Elevated Pro-Inflammatory Cell-Free MicroRNA Levels in Cerebrospinal Fluid of Premature Infants after Intraventricular Hemorrhage
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics of Study Participants
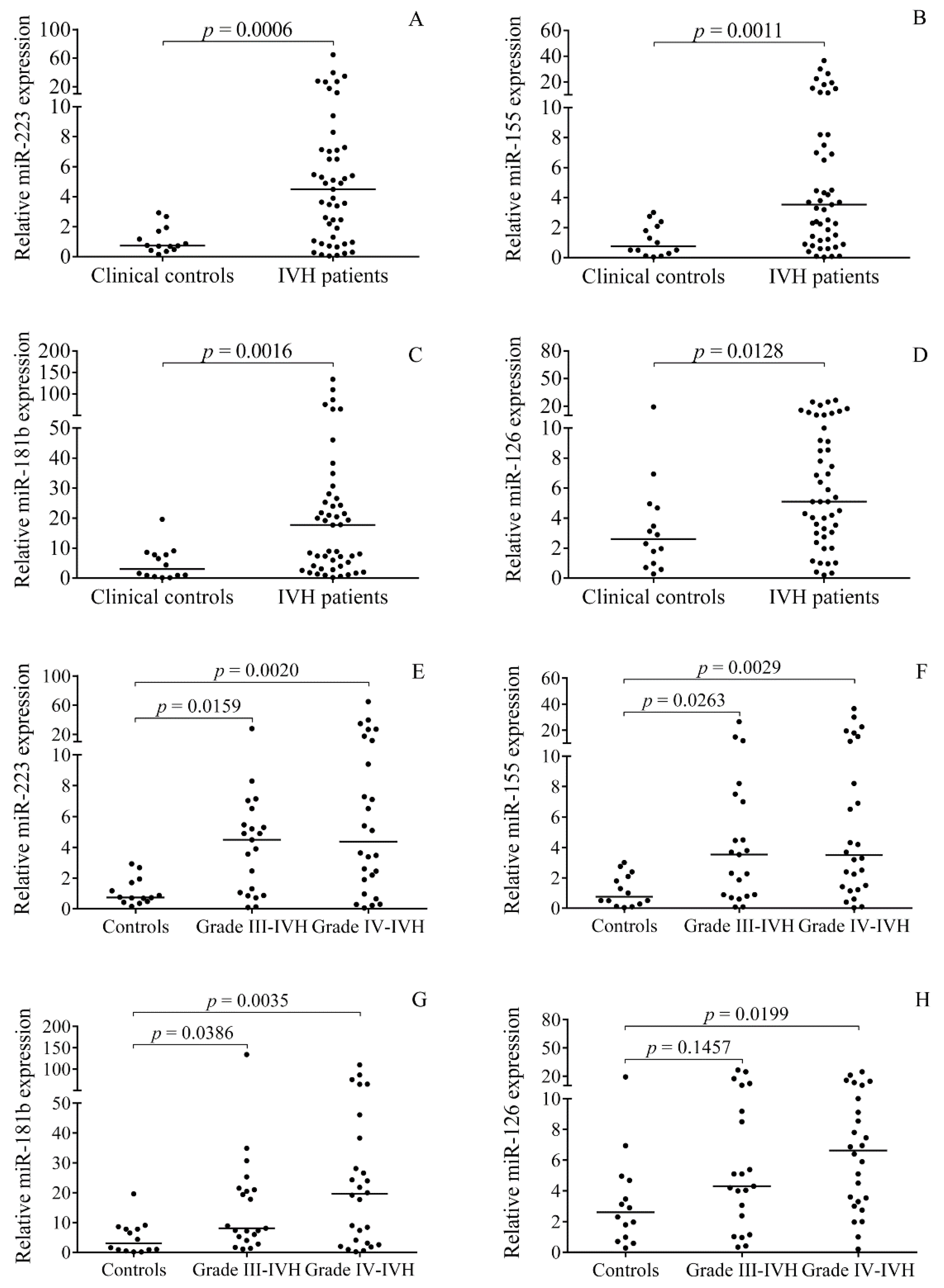
2.2. Elevated miRNA Levels in CSF after the Onset of Preterm IVH
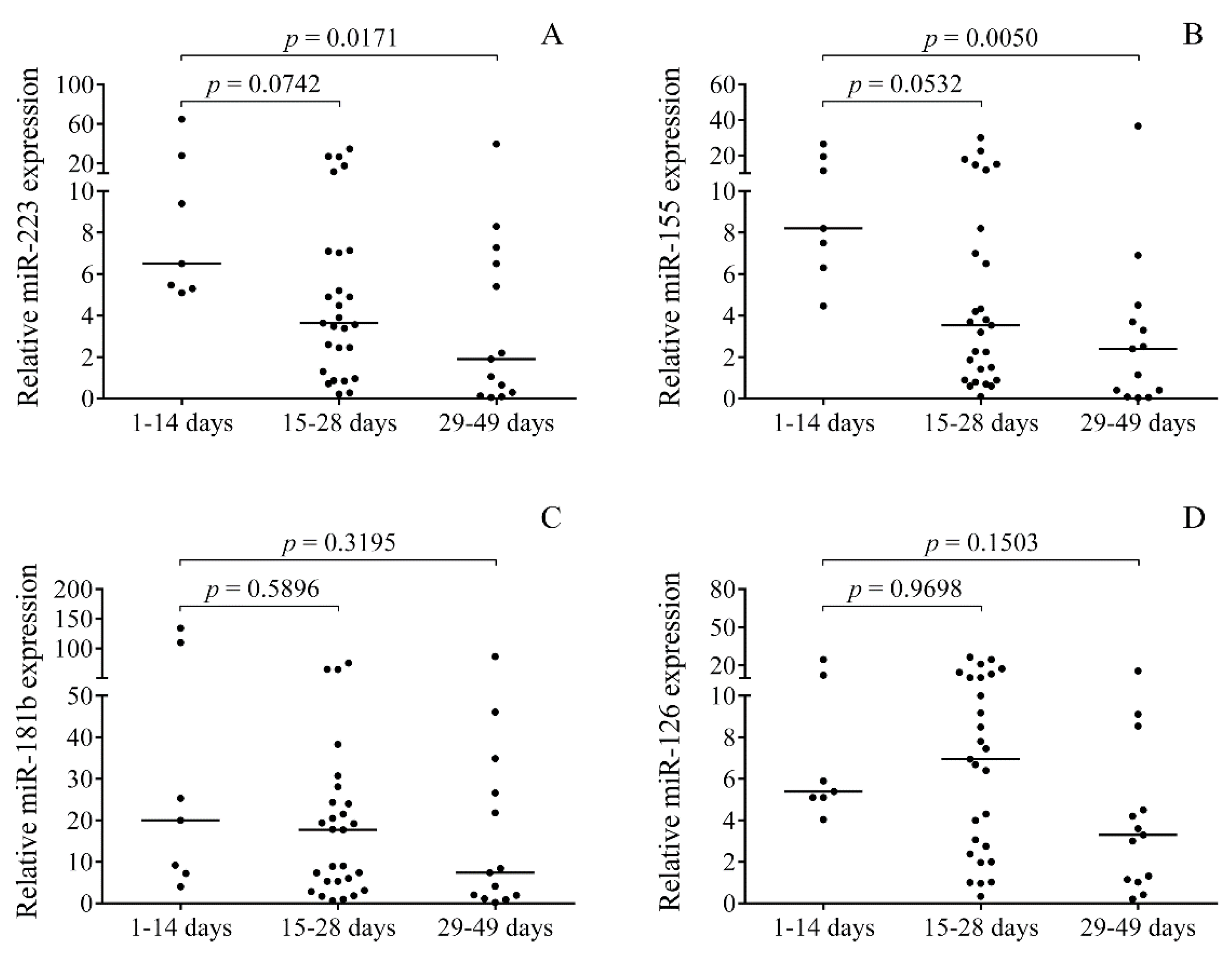
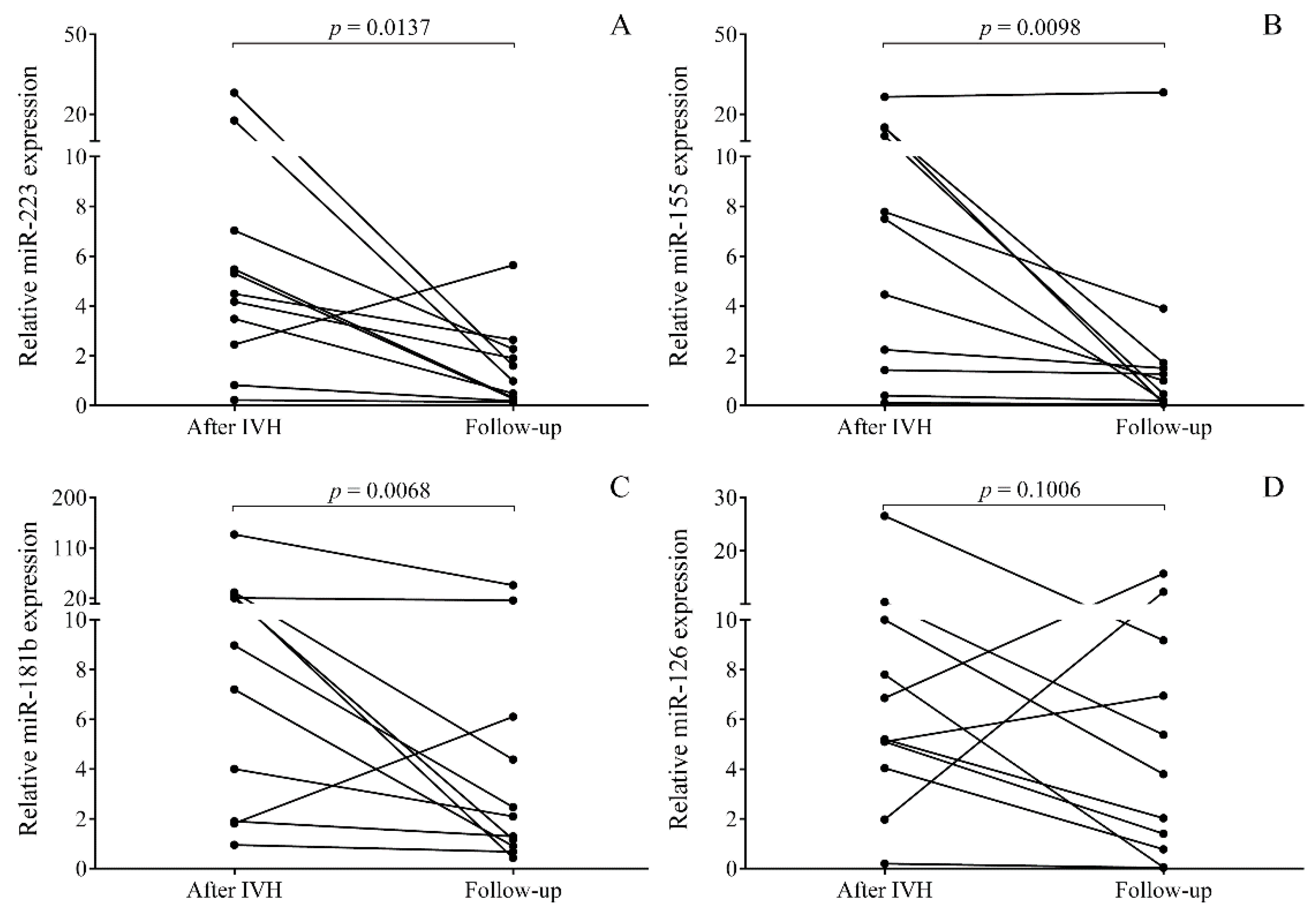
2.3. Time-Dependent Alteration in miRNAs in Post-IVH CSF Samples
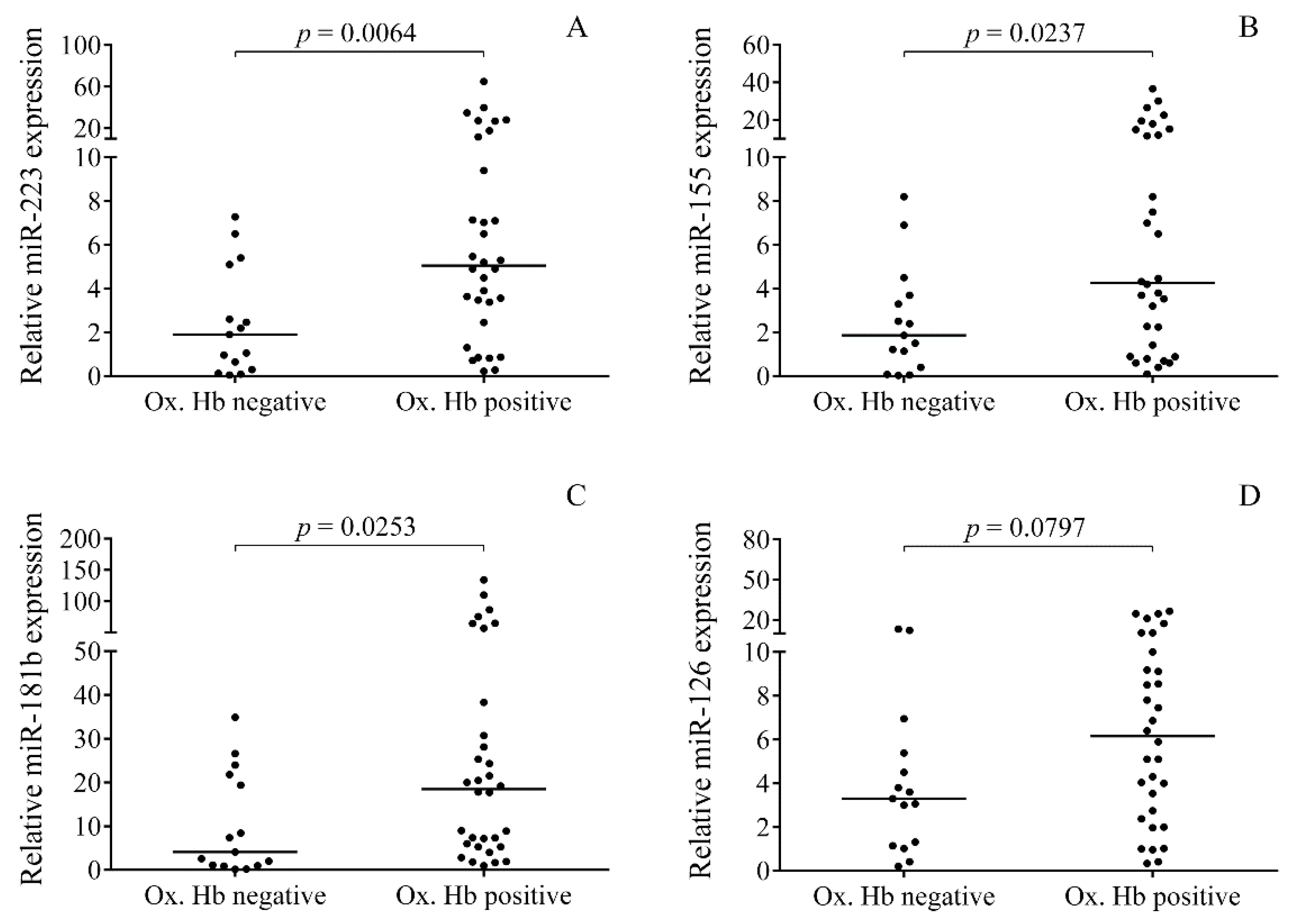
2.4. Relationship between the Levels of Oxidized Hb Forms and miRNAs in CSF after IVH
2.5. Correlations between Pro-Inflammatory Protein Biomarkers and miRNAs in Post-IVH CSF Samples
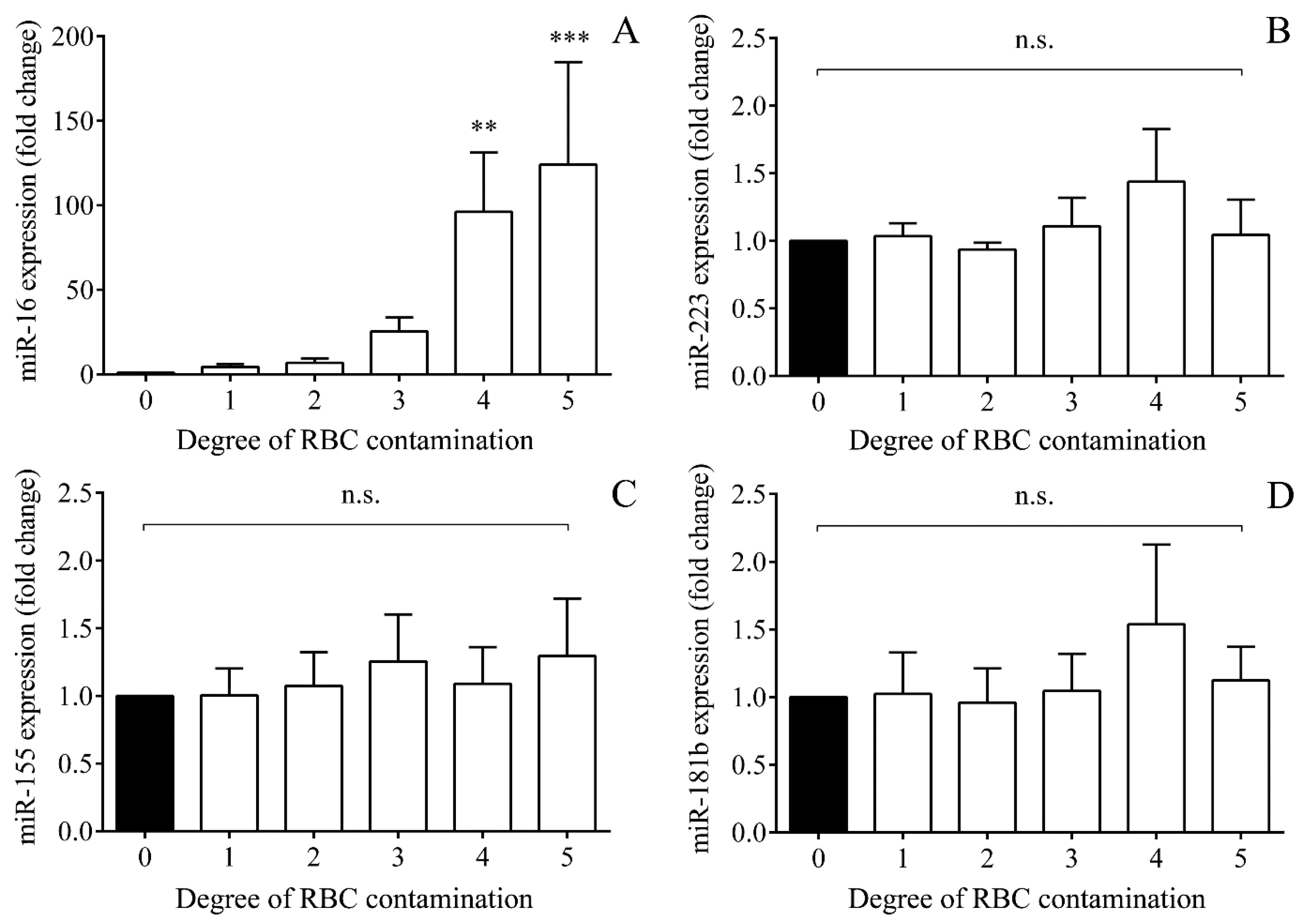
2.6. Impact of Hemolysis on miRNAs Based on In Vitro Controlled Hemolysis Experiments
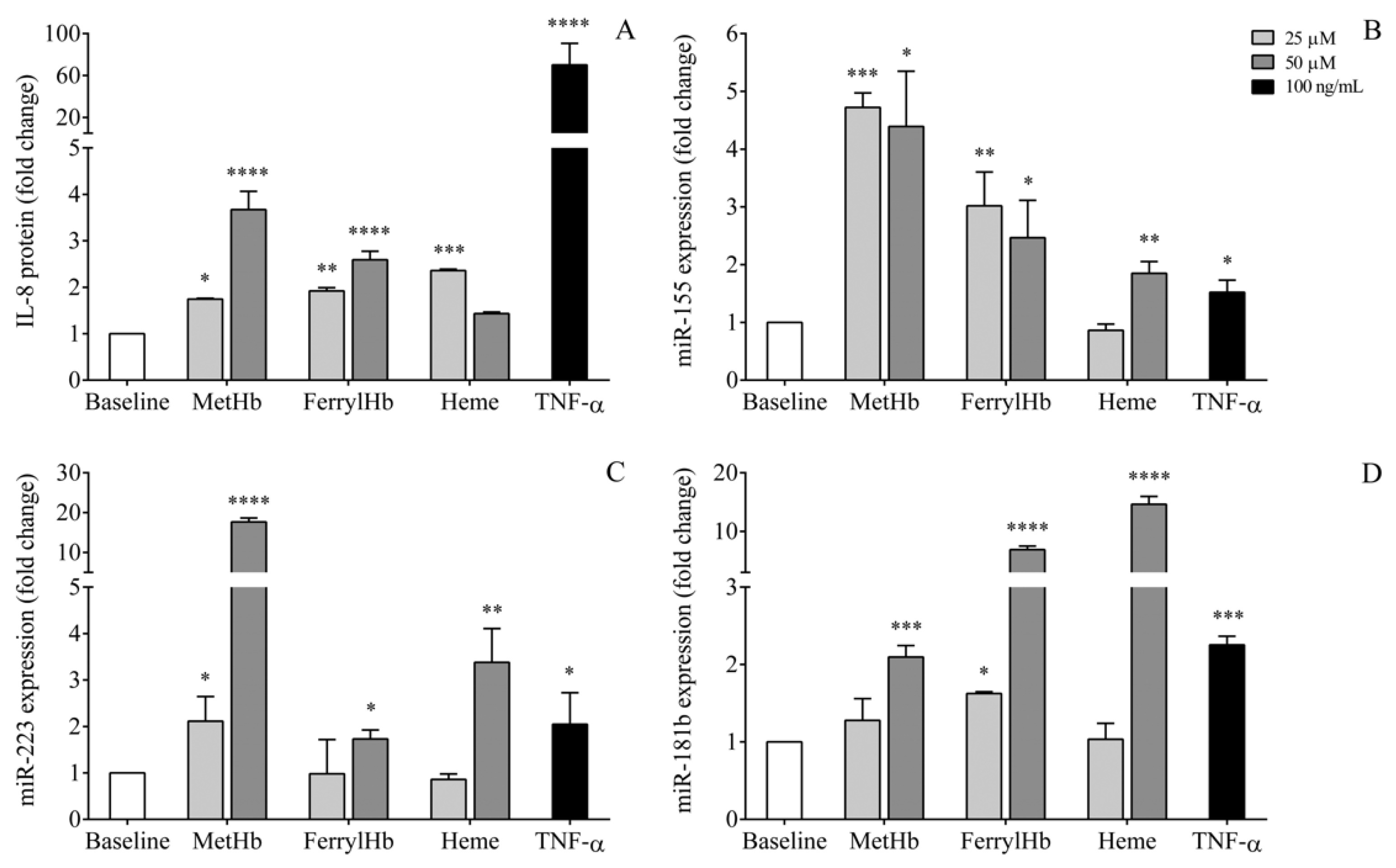
2.7. miRNA Levels in the Supernatant of Human Choroid Plexus Epithelial Cell Cultures after Treatment with Oxidized Hb Forms and Heme
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. CSF Sample Collection and Preparation
4.3. Human Choroid Plexus Epithelial Cell Culture
4.4. Treatment of Human Choroid Plexus Epithelial Cells with Oxidative Hb Forms and Heme
4.5. Extraction of miRNAs from CSF Samples and HCPEpiC Supernatants
4.6. miRNA-Specific Stem-Loop RT-qPCR Analysis
4.7. Determination of Hemoglobin Forms and Heme Levels in CSF Samples
4.8. Measurement of Soluble Adhesion Molecule Concentrations
4.9. Other Laboratory Analyses
4.10. In Vitro Controlled Hemolysis Experiments
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CSF | cerebrospinal fluid |
| CNS | central nervous system |
| CRP | C-reactive protein |
| DCI | delayed cerebral ischemia |
| EC | endothelial cell |
| ELISA | enzyme-linked immunosorbent assay |
| Hb | hemoglobin |
| HCPEpiC | human choroid plexus epithelial cell |
| ICAM-1 | intercellular adhesion molecule 1 |
| IL | interleukin |
| IVH | intraventricular hemorrhage |
| LDH | lactate dehydrogenase |
| miRNA | microRNA |
| NF-κB | nuclear factor kappa-B |
| Ox. Hb | oxidized hemoglobin |
| PCT | procalcitonin |
| RBC | red blood cell |
| RT | room temperature |
| RT-qPCR | real-time quantitative polymerase chain reaction |
| S100B | S100 calcium-binding protein B |
| SAH | subarachnoid hemorrhage |
| TLR4 | Toll-like receptor 4 |
| TNF-α | tumor necrosis factor alpha |
| UPL | Universal ProbeLibrary |
| VCAM-1 | vascular cell adhesion molecule 1 |
| WBC | white blood cell |
References
- Jain, N.J.; Kruse, L.K.; Demissie, K.; Khandelwal, M. Impact of mode of delivery on neonatal complications: Trends between 1997 and 2005. J. Matern. Fetal Neonatal Med. 2009, 22, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P. Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatr. Res. 2010, 67, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, A.J. The role of systemic hemodynamic disturbances in prematurity-related brain injury. J. Child. Neurol. 2009, 24, 1127–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Romantsik, O.; Bruschettini, M.; Ley, D. Intraventricular Hemorrhage and White Matter Injury in Preclinical and Clinical Studies. Neoreviews 2019, 20, e636–e652. [Google Scholar] [CrossRef] [PubMed]
- Jeney, V.; Eaton, J.W.; Balla, G.; Balla, J. Natural history of the bruise: Formation, elimination, and biological effects of oxidized hemoglobin. Oxid. Med. Cell Longev. 2013, 2013, 703571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Gram, M.; Sveinsdottir, S.; Ruscher, K.; Hansson, S.R.; Cinthio, M.; Akerström, B.; Ley, D. Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. J. Neuroinflamm. 2013, 10, 100. [Google Scholar] [CrossRef] [Green Version]
- Gram, M.; Sveinsdottir, S.; Cinthio, M.; Sveinsdottir, K.; Hansson, S.R.; Mörgelin, M.; Akerström, B.; Ley, D. Extracellular hemoglobin–mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. J. Neuroinflamm. 2014, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Milbauer, L.; Abdulla, F.; Alayash, A.I.; Smith, A.; Nath, K.A.; Hebbel, R.P.; Vercellotti, G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 2014, 123, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Leviton, A.; Allred, E.N.; Dammann, O.; Engelke, S.; Fichorova, R.N.; Hirtz, D.; Kuban, K.C.; Ment, L.R.; O’shea, T.M.; Paneth, N.; et al. ELGAN Study Investigators. Systemic inflammation, intraventricular hemorrhage, and white matter injury. J. Child. Neurol. 2013, 28, 1637–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poralla, C.; Hertfelder, H.J.; Oldenburg, J.; Müller, A.; Bartmann, P.; Heep, A. Elevated interleukin-6 concentration and alterations of the coagulation system are associated with the development of intraventricular hemorrhage in extremely preterm infants. Neonatology 2012, 102, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Meeker, R.B.; Williams, K.; Killebrew, D.A.; Hudson, L.C. Cell trafficking through the choroid plexus. Cell Adh. Migr. 2012, 6, 390–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivisäkk, P.; Mahad, D.J.; Callahan, M.K.; Trebst, C.; Tucky, B.; Wei, T.; Wu, L.; Baekkevold, E.S.; Lassmann, H.; Staugaitis, S.M.; et al. Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl. Acad. Sci. USA 2003, 100, 8389–8394. [Google Scholar] [CrossRef] [Green Version]
- Polin, R.S.; Bavbek, M.; Shaffrey, M.E.; Billups, K.; Bogaev, C.A.; Kassell, N.F.; Lee, K.S. Detection of soluble E-selectin, ICAM-1, VCAM-1, and L-selectin in the cerebrospinal fluid of patients after subarachnoid hemorrhage. J. Neurosurg. 1998, 89, 559–567. [Google Scholar] [CrossRef]
- Jaber, S.M.; Hamed, E.A.; Hamed, S.A. Adhesion molecule levels in serum and cerebrospinal fluid in children with bacterial meningitis and sepsis. J. Pediatr. Neurosci. 2009, 4, 76–85. [Google Scholar]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Ann. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Becker Buscaglia, L.E.; Barker, J.R.; Li, Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011, 3, 159–166. [Google Scholar] [CrossRef]
- Tabet, F.; Vickers, K.C.; Cuesta Torres, L.F.; Wiese, C.B.; Shoucri, B.M.; Lambert, G.; Catherinet, C.; Prado-Lourenco, L.; Levin, M.G.; Thacker, S.; et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 2014, 5, 3292. [Google Scholar] [CrossRef] [Green Version]
- Nazari-Jahantigh, M.; Wei, Y.; Schober, A. The role of microRNAs in arterial remodelling. Thromb. Haemost. 2012, 107, 611–618. [Google Scholar] [CrossRef]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Icli, B.; Wara, A.K.; Belkin, N.; He, S.; Kobzik, L.; Hunninghake, G.M.; Vera, M.P.; MICU Registry; Blackwell, T.S.; et al. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J. Clin. Investig. 2012, 122, 1973–1990. [Google Scholar] [PubMed]
- Fejes, Z.; Czimmerer, Z.; Szük, T.; Póliska, S.; Horváth, A.; Balogh, E.; Jeney, V.; Váradi, J.; Fenyvesi, F.; Balla, G.; et al. Endothelial cell activation is attenuated by everolimus via transcriptional and post-transcriptional regulatory mechanisms after drug-eluting coronary stenting. PLoS ONE 2018, 13, e0197890. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.D.; Li, L.; Chan, W.Y. MicroRNAs: Key Regulators in the Central Nervous System and Their Implication in Neurological Diseases. Int. J. Mol. Sci. 2016, 17, 842. [Google Scholar] [CrossRef]
- Saugstad, J.A. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J. Cereb. Blood Flow Metab. 2010, 30, 1564–1576. [Google Scholar] [CrossRef]
- Bache, S.; Rasmussen, R.; Rossing, M.; Laigaard, F.P.; Nielsen, F.C.; Møller, K. MicroRNA Changes in Cerebrospinal Fluid After Subarachnoid Hemorrhage. Stroke 2017, 48, 2391–2398. [Google Scholar] [CrossRef]
- Erdei, J.; Tóth, A.; Nagy, A.; Nyakundi, B.B.; Fejes, Z.; Nagy, B., Jr.; Novák, L.; Bognár, L.; Balogh, E.; Paragh, G.; et al. The Role of Hemoglobin Oxidation Products in Triggering Inflammatory Response Upon Intraventricular Hemorrhage in Premature Infants. Front. Immunol. 2020, 11, 228. [Google Scholar] [CrossRef] [Green Version]
- Habiyaremye, G.; Morales, D.M.; Morgan, C.D.; McAllister, J.P.; CreveCoeur, T.S.; Han, R.H.; Gabir, M.; Baksh, B.; Mercer, D.; Limbrick, D.D., Jr. Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus. Fluids Barriers CNS 2017, 14, 35. [Google Scholar] [CrossRef] [Green Version]
- Kirschner, M.B.; Kao, S.C.; Edelman, J.J.; Armstrong, N.J.; Vallely, M.P.; van Zandwijk, N.; Reid, G. Haemolysis during sample preparation alters microRNA content of plasma. PLoS ONE 2011, 6, e24145. [Google Scholar] [CrossRef]
- Pizzamiglio, S.; Zanutto, S.; Ciniselli, C.M.; Belfiore, A.; Bottelli, S.; Gariboldi, M.; Verderio, P. A methodological procedure for evaluating the impact of hemolysis on circulating microRNAs. Oncol. Lett. 2017, 13, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Mukerji, A.; Shah, V.; Shah, P.S. Periventricular/Intraventricular Hemorrhage and Neurodevelopmental Outcomes: A Meta-analysis. Pediatrics 2015, 136, 1132–1143. [Google Scholar] [CrossRef] [Green Version]
- Perrone, S.; Santacroce, A.; Longini, M.; Proietti, F.; Bazzini, F.; Buonocore, G. The Free Radical Diseases of Prematurity: From Cellular Mechanisms to Bedside. Oxid. Med. Cell Longev. 2018, 2018, 7483062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redell, J.B.; Moore, A.N.; Ward, N.H.; Hergenroeder, G.W.; Dash, P.K. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma 2010, 27, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.S.; Nygaard, A.B.; Nielsen, M.Y.; Jensen, K.; Christensen, T. miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl. Stroke Res. 2014, 5, 711–718. [Google Scholar] [CrossRef]
- Szilágyi, B.; Fejes, Z.; Pócsi, M.; Kappelmayer, J.; Nagy, B., Jr. Role of sepsis modulated circulating microRNAs. EJIFCC 2019, 30, 128–145. [Google Scholar] [PubMed]
- Xiang, J.; Routhe, L.J.; Wilkinson, D.A.; Hua, Y.; Moos, T.; Xi, G.; Keep, R.F. The choroid plexus as a site of damage in hemorrhagic and ischemic stroke and its role in responding to injury. Fluids Barriers CNS 2017, 14, 8. [Google Scholar] [CrossRef] [Green Version]
- Bache, S.; Rasmussen, R.; Wolcott, Z.; Rossing, M.; Møgelvang, R.; Tolnai, D.; Hassager, C.; Forman, J.L.; Køber, L.; Nielsen, F.C.; et al. Elevated miR-9 in Cerebrospinal Fluid Is Associated with Poor Functional Outcome After Subarachnoid Hemorrhage. Transl. Stroke Res. 2020. [Google Scholar] [CrossRef]
- Stylli, S.S.; Adamides, A.A.; Koldej, R.M.; Luwor, R.B.; Ritchie, D.S.; Ziogas, J.; Kaye, A.H. miRNA expression profiling of cerebrospinal fluid in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2017, 126, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.T.H.; Wong, J.Y.Y.; Leung, A.K.T.; Lu, G.; Poon, W.S.; Lau, A.Y.L.; Chan, W.Y.; Wong, G.K.C. Plasma and CSF miRNA dysregulations in subarachnoid hemorrhage reveal clinical courses and underlying pathways. J. Clin. Neurosci. 2019, 62, 155–161. [Google Scholar] [CrossRef]
- Lau, P.; Bossers, K.; Janky, R.; Salta, E.; Frigerio, C.S.; Barbash, S.; Rothman, R.; Sierksma, A.S.; Thathiah, A.; Greenberg, D.; et al. Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 1613–1634. [Google Scholar] [CrossRef] [Green Version]
- Powers, C.J.; Dickerson, R.; Zhang, S.W.; Rink, C.; Roy, S.; Sen, C.K. Human cerebrospinal fluid microRNA: Temporal changes following subarachnoid hemorrhage. Physiol. Genom. 2016, 48, 361–366. [Google Scholar] [CrossRef]
- Nyakundi, B.B.; Erdei, J.; Tóth, A.; Balogh, E.; Nagy, A.; Nagy, B., Jr.; Novák, L.; Bognár, L.; Paragh, G.; Kappelmayer, J.; et al. Formation and Detection of Highly Oxidized Hemoglobin Forms in Biological Fluids during Hemolytic Conditions. Oxid. Med. Cell Longev. 2020, 2020, 8929020. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martinez, E.; Fumagalli, M.; Mohammed Rahim, O.; Passera, S.; Cavallaro, G.; Degraeuwe, P.; Mosca, F.; Villamor, E. Chorioamnionitis Is a Risk Factor for Intraventricular Hemorrhage in Preterm Infants: A Systematic Review and Meta-Analysis. Front. Physiol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laborada, G.; Nesin, M. Interleukin-6 and interleukin-8 are elevated in the cerebrospinal fluid of infants exposed to chorioamnionitis. Biol. Neonat. 2005, 88, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Szpecht, D.; Wiak, K.; Braszak, A.; Szymankiewicz, M.; Gadzinowski, J. Role of selected cytokines in the etiopathogenesis of intraventricular hemorrhage in preterm newborns. Childs Nerv. Syst. 2016, 32, 2097–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaull, R.; McPherson, B.; Gialeli, A.; Clayton, A.; Uney, J.; Heep, A.; Cordero-Llana, Ó. Exosomes populate the cerebrospinal fluid of preterm infants with post-haemorrhagic hydrocephalus. Int. J. Dev. Neurosci. 2019, 73, 59–65. [Google Scholar] [CrossRef]
- Bozza, M.T.; Jeney, V. Pro-inflammatory Actions of Heme and Other Hemoglobin-Derived DAMPs. Front. Immunol. 2020, 11, 1323. [Google Scholar] [CrossRef]
- Silva, G.; Jeney, V.; Chora, A.; Larsen, R.; Balla, J.; Soares, M.P. Oxidized hemoglobin is an endogenous proinflammatory agonist that targets vascular endothelial cells. J. Biol. Chem. 2009, 284, 29582–29595. [Google Scholar] [CrossRef] [Green Version]
- McClugage, S.G.; Laskay, N.M.B.; Donahue, B.N.; Arynchyna, A.; Zimmerman, K.; Aban, I.B.; Alford, E.N.; Peralta-Carcelen, M.; Blount, J.P.; Rozzelle, C.J.; et al. Functional outcomes at 2 years of age following treatment for posthemorrhagic hydrocephalus of prematurity: What do we know at the time of consult? J. Neurosurg. Pediatr. 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Fejes, Z.; Póliska, S.; Czimmerer, Z.; Káplár, M.; Penyige, A.; Gál Szabó, G.; Beke Debreceni, I.; Kunapuli, S.P.; Kappelmayer, J.; Nagy, B., Jr. Hyperglycaemia suppresses microRNA expression in platelets to increase P2RY12 and SELP levels in type 2 diabetes mellitus. Thromb. Haemost. 2017, 117, 529–542. [Google Scholar] [CrossRef]
- Czimmerer, Z.; Hulvely, J.; Simandi, Z.; Varallyay, E.; Havelda, Z.; Szabo, E.; Varga, A.; Dezso, B.; Balogh, M.; Horvath, A.; et al. A versatile method to design stem-loop primer-based quantitative PCR assays for detecting small regulatory RNA molecules. PLoS ONE 2013, 8, e55168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.; Alayash, A.I. Determination of extinction coefficients of human hemoglobin in various redox states. Anal. Biochem. 2017, 521, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | IVH Patients (n = 47) | Clinical Controls (n = 14) | p Value |
|---|---|---|---|
| Gestational age (weeks) | 28.2 ± 3.2 | 32.0 ± 6.8 | 0.3427 |
| Gender (male/female) | 26/21 | 7/7 | 0.7259 |
| Birth weight (g) | 1235 ± 610 | 2046 ± 1281 | 0.240 |
| Severity of IVH (grade III/IV) | 21/26 | ||
| Whole blood WBC count (G/L) | 12.0 (9.77–13.80) | 10.26 (8.64–12.33) | 0.0940 |
| Whole blood Hb (g/L) | 120 (102–135) | 107 (99–130) | 0.4474 |
| Whole blood platelet count (G/L) | 384 (286–484) | 370 (288–509) | 0.963 |
| Serum CRP (mg/L) | 1.34 (0.60–3.76) | 0.50 (0.50–4.51) | 0.2192 |
| Serum PCT (µg/L) | 0.29 (0.19–0.50) | 0.15 (0.10–0.19) | 0.0066 |
| CSF RBC count (cells/µL) | 4267 (1536–10400) | 2 (1–518) | <0.0001 |
| CSF WBC count (cells/µL) | 52 (23–207) | 8 (4–26) | 0.0005 |
| CSF total protein (mg/L) | 1799 (1376–2498) | 758 (448–1510) | 0.0007 |
| CSF S100B (µg/L) | 7.49 (5.01–10.40) | 3.23 (1.12–5.46) | 0.0003 |
| CSF lactate (mmol/L) | 3.47 (2.58–3.70) | 2.16 (1.55–2.45) | 0.0002 |
| CSF oxidized Hb (µmol/L) | 36.98 ± 54.64 | 1.82 ± 5.84 | 0.0005 |
| CSF total heme (µmol/L) | 226.3 ± 262.9 | 0.78 ± 2.81 | <0.0001 |
| CSF RBC | CSF WBC | E-selectin | ICAM-1 | VCAM-1 | IL-8 | Ox. Hb | Total Heme | |
|---|---|---|---|---|---|---|---|---|
| miR-223 | r = 0.5293 p < 0.0001 | r = 0.5135 p < 0.0001 | r = 0.4244 p = 0.0002 | r = 0.3683 p = 0.0009 | r = 0.5101 p < 0.0001 | r = 0.5049 p < 0.0001 | r = 0.5931 p < 0.0001 | r = 0.6281 p < 0.0001 |
| miR-155 | r = 0.4241 p < 0.0001 | r = 0.2874 p = 0.0107 | r = 0.2670 p = 0.0110 | r = 0.2721 p = 0.0070 | r = 0.3733 p = 0.0036 | r = 0.3160 p = 0.0097 | r = 0.4735 p < 0.0001 | r = 0.5394 p < 0.0001 |
| miR-181b | r = 0.3554 p = 0.0014 | r = 0.2254 p = 0.0487 | r = 0.2680 p = 0.0111 | r = 0.2397 p = 0.0187 | r = 0.2994 p = 0.0212 | r = 0.3486 p = 0.0041 | r = 0.4198 p = 0.0001 | r = 0.4510 p < 0.0001 |
| miR-126 | r = 0.2975 p = 0.0057 | r = 0.0865 p = 0.4933 | r = 0.3588 p = 0.0014 | r = 0.2133 p = 0.0514 | r = 0.1832 p = 0.2030 | r = 0.3303 p = 0.0129 | r = 0.2432 p = 0.0323 | r = 0.3558 p = 0.0010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fejes, Z.; Erdei, J.; Pócsi, M.; Takai, J.; Jeney, V.; Nagy, A.; Varga, A.; Bácsi, A.; Bognár, L.; Novák, L.; et al. Elevated Pro-Inflammatory Cell-Free MicroRNA Levels in Cerebrospinal Fluid of Premature Infants after Intraventricular Hemorrhage. Int. J. Mol. Sci. 2020, 21, 6870. https://doi.org/10.3390/ijms21186870
Fejes Z, Erdei J, Pócsi M, Takai J, Jeney V, Nagy A, Varga A, Bácsi A, Bognár L, Novák L, et al. Elevated Pro-Inflammatory Cell-Free MicroRNA Levels in Cerebrospinal Fluid of Premature Infants after Intraventricular Hemorrhage. International Journal of Molecular Sciences. 2020; 21(18):6870. https://doi.org/10.3390/ijms21186870
Chicago/Turabian StyleFejes, Zsolt, Judit Erdei, Marianna Pócsi, Jun Takai, Viktória Jeney, Andrea Nagy, Alíz Varga, Attila Bácsi, László Bognár, László Novák, and et al. 2020. "Elevated Pro-Inflammatory Cell-Free MicroRNA Levels in Cerebrospinal Fluid of Premature Infants after Intraventricular Hemorrhage" International Journal of Molecular Sciences 21, no. 18: 6870. https://doi.org/10.3390/ijms21186870
APA StyleFejes, Z., Erdei, J., Pócsi, M., Takai, J., Jeney, V., Nagy, A., Varga, A., Bácsi, A., Bognár, L., Novák, L., Kappelmayer, J., & Nagy, B., Jr. (2020). Elevated Pro-Inflammatory Cell-Free MicroRNA Levels in Cerebrospinal Fluid of Premature Infants after Intraventricular Hemorrhage. International Journal of Molecular Sciences, 21(18), 6870. https://doi.org/10.3390/ijms21186870






