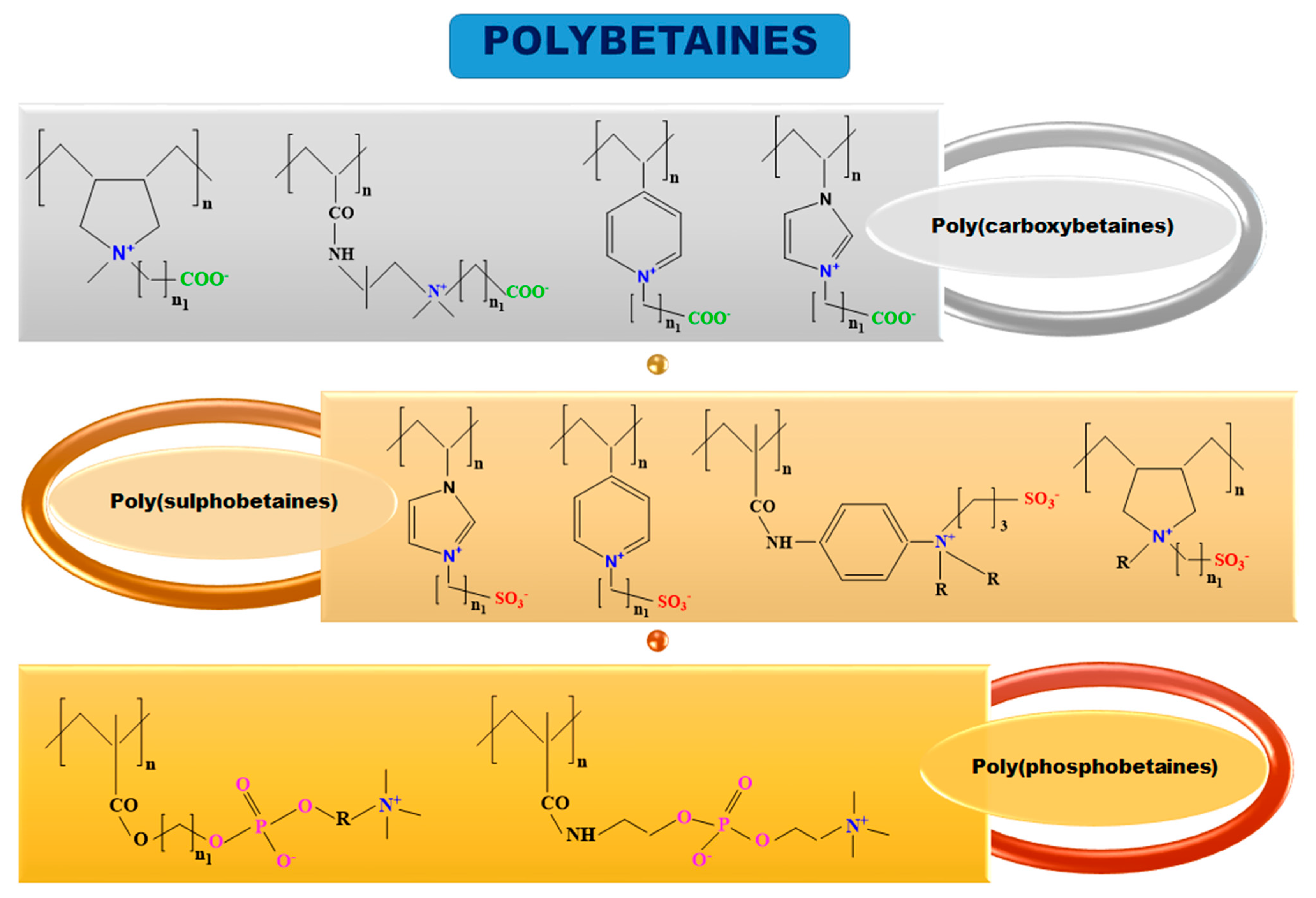
Polybetaines in Biomedical Applications
Abstract
:1. Introduction
- The discovery of new drug delivery based on micro and nano-sized particles that have the ability to respond to stimuli, to carry biologically active targeting principles, to treat cancer or have a multifunctional role in the delivery of therapeutic genes.
- The use of polymeric materials for diagnosis, therapeutic and biomedical applications, particularly in tissue engineering.
- Polymerization of zwitterionic monomer using several polymerization techniques, such as: free radical polymerization (FRP), controlled radical polymerization (CRP), atom transfer radical polymerization (ATRP), group chain-transfer polymerization (GTP), reversible addition fragmentation transfer method (RAFT), distillation-precipitation polymerization (DPP) and reversible-deactivation radical polymerization (RDPR) [7,8,9,10]. By this method polymers with 100% betaine groups are obtained but the polymerization of zwitterionic monomer method has the disadvantage that the molecular mass of synthesized polybetaines cannot be determined accurately by gel permeation chromatography (GPC) and high performance liquid chromatography (HPLC) measurements because polybetaines show strong interactions with the materials found in the chromatographic columns [8].
- Betainization of polymer precursor containing tertiary ammonium groups using polymer-analogous reactions [11]. In this case, the polybetaines are easy to be characterized, being able to obtain the polymers with varied and well-defined chemical structures, but due to the neighbouring groups effects and the complex reactions during the chemical functionalization, the polymer-analogous transformations cannot occur with a yield of 100%.
2. Biomedical Applications
2.1. Antifouling Materials
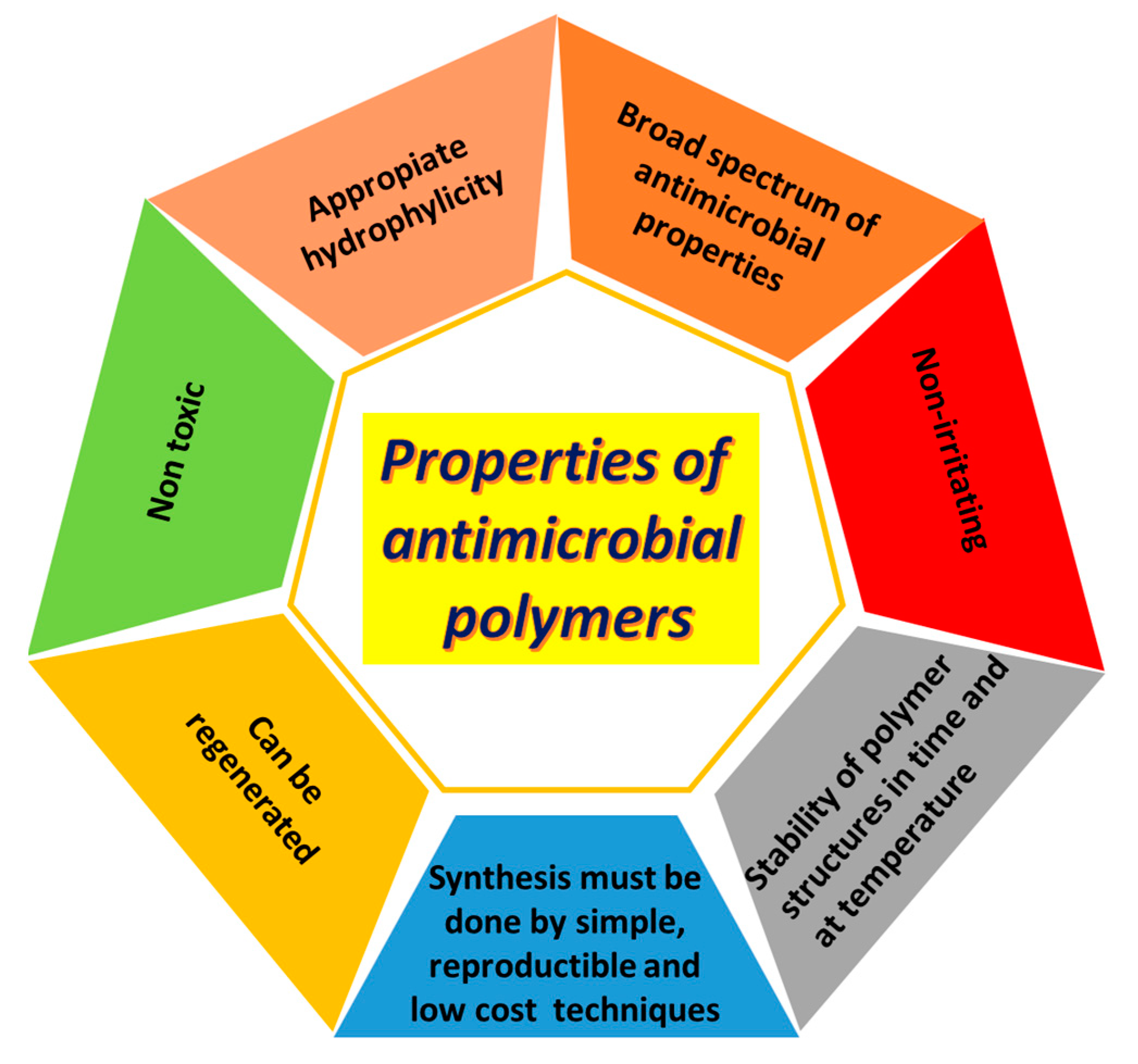
2.2. Antimicrobial Materials
- Extraparietal: capsule, mucus layer, glycocalyx, flagella, fimbriae and pili.
- Biosynthesis of the cell wall. Considering that the cell wall is the one providing the stability and mechanical resistance, attempts have been made to develop antimicrobial materials that would prevent the main component (peptidoglycan) to form the crosslinks in order to weaken the cell wall resistance and ultimately to lead to the destruction of the bacteria.
- Protein synthesis. In this case, the synthesized molecules turned out to be inhibitors that blocked protein biosynthesis especially at the ribosome level.
- DNA replication and repair [54].
- Biocidal polymers are the polymers that contain quaternary ammonium, phosphonium, tertiary sulfonium and guanidinium groups as cationic biocides. The mechanism of antibacterial action can be described as follows: adsorption onto the bacteria cell surface that is usually negatively charged; diffusion through the cell wall; binding and disruption of cytoplasmic membrane; release the component (electrolyte and nucleic acids) of cytoplasmic membrane and finally the death of the bacteria cell [60,61].
- Biocide releasing polymers represent a polymeric carrier for biocide molecules that can be covalently linked or physically entrapped [62].
- Antimicrobial surfaces can be achieved by using two methods: (a) chemical methods that include surface coatings and modification of the surface chemistry through functionalization, derivatization or polymerization; (b) physical methods which are responsible for the modification of the structure architecture [66,67].
- Contact killing surfaces that are functionalized with bactericide (disinfectants, antiseptics or antibiotics) through covalent linkages, physical absorption or coordination bonds [75].
- Anti-adhesion/bacteria repellent surfaces. In this case the surface is coated with hydrophilic polymers that can prevent bacteria accumulation and proliferation by establishing steric repulsion through surface hydration or charge repelling [76].
- Upper layer bactericidal brushes are based on poly[trimethyl amino)ethyl methacrylate chloride] (polyMETAC) or poly[2-(tert-butylamino)ethyl methacrylate] (polyTA).
- Background layer consisting of salt-responsive polybetaine brush based on poly(3-(dimethyl(4-vinylbenzyl)ammonio)propyl sulfonate (polyDVBAPS).
2.3. Drug Delivery Systems
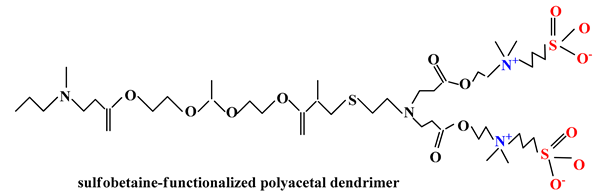
2.3.1. Drug Delivery Systems in Cancer Therapy
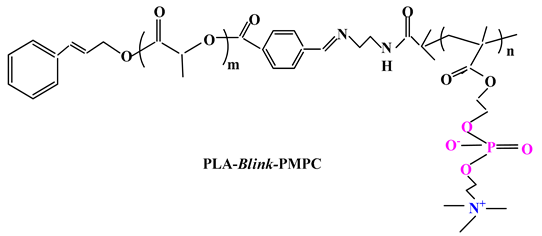
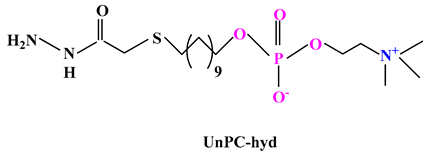
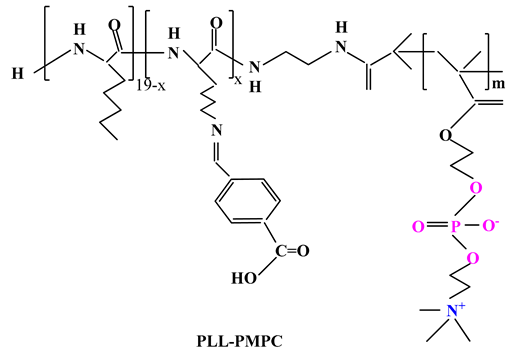
2.3.2. Other Drug Delivery Systems
2.4. Wound Healing
2.5. Implant Coatings
3. Outlook
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, B.; Tang, Q.; Cheng, G. Recent advances of zwitterionic carboxybetaine materials and their derivatives. J. Biomater. Sci. Polym. Ed. 2014, 25, 1502–1513. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4177–4190. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhuang, B.; Yu, J. Functional zwitterionic polymers on surface: Structure and applications. Chem. Asian J. 2020, 15, 2060–2075. [Google Scholar] [CrossRef]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locok, K.E.S. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Stubbs, C.; Bailey, T.L.; Murray, K.; Gibson, M.I. Polyampholytes as emerging macromolecular cryoprotectants. Biomacromolecules 2020, 21, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Luca, C.; Neagu, V.; Vasiliu, S.; Barboiu, V. Synthetic polybetaines: Synthesis and properties. In Focus in Ionic Polymers; Dragan, S., Ed.; Research Singpost: Kerala, India, 2005; pp. 117–153. [Google Scholar]
- Kudaibergenov, S.; Jaeger, W.; Laschewsky, A. Polymeric betains: Synthesis, characterization and application. Adv. Polym. Sci. 2006, 201, 157–224. [Google Scholar] [CrossRef]
- Laschewsky, A. Structures and synthesis of zwitterionic polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Xue, W.; Huglin, M.B.; Liao, B. Observations on the swelling charcateristics of the zwitterionic hydrogel of poly(1-3-sulfopropyl)-2-vinyl-pyridinium-betaine hydrogel. Eur. Polym. J. 2006, 42, 3015–3023. [Google Scholar] [CrossRef]
- Mertoglu, M.; Laschewsky, A.; Skrabania, K.; Wieland, C. New water soluble agents for reversible addition-fragmentation chain transfer polymerization and their application in aqueous solutions. Macromolecules 2005, 38, 3601–3614. [Google Scholar] [CrossRef]
- Luca, C.; Mihailescu, S.; Popa, M. Polymers containing quaternary ammonium groups based on poly(N-vinylimidazole). Eur. Polym. J. 2002, 38, 1501–1507. [Google Scholar] [CrossRef]
- Guo, Y.S.; Mi, Y.F.; Ji, Y.L.; An, Q.F.; Gao, C.J. One-step surface grafting method for preparing zwitterionic nanofiltation membrane via in situ introduction of initiator in interfacial polymerization. ACS Appl. Polym. Mater. 2019, 1, 1022–1033. [Google Scholar] [CrossRef]
- Yu, H.Y.; Kang, Y.; Liu, Y.; Mi, B. Grafting polyzwitterions onto polyamide by click chemistry and nucleophilic substitution on nitrogen: A novel approach to enhance membrane fouling resistance. J. Membr. Sci. 2014, 449, 50–57. [Google Scholar] [CrossRef]
- Cooper, B.M.; Chan-Seng, D.; Samanta, D.; Zhang, X.; Parelkar, S.; Emrick, T. Polyester-graft-phosphorylcholine prepared by ring- opening polymerization and clik chemistry. Chem. Commun. 2009, 7, 815–817. [Google Scholar] [CrossRef] [Green Version]
- Higaki, Y.; Inutsuka, Y.; Sakamaki, T.; Terayama, Y.; Takenaka, A.; Higaki, K.; Yamada, N.L.; Moriwaki, T.; Ikemoto, Y.; Takara, A. Effect of charged group spacer length on hydration state in zwitterionic poly(sulfobetaine) brushes. Langmuir 2017, 33, 8404–8412. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, N.; Singh, M. Advances in synthesis and applications of sulfo and carbo analogues of polybetaines. A review. Rev. Adv. Sci. Eng. 2013, 2, 90–111. [Google Scholar] [CrossRef]
- Ilcikova, M.; Tkac, J.; Kasak, P. Switchable materials containing polyzwitterion moieties. Polymers 2015, 7, 2344–2370. [Google Scholar] [CrossRef]
- Xiao, S.; Ren, B.; Huang, L.; Shen, M.; Zhang, Y.; Zhong, M.; Yang, J.; Zheng, J. Salt-responsive zwitterionic polymer brushes with anti-polyelectrolyte property. Curr. Opin. Chem. Eng. 2018, 19, 86–93. [Google Scholar] [CrossRef]
- Ladenheim, H.; Morawetz, H. A new type of polyampholyte: Poly(4-vinyl pyridine betaine). J. Polym. Sci. 1957, 26, 251–254. [Google Scholar] [CrossRef]
- Hart, R.; Timmerman, D. New polyampholytes: The polysulfobetaines. J. Polym. Sci. 1958, 28, 638–640. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Herrwerth, S.; Rosendahl, T.; Feng, C.; Fick, J.; Eck, W.; Himmelhaus, M.; Dahint, R.; Grunze, M. Covalent coupling of antibodies to self-assembled monolayers of carboxy-functionalized poly(ethylene glycol): protein resistance and specific binding of biomolecules. Langmuir 2003, 19, 1880–1887. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, M.A.; Marchant, R.E. Reduced protein adsorption and platelet adhesion by controlled variation of oligomaltose surfactant polymer coatings. J. Biomed. Mater. Res. 2001, 56, 159–167. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, C.; Hu, R.; Lin, W.; Wang, Q.; Zhao, J.; Bilinovich, S.M.; Leeper, T.C.; Li, L.; Cheung, H.M.; et al. Probing the weak interaction of proteins with neutral and zwitterionic antifouling polymers. Acta Biomater. 2014, 10, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Nam, J.; Won, N.; Jin, H.; Jung, S.; Jung, S.; Cho, S.H.; Kim, S. Compact and stable quantum dots with positive, negative and zwitterionic surface: Specific cell interactions and non-specific adsorptions by the surface charges. Adv. Funct. Mat. 2011, 21, 1558–1566. [Google Scholar] [CrossRef]
- Leng, C.; Huang, H.; Zhang, K.; Hung, H.C.; Xu, Y.; Li, Y.; Jiang, S.; Chen, Z. Effect of surface hydratation on antifouling properties of mixed charged polymers. Langmuir 2018, 34, 6538–6545. [Google Scholar] [CrossRef]
- Tanaka, M.; Morita, S.; Hayashi, T. Role of interfacial water in determining the interactions of proteins and cells with hydrated materials. Colloid Surf. B Biointerfaces 2021, 198, 111449. [Google Scholar] [CrossRef]
- Ladd, J.; Zhang, Z.; Chen, S.; Hower, J.C.; Jiang, S. Zwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasma. Biomacromolecules 2008, 9, 1357–1361. [Google Scholar] [CrossRef]
- Tanaka, M.; Sackmann, E. Polymer-supported membranes as models of the cell surface. Nature 2005, 437, 656–663. [Google Scholar] [CrossRef]
- Wang, R.Y.; Himmelhaus, M.; Fick, J.; Herrwerth, S.; Eck, W.; Grunze, M. Interaction of self-assembled monolayers of oligo(ethylene glycol)-terminated alkanethiols with water studied by vibrational sum-frequency generation. J. Chem. Phys. 2005, 122, 164702. [Google Scholar] [CrossRef] [PubMed]
- Schonemann, E.; Koc, J.; Aldred, N.; Clare, A.S.; Laschewsky, A.; Rosenhahn, A.; Wischerhoff, E. Synthesis of novel sulfobetaine polymers with differing dipole orientations in their side chains, and their effects on the antifouling properties. Macromol. Rapid Commun. 2019, 40, 1900447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Zhou, Y.; Wang, H.; Hu, J. P4VPmodified zwitterionic polymer for the preparation of antifouling functionalized surfaces. Nanomaterials 2019, 9, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Ren, B.; Zhang, D.; Xie, S.; Chang, Y.; Yang, J.; Wu, J.; Xu, L.; Zheng, J. Fundamentals and applications of zwitterionic antifouling polymers. J. Phys. D Appl. Phys. 2019, 52, 403001. [Google Scholar] [CrossRef]
- Heath, D.E.; Cooper, S.L. Design and characterization of sulfobetaine-containing terpolymer biomaterials. Acta Biomater. 2012, 8, 2899–2910. [Google Scholar] [CrossRef]
- Wang, Z.; van Andel, E.; Pujari, S.P.; Feng, H.; Dijksman, J.A.; Smulders, M.M.J.; Zuilhof, H. water-repairable zwitterionic polymer coatings for anti-biofouling surfaces. J. Mat. Chem. B 2017, 5, 6728–6733. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.Q.; Su, Y.L.; Jiang, Z.Y. Antifouling ultrafiltration membrane composed of polyethersulfone and sulfobetaine copolymer. J. Membr. Sci. 2006, 280, 343−350. [Google Scholar] [CrossRef]
- Chen, S.H.; Chang, Y.; Lee, K.R.; Wei, T.C.; Higuchi, A.; Ho, F.M.; Tsou, C.C.; Ho, H.T.; Lai, J.Y. Hemocompatible control of sulfobetaine-grafted polypropylene fibrous membranes in human whole blood via plasma-induced surface zwitterionization. Langmuir 2012, 28, 17733–17742. [Google Scholar] [CrossRef]
- Ye, S.H.; Watanabe, J.; Iwasaki, Y.; Ishihara, K. Antifouling blood purification membrane composed of cellulose acetate and phospholipid polymer. Biomaterials 2003, 24, 4143−4152. [Google Scholar] [CrossRef]
- Paschke, S.; Lienkamp, K. Polyzwitterions: From surface properties and bioactivity profiles to biomedical applications. ACS Appl. Polym. Mater. 2020, 2, 129–151. [Google Scholar] [CrossRef]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Gomez-Vallejo, H.J.; Uriel-Latorre, B.; Sande-Meijide, M.; Villamarin-Bello, B.; Pavon, R.; Fdez-Riverela, F.; Glez-Pena, D. A case-based reasoning system for aiding detection and classification of nosocomial infections. Decis. Supp. Lyst. 2016, 84, 104–116. [Google Scholar] [CrossRef]
- Parry, B.R.; Surovtsev, I.V.; Cabeen, M.T.; O´Hern, C.S.; Dufresne, E.R.; Jacobs-Wagner, C. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 2014, 156, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, T.D. The bacterial nucleus: A history. Microbiol. Rev. 1988, 52, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Kurland, C. Molecular characterization of ribonucleic acid from Escherichia coli ribosomes. J. Mol. Biol. 1960, 2, 83–91. [Google Scholar] [CrossRef]
- Meroueh, S.O.; Bencze, K.Z.; Hesek, D.; Lee, M.; Fisher, J.F.; Stemmler, T.L.; Mobashery, S. Three dimensional structure of the bacterial cell wall peptidoglycan. Proc. Natl. Acad. Sci. USA 2006, 103, 4404–4409. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [Green Version]
- Huanga, K.C.; Mukhopadhyayb, R.; Wena, B.; Gitaia, Z.; Wingreena, N.S. Cell shape and cell-wall organization in gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 19282–19287. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Sjollema, J.; Sharma, P.K.; Kaper, H.J.; van der Mei, H.C.; Busscher, H.J. Nanoscopic vibrations of bacteria with different cell-wall properties adhering to surfaces under flow and static conditions. ACS Nano 2014, 8, 8457–8467. [Google Scholar] [CrossRef]
- Lemonche, L.P.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J. Structure of Gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, M.; Liang, Y.; Lv, X.; Li, C.; Yang, Y.Y.; Yuan, P.; Ding, X. Recent advances in hydrogel-based anti-infective coatings. J. Mat. Sci. Technol. 2021, 85, 169–183. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Worley, S.D. The chemistry and applications of antimicrobial polymers: State of theart review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta. 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.S.; Yang, C.H.; Huang, S.L.; Chen, C.Y.; Lu, Y.Y.; Lin, Y.S. Recent advances in antimicrobial polymers: A mini review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francolini, I.; Donelli, G.; Crisante, F.; Taresco, V.; Piozzi, A. Antimicrobial polymers for anti-biofilm medical devices: State-of-art and perspective. Adv. Exp. Med. Biol. 2015, 831, 93–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, T.; Yamaguchi, H.; Tazuke, S. New polymeric biocides: Synthesis and antibacterial activities of polycations with pendant biguanide groups. Antimicrob. Agents Chemother. 1984, 26, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babutan, I.; Lucaci, A.D.; Botiz, I. Antimicrobial polymeric structures assembled on surfaces. Polymers 2021, 13, 1552. [Google Scholar] [CrossRef]
- Ali, A.; Jamil, M.I.; Jiang, J.; Shoaib, M.; Amin, B.U.; Luo, S.; Zhan, X.; Chen, F.; Zhang, Q. An overview of conttrolled-bioacide-release coating based on polymer resin for marine antifouling applications. J. Polym. Res. 2020, 27, 85. [Google Scholar] [CrossRef]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk derived antimicrobial bioactive peptides: A review. Int. J. Food Prop. 2015, 19, 837–846. [Google Scholar] [CrossRef]
- Sanchez, A.; Vazquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Gddoa Al-sahlany, S.T.; Altemimi, A.B.; Abd Al Manhel, A.J.; Niamah, A.K.; Lakhssasi, N.; Ibrahim, S.A. Purification et bioactive peptide with antimicrobial properties produced by Saccharomyces cerevisiae. Foods 2020, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, H.; Xu, Z.; Li, F. Surface modification of polyacrylonitrile membrane by chemical reactions and physical coating: Comparison between static and pore-flowing procedures. ACS Omega 2018, 3, 4231–4241. [Google Scholar] [CrossRef] [PubMed]
- Ran, B.; Jing, C.; Yang, C.; Li, X.; Li, Y. Synthesis of efficient bacterial adhesion-resistant coatings by one-step polydopamine-assisted deposition of branched polyethylenimine-g-poly(sulfobetaine methacrylate) copolymers. Appl. Surf. Sci. 2018, 450, 77–84. [Google Scholar] [CrossRef]
- Yin, X.; Liang, W.; Wang, Y.; Xiao, Y.; Zhou, Y.; Lang, M. Zwitterionic terpolymer based coating potentially for antibacterial and antifouling applications. Mat. Chem. Phys. 2021, 124102. [Google Scholar] [CrossRef]
- Eslamian, M.; Soltani-Kordshuli, F. Development of multiple-droplet-casting method for the facbrication of coatings and thin solid films. J. Coat. Technol. Res. 2018, 15, 271–280. [Google Scholar] [CrossRef]
- Siemann, U. Solvent cast technology-a versatile tool for thin film production. Progr. Colloid Polym. Sci. 2005, 130, 1–14. [Google Scholar] [CrossRef]
- Erkoc, P.; Ulucan-Karnak, F. Nanotechnology-based antimicrobial and antiviral surface coating strategies. Prosthesis 2021, 3, 25–52. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, S.; Moon, H.C.; Seo, H.; Kim, Y.Y.; Hong, S.P.; Lee, B.S.; Kang, E.; Lee, J.; Ryu, D.H.; et al. Antimicrobial spray nanocoating of supramolecular Fe(III)-tannic acid metal-organic coordination complex: Applications to shoe insoles and fruits. Sci. Rep. 2017, 7, 6980. [Google Scholar] [CrossRef]
- Kausar, A. Polymer coating technology for high performance applications: Fundamentals and advances. J. Macromol. Sci. Part A 2018, 55, 440–448. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G.B.; Uppu, D.S.; Konai, M.M.; Yarlagadda, V.; Sanyal, K.; Haldar, J. Broad spectrum antibacterial and antifungal polymeric paint materials: Synthesis structure-activity relationship and membrane-active mode of action. ACS Appl. Mater. Interfaces 2015, 7, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Mangal, U.; Kwon, J.S.; Choi, S.H. Bio-interactive zwitterionic dental biomaterials for improving biofilm resistance: Characteristics and applications. Int. J. Mol. Sci. 2020, 21, 9087. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. Acs Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, L.; Xiao, S.; Yang, Y.; Chen, F.; Fan, P.; Zhao, Z.; Zhong, M.; Yang, Y. Bacteria killing and release of salt-responsive regenerative, double-layered polyzwitterionic brushes. Chem. Eng. J. 2018, 333, 1–10. [Google Scholar] [CrossRef]
- Schneider-Chaabane, A.; Bleicher, V.; Ran, S.; Al-Ahmad, A.; Lienkamp, K. Stimulus-responsive polyxwitterionic surfaces made from itaconic acid: Self-triggered antimicrobial activity, protein repellency and cell compatibility. ACS Appl. Mater. Interfaces 2020, 12, 21242–21253. [Google Scholar] [CrossRef]
- Zheng, L.; Sundaram, H.S.; Wei, Z.; Li, C.; Yuan, Z. Applications of zwitterionic polymers. React. Funct. Polym. 2017, 118, 51–61. [Google Scholar] [CrossRef]
- Sobolciak, P.; Spirek, M.; Katrlik, J.; Gemeiner, P.; Lacik, I.; Kasak, P. Light-switchable polymer from cationic to zwitterionic form: Synthesis, characterization and interactions with DNA and bacterial cells. Macromol. Rapid Coomun. 2013, 34, 635–639. [Google Scholar] [CrossRef]
- Cao, Q.; Mi, L.; Mendiola, J.; Ella-Menye, J.R.; Zhang, L.; Xue, H.; Jiang, S.Y. Reversibly switching the function of a surface between attacking and defending against bacteria. Angew. Chem. Int. Ed. 2012, 51, 2602–2605. [Google Scholar] [CrossRef]
- Jia, G.; Cao, Z.; Xue, H.; Xu, Y.; Jiang, S. Novel zwitterionic-polymer-coated silica nanoparticles. Langmuir 2009, 25, 3196–3199. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Xiao, S.; Shen, M.; Chen, F.; Fan, P.; Zhong, M.; Zheng, J. Salt responsive zwitterionic polymer brushes with tunable friction and antifouling properties. Langmuir 2015, 31, 9125–9133. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Jiang, S. Synchronizing nonfouling and antimicrobial properties in a zwitterionic hydrogel. Biomaterials 2012, 33, 8928–8933. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; Sanchez, M.; Elasri, M.O.; Lowe, A.B. Antimicrobial activity of statistical polymethacrylic sulfopropylbetaine against Gram positive and Gram negative bacteria. J. Appl. Polym. Sci. 2006, 101, 1036–1041. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Goda, T.; Matsumoto, A.; Takeuchi, H.; Yamaoka, S.; Miyahara, Y. Gold nanoparticles with ligand/zwitterion hybrid layer for individual counting of Influenza A H1N1 subtype using resistive pulse sensing. Langmuir 2019, 35, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Srivastava, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An update review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, S.; Kim, G.; Singha, K.; Park, S.; Ree, M.; Kim, W.J. Artificial cell membrane-mimicking nanostructure facilitates efficient gene delivery through fusogenic interaction with the plasma membrane of living cells. Small 2011, 7, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Ishihara, K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci. Technol. Adv. Mater. 2012, 13, 064101. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Watanabe, J.; Ishihara, K. Enhanced solubility of paclitaxel using water-soluble and biocompatible 2-methacryloyloxyethyl phosphorylcholine polymers. J. Biomed. Mater. Res. A 2003, 65, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Hsiue, G.H.; Lo, C.L.; Cheng, C.H.; Lin, C.P.; Huang, C.K.; Chen, H.H. Preparation and characterization of poly (2-methacryloyloxyethyl phosphorylcholine)-block-poly(D, L-lactide) polymer nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 688–698. [Google Scholar] [CrossRef]
- Liu, G.Y.; Lv, L.P.; Chen, C.J.; Liu, X.S.; Hu, X.F.; Ji, J. Biocompatible and biodegradable polymersomes for pH-triggered drug release. Soft. Matter. 2011, 7, 6629–6636. [Google Scholar] [CrossRef]
- Ma, B.; Zhuang, W.; Liu, G.; Wang, Y. A biomimetic and pH-sensitive polymeric micelle as carrier for paclitaxel delivery. Regen. Biomater. 2018, 5, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Drug-polymer conjugates: Potential for improved chemotherapy. Anti-Cancer Drugs 1992, 3, 175–210. [Google Scholar] [CrossRef]
- Wang, H.B.; Liu, X.S.; Wang, Y.; Chen, Y.J.; Jin, Q.; Ji, J. Doxorubicin conjugated phospholipid prodrugs as smart nanomedicine platforms for cancer therapy. J. Mater. Chem. B 2015, 3, 3297–3305. [Google Scholar] [CrossRef]
- Ma, B.; Zhuang, W.; Wang, Y.; Luo, R.; Wang, Y. pH-sensitive doxorubicin-conjugated prodrug micelles with charge-conversion for cancer therapy. Acta Biomater. 2018, 70, 186–196. [Google Scholar] [CrossRef]
- Chen, Y.; Han, H.; Tong, H.; Chen, T.; Wang, H.; Ji, J.; Jin, Q. Zwitterionic phosphorylcholine-TPE conjugate for pH-responsive drug delivery and AIE active imaging. ACS Appl. Mater. Interfaces 2016, 8, 21185–21192. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, D.; Wang, X.; Yang, F.; Shen, H.; Wu, D. Fabrication of zwitterionic and pH-responsive polyacetal dendrimers for anticancer drug delivery. Biomater. Sci. 2019, 7, 3238–3248. [Google Scholar] [CrossRef]
- Men, Y.; Peng, S.; Yang, P.; Jiang, Q.; Zhang, Y.; Shen, B.; Dong, P.; Pang, Z.; Yang, W. Biodegradable zwitterionic nanogels with long circulation for antitumor drug delivery. ACS Appl. Mater. Inter. 2018, 10, 23509–23521. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular understanding and design of zwitterionic materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Jiang, S. Dual-functional biomimetic materials: Nonfouling poly(carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules 2006, 7, 3311–3315. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kang, K.; Zhang, Y.; Yi, Q.; Gu, Z. Detachable polyzwitterion-coated ternary nanoparticles based on peptide dendritic carbon dots for efficient drug delivery in cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 43923–43935. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Li, R.; Ma, Y.; Li, N.; Zhang, T.; Cheng-Mei, X.; Jiang, H.T.; Gong, Y.K. Folate ligand orientation optimized during cell membrane mimetic micelle formation for enhanced tumor cell targeting. Langmuir 2019, 35, 1257–1265. [Google Scholar] [CrossRef]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
- Jinrong Peng, Q.Y.; Xiao, Y.; Shi, K.; Liu, Q.; Hao, Y.; Yang, F.; Han, R.; Qian, Z. Tumor microenvironment responsive drug-dye-peptide nanoassembly for enhanced tumor-targeting, penetration, and photo-chemo-immunotherapy. Adv. Funct. Mater. 2019, 29, 1900004.1–1900004.16. [Google Scholar] [CrossRef]
- Noh, T.; Kook, Y.H.; Park, C.; Youn, H.; Kim, H.; Oh, E.T.; Choi, E.K.; Park, H.J.; Kim, C. Block copolymer micelles conjugated with anti-EGFR antibody for targeted delivery of anticancer drug. J. Polym. Sci. Part A Pol. Chem. 2008, 46, 7321–7331. [Google Scholar] [CrossRef]
- Massignani, M.; LoPresti, C.; Blanazs, A.; Madsen, J.; Armes, S.P.; Lewis, A.L.; Battaglia, G. Controlling cellular uptake by surface chemistry, size, and surface topology at the nanoscale. Small 2009, 5, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Le Men, L.; Borsali, R.; Lai-Kee-Him, J.; Brisson, A.; Armes, S.P.; Lewis, A.L. Phosphorylcholine-based pH-responsive diblock copolymer micelles as drug delivery vehicles: Light scattering, electron microscopy, and fluorescence experiments. Biomacromolecules 2006, 7, 817–828. [Google Scholar] [CrossRef]
- Pegoraro, C.; Cecchin, D.; Gracia, L.S.; Warren, N.; Madsen, J.; Armes, S.P.; Lewis, A.; MacNeil, S.; Battaglia, G. Enhanced drug delivery to melanoma cells using PMPC-PDPA polymersomes. Cancer Lett. 2013, 334, 328–337. [Google Scholar] [CrossRef]
- Reddy, J.A.; Low, P.S. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit. Rev. Ther. Drug Carr. Syst. 1998, 15, 587–627. [Google Scholar] [CrossRef]
- Licciardi, M.; Craparo, E.F.; Giammona, G.; Armes, S.P.; Tang, Y.; Lewis, A.L. In vitro biological evaluation of folate-functionalized block copolymer micelles for selective anti-cancer drug delivery. Macromol. Biosci. 2008, 8, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Giammona, G.; Du, J.Z.; Armes, S.P.; Tang, Y.; Lewis, A.L. New folate-functionalized biocompatible block copolymer micelles as potential anti-cancer drug delivery systems. Polymer 2006, 47, 2946–2955. [Google Scholar] [CrossRef]
- Colombo, M.; Bianchi, A. Click chemistry for the synthesis of RGD-containing integrin ligands. Molecules 2010, 15, 178–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Song, H.; Wang, W.; Sun, Y.; Zhou, J.; Wang, X.; Liu, J.; Liu, J.; Kong, D.; Dong, A. Integrin-targeted zwitterionic polymeric nanoparticles with acid-induced disassembly property for enhanced drug accumulation and release in tumor. Biomacromolecules 2014, 15, 3128–3138. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of long-curculating zwitterionic cross-linked micelles for active-targeted drug delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef]
- Wu, M.; Cao, Z.; Zhao, Y.; Zeng, R.; Tu, M.; Zhao, J. Novel self-assembled pH-responsive biomimetic nanocarriers for drug delivery. Mater. Sci. Eng. C 2016, 64, 346–353. [Google Scholar] [CrossRef]
- Segregur, D.; Flanagan, T.; Mann, J.; Moir, A.; Karlsson, E.M.; Hoch, M.; Carlile, D.; Sayah-Jeanne, S.; Dressman, J. Impact of acid-reducing agents on gastrointestinal physiology and design of biorelevant dissolution tests to reflect these changes. J. Pharm. Sci. 2019, 108, 3461–3477. [Google Scholar] [CrossRef]
- Zhanga, P.; Suna, F.; Tsaoa, C.; Liub, S.; Jaina, P.; Sinclaira, A.; Hunga, H.C.; Bai, T.; Wu, K.; Jiang, S. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc. Natl. Acad. Sci. USA 2015, 112, 12046–12051. [Google Scholar] [CrossRef] [Green Version]
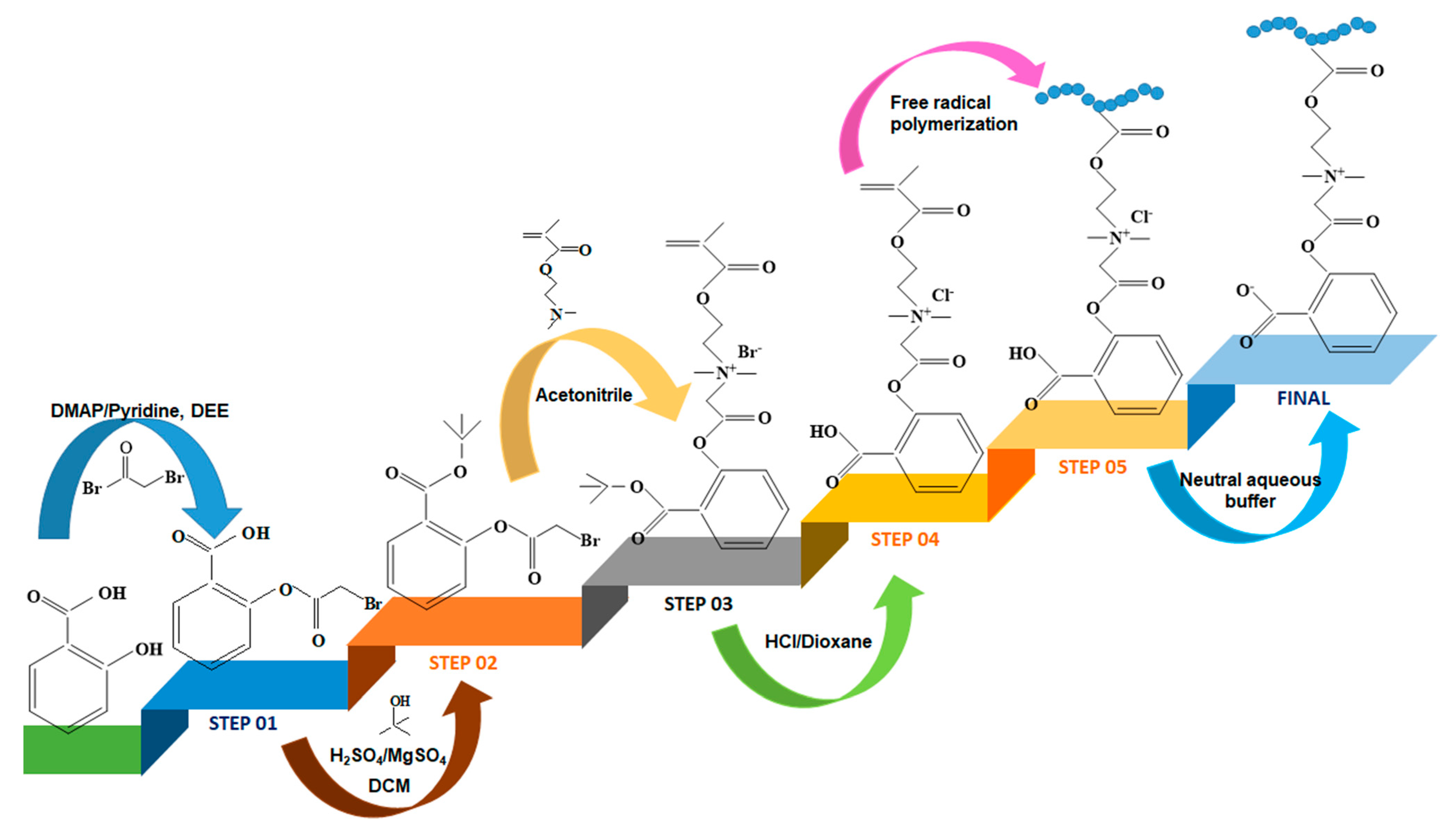
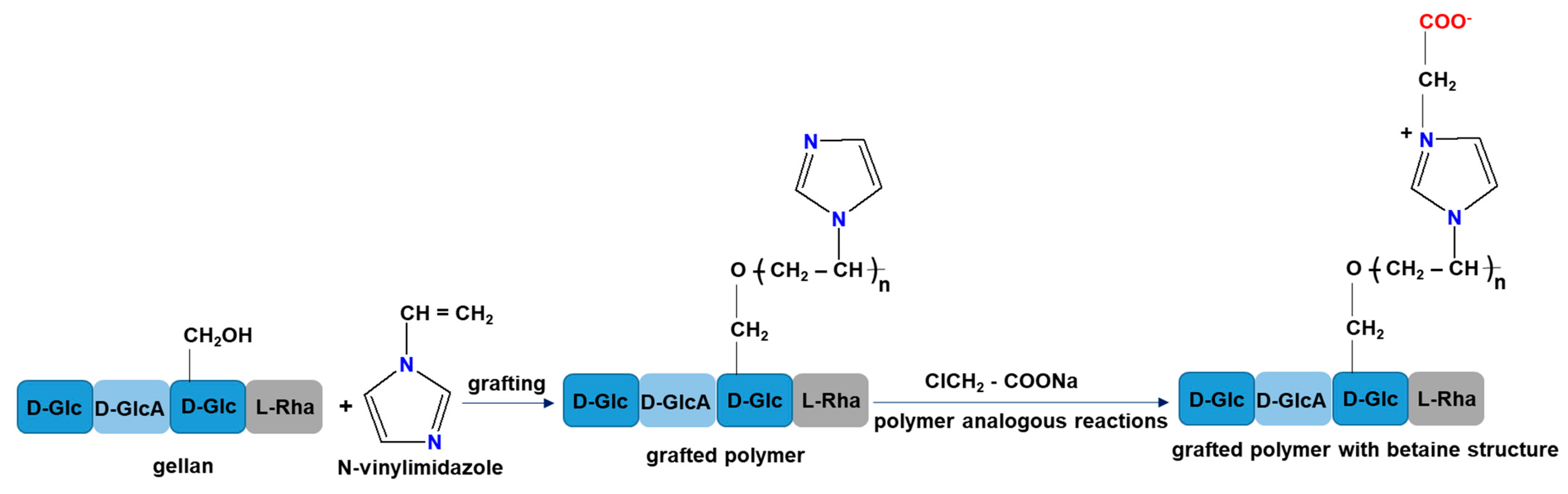
- Racovita, S.; Baranov, N.; Macsim, A.M.; Lionte, C.; Cheptea, C.; Sunel, V.; Popa, M.; Vasiliu, S.; Desbrieres, J. New grafted copolymers carrying betaine units based on gellan and N-vinylimidazole as precursors for design of drug delivery systems. Molecules 2020, 25, 5451. [Google Scholar] [CrossRef] [PubMed]
- Baranov, N.; Racovita, S.; Vasiliu, S.; Macsim, A.M.; Lionte, C.; Sunel, V.; Popa, M.; Desbrieres, J.; Cheptea, C. Immobilization and release studies of triazole derivatives from grafted copolymer based on gellan-carrying betaine units. Molecules 2021, 26, 3330. [Google Scholar] [CrossRef]
- Racovita, S.; Vasiliu, S.; Vlad, C.D. New drugs delivery systems based on polyelectrolyte complexes. Rev. Roum. Chim. 2010, 55, 659–666. [Google Scholar]
- Tsuboi, R.; Rifkin, D.B. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J. Exp. Med. 1990, 172, 245–251. [Google Scholar] [CrossRef]
- Kitano, H.; Tada, S.; Mori, T.; Takaha, K.; Gemmei-Ide, M.; Tanaka, M.; Fukuda, M.; Yokoyama, Y. Correlation between the structure of water in the vicinity of carboxybetaine polymers and their blood-compatibility. Langmuir 2005, 21, 11932—11940. [Google Scholar] [CrossRef] [PubMed]
- Fujishita, S.; Inaba, C.; Tada, S.; Gemmei-Ide, M.; Kitano, H.; Saruwatari, Y. Effect of zwitterionic polymers on wound healing. Biol. Pharm. Bull. 2008, 31, 2309–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Zhang, J.; Yang, J.; Pan, C.; Xu, T.; Zhang, L. Zwitterionic hydrogels promote skin wound healing. J. Mater. Chem. B 2016, 4, 5105–5111. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xiao, Z.; Zhou, Y.; Chen, A.; Xuan, X.; Li, Y.; Guo, X.; Zheng, J.; Xiao, J.; Wu, J. Zwitterionic poly(sulfobetaine methacrylate) hydrogels with optimal mechanical properties for improving wound healing in vivo. J. Mater. Chem. B 2019, 7, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Pazoki-Toroudi, H.; Bakhshayesh, A.R.D.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular-based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef]
- Langer, R. Perspectives and challenges in tissue engineering and regenerative medicine. Adv. Mater. 2009, 21, 3235–3236. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanism of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef]
- Xu, J.P.; Ji, J.; Chen, W.D.; Fan, D.Z.; Sun, Y.F.; Shen, J.C. Phospholipid based polymer as drug release coating for cardiovascular device. Eur. Polym. J. 2004, 40, 291−298. [Google Scholar] [CrossRef]
- Kitano, H.; Kawasaki, A.; Kawasaki, H.; Morokoshi, S. Resistance of zwitterionic telomers accumulated on metal surfaces against nonspecific adsorption of proteins. J. Colloid Interface Sci. 2005, 15, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Kong, B.; Choi, I.S. Highly efficient non-biofouling coating of zwitterionic polymers: Poly((3-(methacryloylamino)propyl)-dimethyl(3-sulfopropyl)ammonium hydroxide). Langmuir 2007, 23, 5678–5682. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, H.; Liu, Y.; Xia, Q.; Yang, Y.; Chen, N.; Yang, M.; Luo, R.; Liu, G.; Wang, Y. A robust mussel-inspired zwitterionic coating on biodegradable poly(L-lactide) stent with enhanced anticoagulant, anti-inflammatory, and anti-hyperplasia properties. Chem. Eng. J. 2022, 427, 130910. [Google Scholar] [CrossRef]
- Xu, R.; Cui, X.; Xin, Q.; Lu, M.; Li, Z.; Li, J.; Chen, X. Zwitterionic PMCP-functionalized titanium surface resists protein adsorption, promotes cell adhesion, and enhances osteogenic activity. Colloids Surf. B Biointerfaces 2021, 206, 111928. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gao, Q.; Long, Z.; Huo, Q.; Ge, Y.; Vianney, N.; Daliko, N.A.; Meng, Y.; Qu, J.; Chen, H.; et al. Polydopamine/poly(sulfobetaine methacrylate) Co-deposition coatings triggered by CuSO4/H2O2 on implants for improved surface hemocompatibility and antibacterial activity. Bioact. Mater. 2021, 6, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xue, H.; Carr, L.R.; Wang, J.; Jiang, S. Zwitterionic poly(carboxybetaine) hydrogels for glucose biosensors in complex media. Biosens. Bioelectron. 2011, 26, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- Neagu, V.; Vasiliu, S.; Racovita, S. Adsorption studies of some inorganic and organic salts on new zwitterionic ion exchangers with carboxybetaine moieties. Chem. Eng. J. 2010, 162, 965–973. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, V.K.; Singh, M. Zwitterionic polyelectrolytes: A review. e-Polymers 2007, 7, 1–34. [Google Scholar] [CrossRef]
- Garg, G.; Chauhan, G.S.; Ahn, J.H. Polysylfobetaines as extractants for Sr(II) ions from aqueous solutions. Polym. Adv. Technol. 2011, 22, 1794–1801. [Google Scholar] [CrossRef]
- Lloyd, A.W.; Baker, J.A.; Smith, G.; Olliff, C.J.; Rutt, K.J. A comparison of glycine, sarcosine, N,N-dimethylglycine, glycine betaine and N-modified betaines as liposome cryoprotectants. J. Pharm. Pharmacol. 1992, 44, 507–511. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitterion: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]

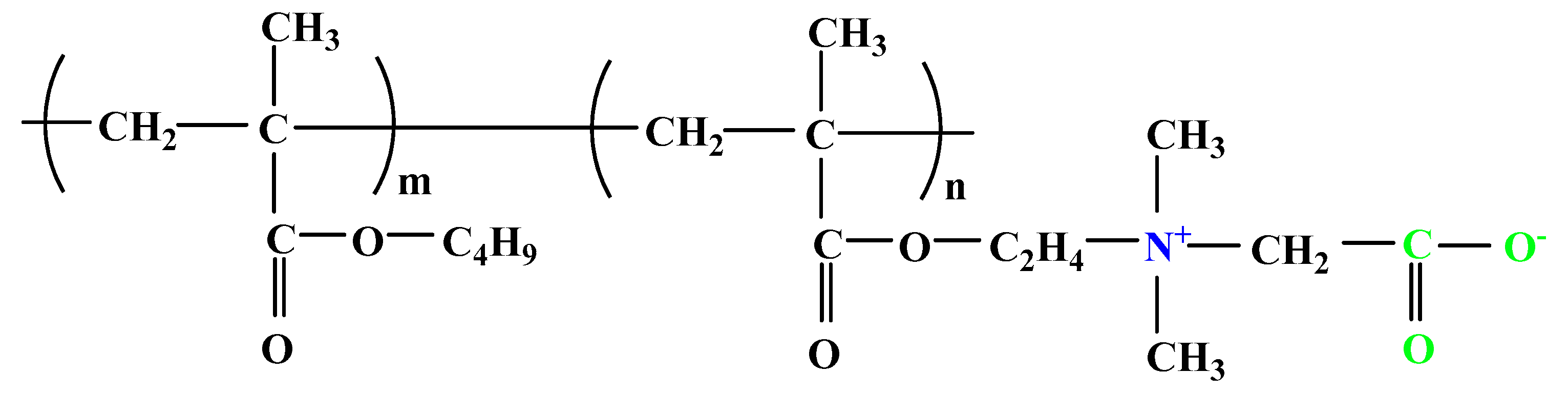
| Name and Chemical Structures | Ref. | |||
|---|---|---|---|---|
| Sulfobetaines Monomers | ||||
| 1 | 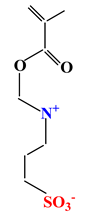 | 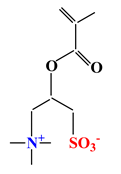 | 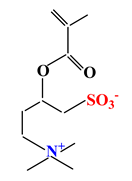 | [33] |
| P4VPPC-co-PDMAPC copolymer | ||||
| 2 | 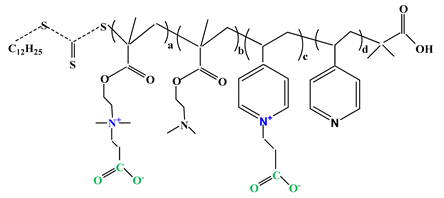 | [34] | ||
| Polybetaines based on poly(ethylene glycol) | ||||
| 3 | 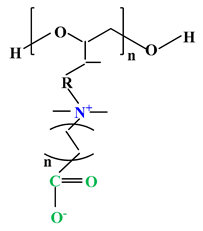 | 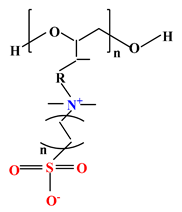 | 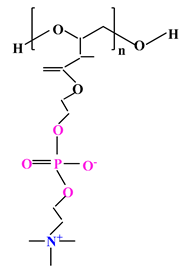 | [35] |
| Polybetaines based on poly(acrylates) (PA) | ||||
| 4 |  | 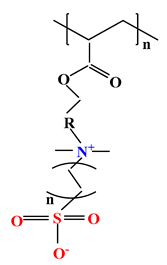 | 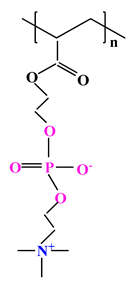 | [35] |
| Polybetaines based on poly(acrylamides) (PAA) | ||||
| 5 | 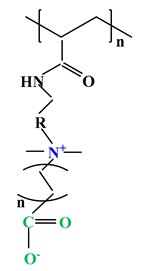 | 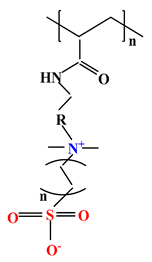 | 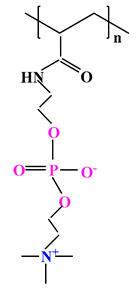 | [35] |
| Sulfobetaine-containing terpolymer | ||||
| 6 | 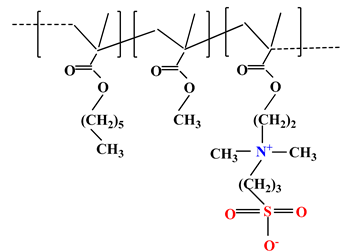 | [36] | ||
| Chemical Structure | Name | |
|---|---|---|
| 1 | 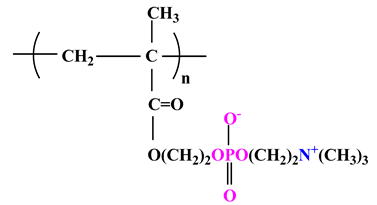 | Poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) |
| 2 | 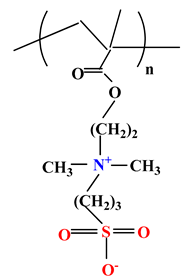 | Poly(sulfobetaine methacrylate) (PSBMA) |
| 3 | 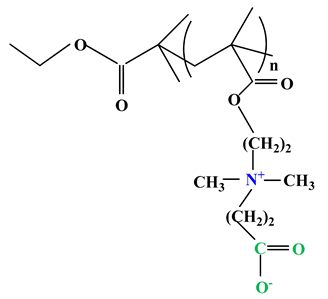 | Poly(carboxybetainem methacrylate) (PCBMA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racovita, S.; Trofin, M.-A.; Loghin, D.F.; Zaharia, M.-M.; Bucatariu, F.; Mihai, M.; Vasiliu, S. Polybetaines in Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 9321. https://doi.org/10.3390/ijms22179321
Racovita S, Trofin M-A, Loghin DF, Zaharia M-M, Bucatariu F, Mihai M, Vasiliu S. Polybetaines in Biomedical Applications. International Journal of Molecular Sciences. 2021; 22(17):9321. https://doi.org/10.3390/ijms22179321
Chicago/Turabian StyleRacovita, Stefania, Marin-Aurel Trofin, Diana Felicia Loghin, Marius-Mihai Zaharia, Florin Bucatariu, Marcela Mihai, and Silvia Vasiliu. 2021. "Polybetaines in Biomedical Applications" International Journal of Molecular Sciences 22, no. 17: 9321. https://doi.org/10.3390/ijms22179321