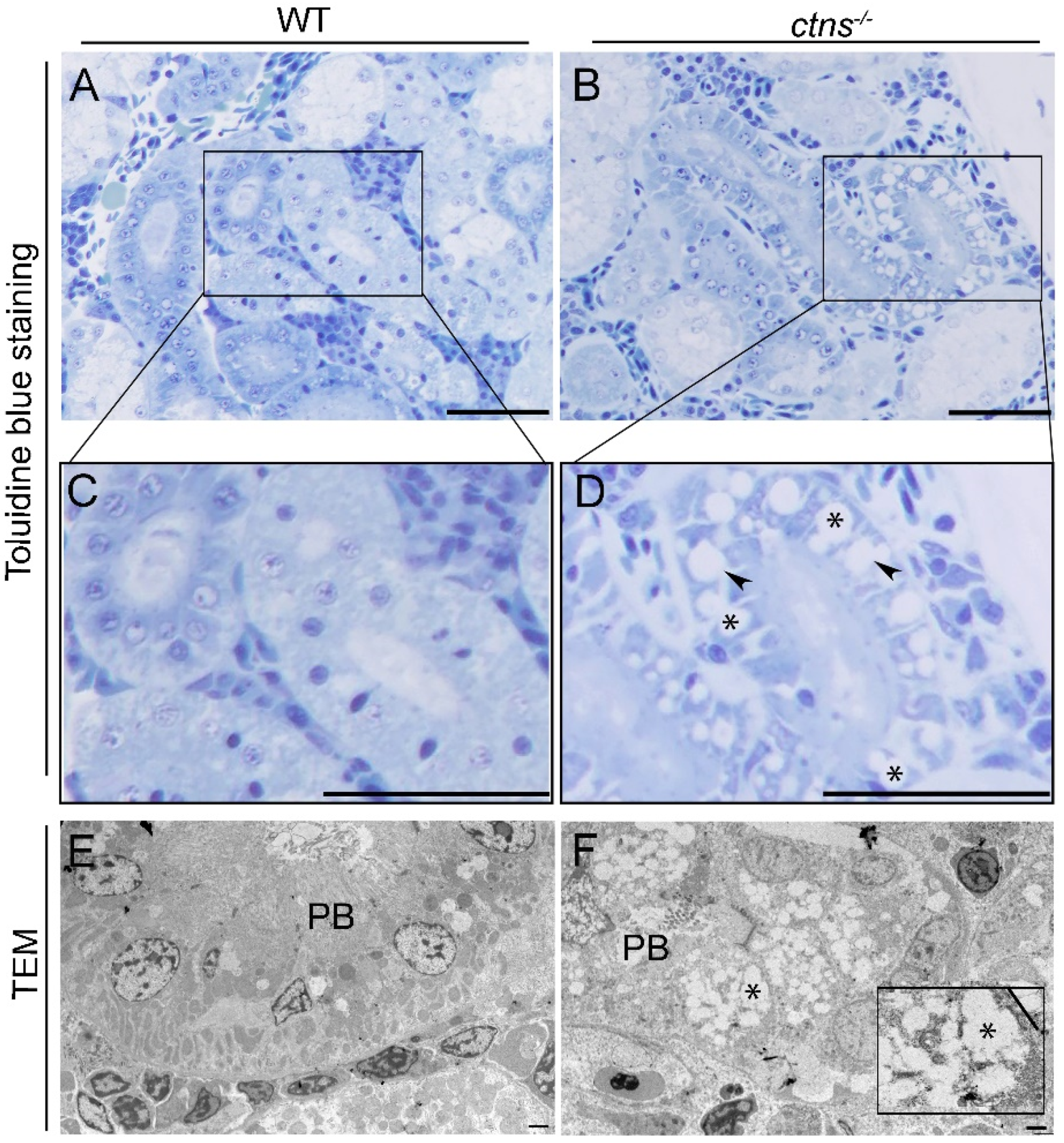
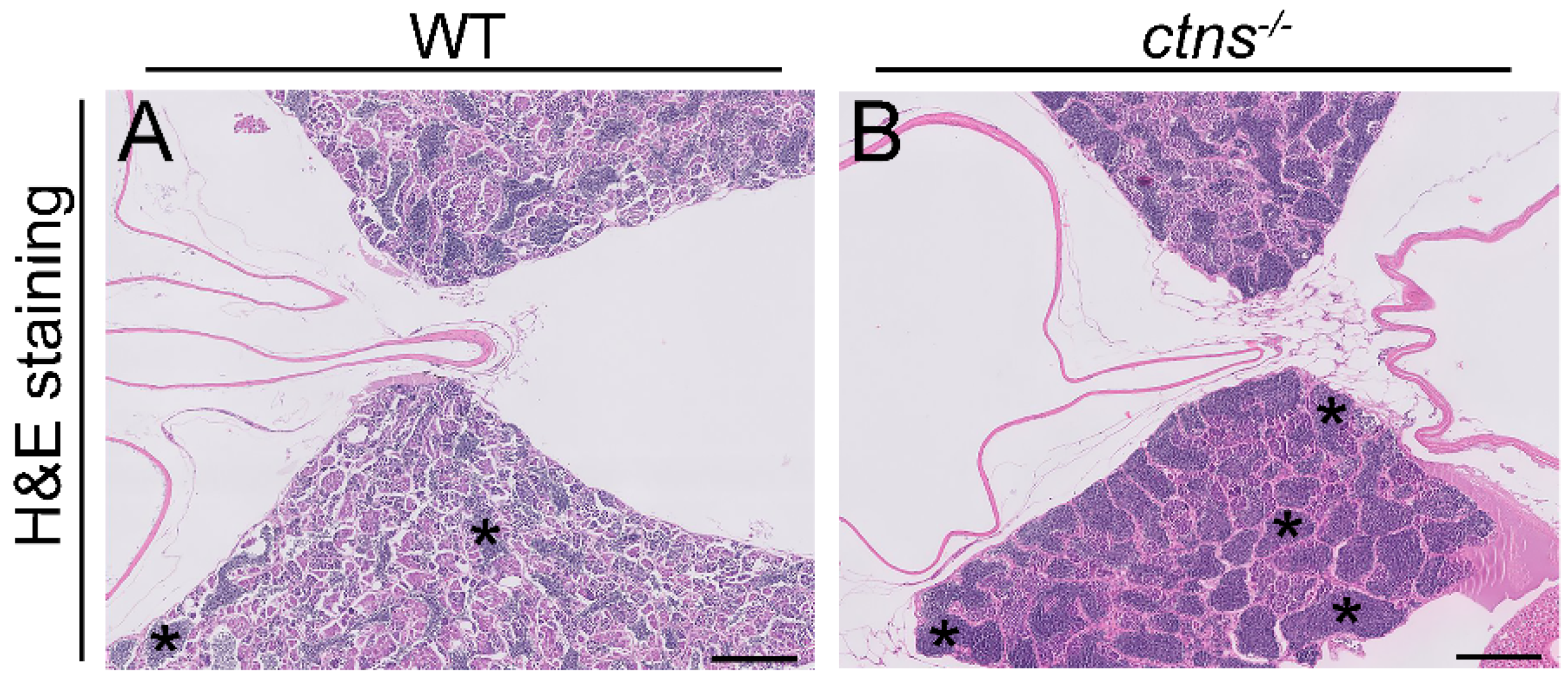
2.3.2. Ctns−/− Zebrafish Show Spermatogenic Cysts Enriched in Spermatozoa
2.3.3. Ctns−/− Female Zebrafish Show Decreased Egg Production
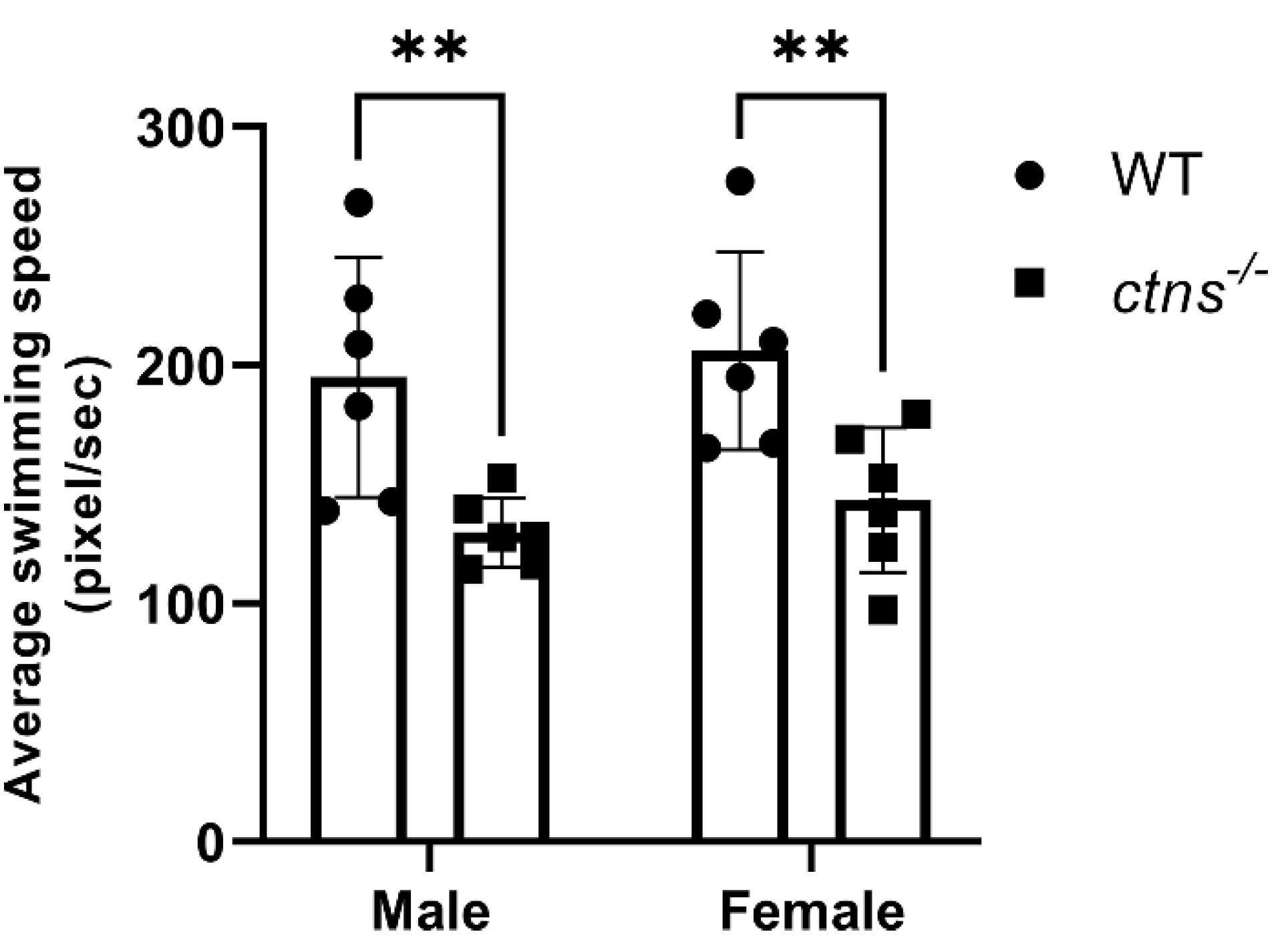
2.3.4. Ctns−/− Zebrafish Display Decreased Locomotor Activity
2.3.5. Ctns−/− Zebrafish Present Increased Thickness of THE Cornea
2.3.6. Additional Ctns−/− Zebrafish Phenotypes
3. Discussion
4. Materials and Methods
4.1. Zebrafish Mainteinance and Breeding
4.2. Cystine Measurement
4.3. Hematoxilin and Eosin and Periodic Acid-Shiff Staining
4.4. Immunohistochemistry (Cleaved Caspase-3 Staining)
4.5. Toluidine Blue Staining and TEM
4.6. Digital Image Analysis
4.6.1. Glomerular Hypertrophy Analysis
4.6.2. Cleaved Caspase-3 Expression Analysis
4.6.3. Thickness of the Corneal Stroma Analysis
4.7. Fertility Study
4.8. Locomotory Activity
4.9. Body Weight and Length Measurements
4.10. Statistical Analysis
4.11. Fertility Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Elmonem, M.A.; Veys, K.R.; Soliman, N.A.; van Dyck, M.; van den Heuvel, L.P.; Levtchenko, E. Cystinosis: A review. Orphanet J. Rare Dis. 2016, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Jezegou, A.; Llinares, E.; Anne, C.; Kieffer-Jaquinod, S.; O’Regan, S.; Aupetit, J.; Chabli, A.; Sagne, C.; Debacker, C.; Chadefaux-Vekemans, B.; et al. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc. Natl. Acad. Sci. USA 2012, 109, E3434–E3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besouw, M.T.; Levtchenko, E.N. Improving the prognosis of nephropathic cystinosis. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Brodin-Sartorius, A.; Tete, M.J.; Niaudet, P.; Antignac, C.; Guest, G.; Ottolenghi, C.; Charbit, M.; Moyse, D.; Legendre, C.; Lesavre, P.; et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012, 81, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Cherqui, S. Cysteamine therapy: A treatment for cystinosis, not a cure. Kidney Int. 2012, 81, 127–129. [Google Scholar] [CrossRef] [Green Version]
- David, D.; Princiero Berlingerio, S.; Elmonem, M.A.; Oliveira Arcolino, F.; Soliman, N.; van den Heuvel, B.; Gijsbers, R.; Levtchenko, E. Molecular Basis of Cystinosis: Geographic Distribution, Functional Consequences of Mutations in the CTNS Gene, and Potential for Repair. Nephron 2019, 141, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Yanobu-Takanashi, R.; Nakano, K.; Hamase, K.; Shimizu, T.; Okamura, T. A deletion in the Ctns gene causes renal tubular dysfunction and cystine accumulation in LEA/Tohm rats. Mamm. Genome 2019, 30, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Nevo, N.; Chol, M.; Bailleux, A.; Kalatzis, V.; Morisset, L.; Devuyst, O.; Gubler, M.C.; Antignac, C. Renal phenotype of the cystinosis mouse model is dependent upon genetic background. Nephrol. Dial. Transplant 2010, 25, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmonem, M.A.; Berlingerio, S.P.; van den Heuvel, L.P.; de Witte, P.A.; Lowe, M.; Levtchenko, E.N. Genetic Renal Diseases: The Emerging Role of Zebrafish Models. Cells 2018, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmonem, M.A.; Khalil, R.; Khodaparast, L.; Khodaparast, L.; Arcolino, F.O.; Morgan, J.; Pastore, A.; Tylzanowski, P.; Ny, A.; Lowe, M.; et al. Cystinosis (ctns) zebrafish mutant shows pronephric glomerular and tubular dysfunction. Sci. Rep. 2017, 7, 42583. [Google Scholar] [CrossRef] [Green Version]
- Lusco, M.A.; Najafian, B.; Alpers, C.E.; Fogo, A.B. AJKD Atlas of Renal Pathology: Cystinosis. Am. J. Kidney Dis. 2017, 70, e23–e24. [Google Scholar] [CrossRef]
- Park, M.A.; Pejovic, V.; Kerisit, K.G.; Junius, S.; Thoene, J.G. Increased apoptosis in cystinotic fibroblasts and renal proximal tubule epithelial cells results from cysteinylation of protein kinase Cdelta. J. Am. Soc. Nephrol. 2006, 17, 3167–3175. [Google Scholar] [CrossRef]
- Veys, K.R.P.; Elmonem, M.A.; Dhaenens, F.; Van Dyck, M.; Janssen, M.; Cornelissen, E.A.M.; Hohenfellner, K.; Reda, A.; Quatresooz, P.; van den Heuvel, B.; et al. Enhanced Intrinsic Skin Aging in Nephropathic Cystinosis Assessed by High-Definition Optical Coherence Tomography. J. Invest. Dermatol. 2019, 139, 2242–2245. [Google Scholar] [CrossRef]
- Besouw, M.T.; Kremer, J.A.; Janssen, M.C.; Levtchenko, E.N. Fertility status in male cystinosis patients treated with cysteamine. Fertil. Steril. 2010, 93, 1880–1883. [Google Scholar] [CrossRef] [Green Version]
- Veys, K.R.; D’Hauwers, K.W.; van Dongen, A.; Janssen, M.C.; Besouw, M.T.P.; Goossens, E.; van den Heuvel, L.P.; Wetzels, A.; Levtchenko, E.N. First Successful Conception Induced by a Male Cystinosis Patient. JIMD Rep. 2018, 38, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohayem, J.; Haffner, D.; Cremers, J.F.; Huss, S.; Wistuba, J.; Weitzel, D.; Kliesch, S.; Hohenfellner, K. Testicular function in males with infantile nephropathic cystinosis. Hum. Reprod. 2021, 36, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Blakey, H.; Proudfoot-Jones, J.; Knox, E.; Lipkin, G. Pregnancy in women with cystinosis. Clin. Kidney J. 2019, 12, 855–858. [Google Scholar] [CrossRef]
- Dogulu, C.F.; Tsilou, E.; Rubin, B.; Fitzgibbon, E.J.; Kaiser-Kupper, M.I.; Rennert, O.M.; Gahl, W.A. Idiopathic intracranial hypertension in cystinosis. J. Pediatr. 2004, 145, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Christopher, K.; Chauhan, A. Potential role of stromal collagen in cystine crystallization in cystinosis patients. Int. J. Pharm. 2018, 551, 232–240. [Google Scholar] [CrossRef]
- Katz, B.; Melles, R.B.; Schneider, J.A.; Rao, N.A. Corneal thickness in nephropathic cystinosis. Br. J. Ophthalmol. 1989, 73, 665–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesterova, G.; Gahl, W.A. Cystinosis: The evolution of a treatable disease. Pediatr. Nephrol. 2013, 28, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariceta, G.; Giordano, V.; Santos, F. Effects of long-term cysteamine treatment in patients with cystinosis. Pediatr. Nephrol. 2019, 34, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, M.J.; Zerulla, T.C.; Tierney, K.B. Zebrafish (Danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp. Gerontol. 2014, 50, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhard, G.S.; Kauffman, E.J.; Wang, X.; Stewart, R.; Moore, J.L.; Kasales, C.J.; Demidenko, E.; Cheng, K.C. Life spans and senescent phenotypes in two strains of Zebrafish (Danio rerio). Exp. Gerontol. 2002, 37, 1055–1068. [Google Scholar] [CrossRef]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef]
- Cherqui, S.; Sevin, C.; Hamard, G.; Kalatzis, V.; Sich, M.; Pequignot, M.O.; Gogat, K.; Abitbol, M.; Broyer, M.; Gubler, M.C.; et al. Intralysosomal cystine accumulation in mice lacking cystinosin, the protein defective in cystinosis. Mol. Cell. Biol. 2002, 22, 7622–7632. [Google Scholar] [CrossRef] [Green Version]
- Raggi, C.; Luciani, A.; Nevo, N.; Antignac, C.; Terryn, S.; Devuyst, O. Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis. Hum. Mol. Genet. 2014, 23, 2266–2278. [Google Scholar] [CrossRef] [Green Version]
- Hard, G.C. Some aids to histological recognition of hyaline droplet nephropathy in ninety-day toxicity studies. Toxicol. Pathol. 2008, 36, 1014–1017. [Google Scholar] [CrossRef]
- Sakarcan, A.; Timmons, C.; Baum, M. Intracellular distribution of cystine in cystine-loaded proximal tubules. Pediatr. Res. 1994, 35, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Kroeger, P.T., Jr.; Wingert, R.A. Using zebrafish to study podocyte genesis during kidney development and regeneration. Genesis. 2014, 52, 771–792. [Google Scholar] [CrossRef] [Green Version]
- Sumayao, R.; McEvoy, B.; Newsholme, P.; McMorrow, T. Lysosomal cystine accumulation promotes mitochondrial depolarization and induction of redox-sensitive genes in human kidney proximal tubular cells. J. Physiol. 2016, 594, 3353–3370. [Google Scholar] [CrossRef] [Green Version]
- Chiaverini, C.; Sillard, L.; Flori, E.; Ito, S.; Briganti, S.; Wakamatsu, K.; Fontas, E.; Berard, E.; Cailliez, M.; Cochat, P.; et al. Cystinosin is a melanosomal protein that regulates melanin synthesis. FASEB J. 2012, 26, 3779–3789. [Google Scholar] [CrossRef] [Green Version]
- Adelmann, C.H.; Traunbauer, A.K.; Chen, B.; Condon, K.J.; Chan, S.H.; Kunchok, T.; Lewis, C.A.; Sabatini, D.M. MFSD12 mediates the import of cysteine into melanosomes and lysosomes. Nature 2020, 588, 699–704. [Google Scholar] [CrossRef]
- Leal, M.C.; Cardoso, E.R.; Nobrega, R.H.; Batlouni, S.R.; Bogerd, J.; Franca, L.R.; Schulz, R.W. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol. Reprod. 2009, 81, 177–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansanwal, P.; Sarwal, M.M. Abnormal mitochondrial autophagy in nephropathic cystinosis. Autophagy 2010, 6, 971–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellomo, F.; Signorile, A.; Tamma, G.; Ranieri, M.; Emma, F.; De Rasmo, D. Impact of atypical mitochondrial cyclic-AMP level in nephropathic cystinosis. Cell. Mol. Life Sci. 2018, 75, 3411–3422. [Google Scholar] [CrossRef] [PubMed]
- De Rasmo, D.; Signorile, A.; De Leo, E.; Polishchuk, E.V.; Ferretta, A.; Raso, R.; Russo, S.; Polishchuk, R.; Emma, F.; Bellomo, F. Mitochondrial Dynamics of Proximal Tubular Epithelial Cells in Nephropathic Cystinosis. Int. J. Mol. Sci. 2019, 21, 192. [Google Scholar] [CrossRef] [Green Version]
- Kalatzis, V.; Serratrice, N.; Hippert, C.; Payet, O.; Arndt, C.; Cazevieille, C.; Maurice, T.; Hamel, C.; Malecaze, F.; Antignac, C.; et al. The ocular anomalies in a cystinosis animal model mimic disease pathogenesis. Pediatr. Res. 2007, 62, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Romero-Ferrero, F.; Bergomi, M.G.; Hinz, R.C.; Heras, F.J.H.; de Polavieja, G.G. idtracker.ai: Tracking all individuals in small or large collectives of unmarked animals. Nat. Methods 2019, 16, 179–182. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlingerio, S.P.; He, J.; De Groef, L.; Taeter, H.; Norton, T.; Baatsen, P.; Cairoli, S.; Goffredo, B.; de Witte, P.; van den Heuvel, L.; et al. Renal and Extra Renal Manifestations in Adult Zebrafish Model of Cystinosis. Int. J. Mol. Sci. 2021, 22, 9398. https://doi.org/10.3390/ijms22179398
Berlingerio SP, He J, De Groef L, Taeter H, Norton T, Baatsen P, Cairoli S, Goffredo B, de Witte P, van den Heuvel L, et al. Renal and Extra Renal Manifestations in Adult Zebrafish Model of Cystinosis. International Journal of Molecular Sciences. 2021; 22(17):9398. https://doi.org/10.3390/ijms22179398
Chicago/Turabian StyleBerlingerio, Sante Princiero, Junling He, Lies De Groef, Harold Taeter, Tomas Norton, Pieter Baatsen, Sara Cairoli, Bianca Goffredo, Peter de Witte, Lambertus van den Heuvel, and et al. 2021. "Renal and Extra Renal Manifestations in Adult Zebrafish Model of Cystinosis" International Journal of Molecular Sciences 22, no. 17: 9398. https://doi.org/10.3390/ijms22179398





