Migrating Myofibroblastic Iliotibial Band-Derived Fibroblasts Represent a Promising Cell Source for Ligament Reconstruction
Abstract
:1. Introduction
2. Results
2.1. Histological and Histopathological Analysis
2.2. Myofibroblasts in Harvested ITB and ACL
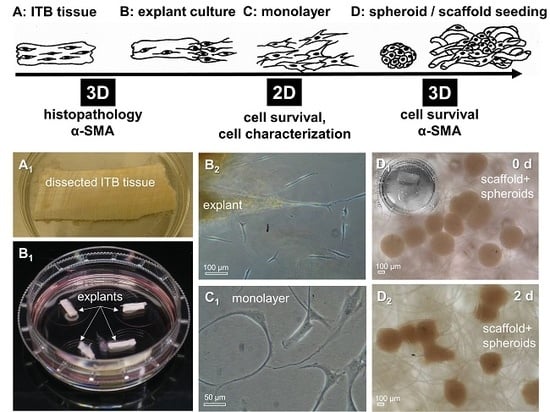
2.3. ITB Tissue and Cultured ITB Explants
2.4. ITB Fibroblasts Expressed Typical Ligament Markers
2.5. Gene Expression Analysis
2.6. Demonstration of Multilineage Differentiation Capacity
2.7. Cell Viability and αSMA in 2-D and 3D Cultures
3. Discussion
4. Materials and Methods
4.1. Tissues
4.2. Histology
4.3. Histopathology
4.4. Fibroblast Isolation by Explant Culture and Cell Culturing
4.5. Culture of ITB and ACL Fibroblasts on Cover Slips
4.6. Vitality Assay
4.7. Immunofluorescence Analysis of Marker Expression
4.8. Gene Expression Analysis of Marker Expression Using Realtime Detection PCR
4.9. Multipotency Testing of ITB Fibroblasts
4.9.1. Alizarin Red Staining
4.9.2. Oil Red Staining
4.10. Spheroid Culture and Scaffold Seeding Using ITB Fibroblasts
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACL | anterior cruciate ligament |
| A. dest. | distilled water |
| AB | Alcian blue |
| ABI | applied biosystems |
| αSMA | alpha smooth muscle actin |
| ALK1 | activin receptor-like kinase 1 |
| 2D | two-dimensional |
| 3D | three-dimensional |
| DAPI | 4’,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ECM | extracellular matrix |
| FCS | fetal calf serum |
| FDA | fluorescein diacetate |
| ITB | iliotibial band |
| HE | hematoxylin and eosin |
| MMP | matrix-metalloproteinases |
| MSC | mesenchymal stromal cells |
| MNE | mean normalized expression |
| OA | osteoarthritis |
| PBS | phosphate buffered saline |
| PFA | paraformaldehyde |
| PGA | polyglycolic acid |
| PI | propidium iodide |
| PRG4 | lubricin |
| RT | room temperature |
| SCX | scleraxis |
| SD | standard deviation |
| TBS | TRIS buffered saline |
| TGFβ1 | transforming growth factor beta1 |
| VEGF | vascular endothelial growth factor |
References
- Fairclough, J.; Hayashi, K.; Toumi, H.; Lyons, K.; Bydder, G.; Phillips, N.; Best, T.M.; Benjamin, M. The functional anatomy of the iliotibial band during flexion and extension of the knee: Implications for understanding iliotibial band syndrome. J. Anat. 2006, 208, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Hammer, N.; Huster, D.; Boldt, A.; Hadrich, C.; Koch, H.; Mobius, R.; Schulze-Tanzil, G.; Scheidt, H.A. A preliminary technical study on sodium dodecyl sulfate-induced changes of the nano-structural and macro-mechanical properties in human iliotibial tract specimens. J. Mech. Behav. Biomed. Mater. 2016, 61, 164–173. [Google Scholar] [CrossRef]
- Frank, C.B. Ligament structure, physiology and function. J. Musculoskelet. Neuronal Interact. 2004, 4, 199–201. [Google Scholar] [PubMed]
- Waggett, A.D.; Benjamin, M.; Ralphs, J.R. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur. J. Cell Biol. 2006, 85, 1145–1154. [Google Scholar] [CrossRef]
- Delcroix, G.J.; Kaimrajh, D.N.; Baria, D.; Cooper, S.; Reiner, T.; Latta, L.; D’Ippolito, G.; Schiller, P.C.; Temple, H.T. Histologic, biomechanical, and biological evaluation of fan-folded iliotibial band allografts for anterior cruciate ligament reconstruction. Arthroscopy 2013, 29, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Posthumus, M.; September, A.V.; O’Cuinneagain, D.; van der Merwe, W.; Schwellnus, M.P.; Collins, M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. Am. J. Sports Med. 2009, 37, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Rees, S.G.; Curtis, C.L.; Dent, C.M.; Caterson, B. Catabolism of aggrecan proteoglycan aggregate components in short-term explant cultures of tendon. Matrix Biol. 2005, 24, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Ren, Y.J.; Yan, L.; Xiao, Z.H.; Ding, F.; Li, F.; Han, Q.; Cheng, W.J.; Kan, W.S. A free anterolateral thigh flap and iliotibial band for reconstruction of soft tissue defects at children’s feet and ankles. Injury 2015, 46, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Wuerz, T.H.; Shewman, E.; McCormick, F.M.; Salata, M.J.; Philippon, M.J.; Nho, S.J. Labral reconstruction with iliotibial band autografts and semitendinosus allografts improves hip joint contact area and contact pressure: An in vitro analysis. Am. J. Sports Med. 2015, 43, 98–104. [Google Scholar] [CrossRef]
- Mascarenhas, R.; McConkey, M.O.; Forsythe, B.; Harner, C.D. Revision anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft and extra-articular iliotibial band tenodesis. Am. J. Orthop. 2015, 44, E89–E93. [Google Scholar] [PubMed]
- Lutz, C.; Sonnery-Cottet, B.; Imbert, P.; Barbosa, N.C.; Tuteja, S.; Jaeger, J.H. Combined Anterior and Anterolateral Stabilization of the Knee with the Iliotibial Band. Arthrosc. Tech. 2016, 5, e251–e256. [Google Scholar] [CrossRef]
- Szotek, S.; Dawidowicz, J.; Eyden, B.; Matysiak, N.; Czogalla, A.; Dudzik, G.; Lesniewicz, A.; Maksymowicz, K. Morphological features of fascia lata in relation to fascia diseases. Ultrastruct. Pathol. 2016, 40, 297–310. [Google Scholar] [CrossRef]
- Weiss, M.; Unterhauser, F.N.; Weiler, A. Crimp frequency is strongly correlated to myofibroblast density in the human anterior cruciate ligament and its autologous tendon grafts. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Legerlotz, K.; Dorn, J.; Richter, J.; Rausch, M.; Leupin, O. Age-dependent regulation of tendon crimp structure, cell length and gap width with strain. Acta Biomater. 2014, 10, 4447–4455. [Google Scholar] [CrossRef]
- Patterson-Kane, J.C.; Firth, E.C.; Goodship, A.E.; Parry, D.A. Age-related differences in collagen crimp patterns in the superficial digital flexor tendon core region of untrained horses. Aust. Vet. J. 1997, 75, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Kane, J.C.; Parry, D.A.; Goodship, A.E.; Firth, E.C. Exercise modifies the age-related change in crimp pattern in the core region of the equine superficial digital flexor tendon. N. Z. Vet. J. 1997, 45, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; Mobasheri, A.; Clegg, P.D.; Sendzik, J.; John, T.; Shakibaei, M. Cultivation of human tenocytes in high-density culture. Histochem. Cell Biol. 2004, 122, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Gittel, C.; Brehm, W.; Burk, J.; Juelke, H.; Staszyk, C.; Ribitsch, I. Isolation of equine multipotent mesenchymal stromal cells by enzymatic tissue digestion or explant technique: Comparison of cellular properties. BMC Vet. Res. 2013, 9, 221. [Google Scholar] [CrossRef]
- Gögele, C.; Schwarz, S.; Ondruschka, B.; Hammer, N.; Schulze-Tanzil, G. Decellularized Iliotibial Band Recolonized with Allogenic Homotopic Fibroblasts or Bone Marrow-Derived Mesenchymal Stromal Cells. Methods Mol. Biol. 2017, 1577, 55–69. [Google Scholar]
- Chard, M.D.; Wright, J.K.; Hazleman, B.L. Isolation and growth characteristics of adult human tendon fibroblasts. Ann. Rheum. Dis. 1987, 46, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.Z.; Martinac, B.; Samiric, T.; Handley, C.J. Effects of glucosamine on proteoglycan loss by tendon, ligament and joint capsule explant cultures. Osteoarthr. Cartil. 2008, 16, 1501–1508. [Google Scholar] [CrossRef]
- Lee, M.Y.; Ehrlich, H.P. Influence of vanadate on migrating fibroblast orientation within a fibrin matrix. J. Cell. Physiol. 2008, 217, 72–76. [Google Scholar] [CrossRef]
- Leigh, D.R.; Abreu, E.L.; Derwin, K.A. Changes in gene expression of individual matrix metalloproteinases differ in response to mechanical unloading of tendon fascicles in explant culture. J. Orthop. Res. 2008, 26, 1306–1312. [Google Scholar] [CrossRef]
- Wong, M.W.; Lui, W.T.; Fu, S.C.; Lee, K.M. The effect of glucocorticoids on tendon cell viability in human tendon explants. Acta Orthop. 2009, 80, 363–367. [Google Scholar] [CrossRef]
- Ikeda, J.; Zhao, C.; Moran, S.L.; An, K.N.; Amadio, P.C. Effects of synovial interposition on healing in a canine tendon explant culture model. J. Hand Surg. 2010, 35, 1153–1159. [Google Scholar] [CrossRef]
- Yao, L.; Bestwick, C.S.; Bestwick, L.A.; Maffulli, N.; Aspden, R.M. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006, 12, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Martin, S.D.; Martin, T.L.; Spector, M. Histological changes in the human anterior cruciate ligament after rupture. J. Bone Jt. Surg. 2000, 82, 1387–1397. [Google Scholar] [CrossRef]
- Poehling-Monaghan, K.L.; Salem, H.; Ross, K.E.; Secrist, E.; Ciccotti, M.C.; Tjoumakaris, F.; Ciccotti, M.G.; Freedman, K.B. Long-term outcomes in anterior cruciate ligament reconstruction: A systematic review of patellar tendon versus Hamstring autografts. Orthop. J. Sports Med. 2017, 5, 2325967117709735. [Google Scholar] [CrossRef]
- Sugimoto, D.; Heyworth, B.E.; Collins, S.E.; Fallon, R.T.; Kocher, M.S.; Micheli, L.J. Comparison of lower extremity recovery after anterior cruciate ligament reconstruction with transphyseal hamstring versus extraphyseal Iliotibial band techniques in skeletally immature athletes. Orthop. J. Sports Med. 2018, 6, 2325967118768044. [Google Scholar] [CrossRef]
- Naraoka, T.; Ishibashi, Y.; Tsuda, E.; Yamamoto, Y.; Kusumi, T.; Kakizaki, I.; Toh, S. Time-dependent gene expression and immunohistochemical analysis of the injured anterior cruciate ligament. Bone Jt. Res. 2012, 1, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Rees, S.G.; Waggett, A.D.; Dent, C.M.; Caterson, B. Inhibition of aggrecan turnover in short-term explant cultures of bovine tendon. Matrix Biol. 2007, 26, 280–290. [Google Scholar] [CrossRef]
- Rouwkema, J.; Koopman, B.; Blitterswijk, C.; Dhert, W.; Malda, J. Supply of nutrients to cells in engineered tissues. Biotechnol. Genet. Eng. Rev. 2010, 26, 163–178. [Google Scholar] [CrossRef]
- Watanabe, T.; Yasue, A.; Tanaka, E. Hypoxia-inducible factor-1alpha is required for transforming growth factor-beta1-induced type I collagen, periostin and alpha-smooth muscle actin expression in human periodontal ligament cells. Arch. Oral Biol. 2014, 59, 595–600. [Google Scholar] [CrossRef]
- Meyer-Ter-Vehn, T.; Gebhardt, S.; Sebald, W.; Buttmann, M.; Grehn, F.; Schlunck, G.; Knaus, P. p38 inhibitors prevent TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1500–1509. [Google Scholar] [CrossRef]
- Krause, C.; Kloen, P.; Ten Dijke, P. Elevated transforming growth factor beta and mitogen-activated protein kinase pathways mediate fibrotic traits of Dupuytren’s disease fibroblasts. Fibrogenes. Tissue Repair 2011, 4, 14. [Google Scholar] [CrossRef]
- Pannu, J.; Nakerakanti, S.; Smith, E.; ten Dijke, P.; Trojanowska, M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J. Biol. Chem. 2007, 282, 10405–10413. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Felix, J.M.; Gonzalez-Nunez, M.; Lopez-Novoa, J.M. ALK1-Smad1/5 signaling pathway in fibrosis development: Friend or foe? Cytokine Growth Factor Rev. 2013, 24, 523–537. [Google Scholar] [CrossRef]
- Hasegawa, A.; Nakahara, H.; Kinoshita, M.; Asahara, H.; Koziol, J.; Lotz, M.K. Cellular and extracellular matrix changes in anterior cruciate ligaments during human knee aging and osteoarthritis. Arthritis Res. Ther. 2013, 15, R29. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M. The fascia of the limbs and back—A review. J. Anat. 2009, 214, 1–18. [Google Scholar] [CrossRef]
- Dawidowicz, J.; Szotek, S.; Matysiak, N.; Mielanczyk, L.; Maksymowicz, K. Electron microscopy of human fascia lata: Focus on telocytes. J. Cell. Mol. Med. 2015, 19, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Mauro, A.; Martelli, A.; Di Giacinto, O.; Di Marcantonio, L.; Nardinocchi, D.; Berardinelli, P.; Barboni, B. Cellular and molecular maturation in fetal and adult ovine calcaneal tendons. J. Anat. 2015, 226, 126–142. [Google Scholar] [CrossRef]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Satchell, L.; Kumar, A.; Pathmanathan, L.; Beason, D.P.; Iozzo, R.V.; et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013, 32, 3–13. [Google Scholar] [CrossRef]
- Laumonier, T.; Michel, M.; Gabbiani, G.; Hoffmeyer, P.; Bochaton-Piallat, M.L.; Menetrey, J. Autologous transplantation of culture-born myofibroblasts into intact and injured rabbit ligaments. Int. Orthop. 2012, 36, 1733–1738. [Google Scholar] [CrossRef]
- Faryniarz, D.A.; Chaponnier, C.; Gabbiani, G.; Yannas, I.V.; Spector, M. Myofibroblasts in the healing lapine medial collateral ligament: Possible mechanisms of contraction. J. Orthop. Res. 1996, 14, 228–237. [Google Scholar] [CrossRef]
- Dakin, S.G.; Buckley, C.D.; Al-Mossawi, M.H.; Hedley, R.; Martinez, F.O.; Wheway, K.; Watkins, B.; Carr, A.J. Persistent stromal fibroblast activation is present in chronic tendinopathy. Arthritis Res. Ther. 2017, 19, 16. [Google Scholar] [CrossRef]
- Lee, K.J.; Clegg, P.D.; Comerford, E.J.; Canty-Laird, E.G. A comparison of the stem cell characteristics of murine tenocytes and tendon-derived stem cells. BMC Musculoskelet. Disord. 2018, 19, 116. [Google Scholar] [CrossRef]
- Abrahamsson, S.O.; Lundborg, G.; Lohmander, L.S. Long-term explant culture of rabbit flexor tendon: Effects of recombinant human insulin-like growth factor-I and serum on matrix metabolism. J. Orthop. Res. 1991, 9, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Samiric, T.; Ilic, M.Z.; Handley, C.J. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004, 23, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, M.; Meier, C.; Kohl, B.; Lohan, A.; Kokozidou, M.; Schulze-Tanzil, G. Histological and biochemical characteristics of the rabbit anterior cruciate ligament in comparison to potential autografts. Histol. Histopathol. 2016, 31, 867–877. [Google Scholar] [PubMed]
- Vogel, K.G.; Sandy, J.D.; Pogany, G.; Robbins, J.R. Aggrecan in bovine tendon. Matrix Biol. 1994, 14, 171–179. [Google Scholar] [CrossRef]
- Mierke, C.T. The role of vinculin in the regulation of the mechanical properties of cells. Cell Biochem. Biophys. 2009, 53, 115–126. [Google Scholar] [CrossRef]
- Carisey, A.; Ballestrem, C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur. J. Cell Biol. 2011, 90, 157–163. [Google Scholar] [CrossRef]
- Ruschke, K.; Meier, C.; Ullah, M.; Krebs, A.C.; Silberreis, K.; Kohl, B.; Knaus, P.; Jagielski, M.; Arens, S.; Schulze-Tanzil, G. Bone morphogenetic protein 2/SMAD signalling in human ligamentocytes of degenerated and aged anterior cruciate ligaments. Osteoarthr. Cartil. 2016, 24, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Kopf, J.; Petersen, A.; Duda, G.N.; Knaus, P. BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway. BMC Biol. 2012, 10, 37. [Google Scholar] [CrossRef]
- Ogata, Y.; Mabuchi, Y.; Shinoda, K.; Horiike, Y.; Mizuno, M.; Otabe, K.; Suto, E.G.; Suzuki, N.; Sekiya, I.; Akazawa, C. Anterior cruciate ligament-derived mesenchymal stromal cells have a propensity to differentiate into the ligament lineage. Regen. Ther. 2018, 8, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Clegg, P.D.; Comerford, E.J.; Canty-Laird, E.G. Ligament-derived stem cells: Identification, characterisation, and therapeutic application. Stem Cells Int. 2017, 2017, 1919845. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Kunz, M.; Prager, P.; Barthel, T.; Jakob, F.; Noth, U.; Murray, M.M.; Evans, C.H.; Porter, R.M. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng. Part A. 2011, 17, 1375–1388. [Google Scholar] [CrossRef]
- Huang, T.F.; Chen, Y.T.; Yang, T.H.; Chen, L.L.; Chiou, S.H.; Tsai, T.H.; Tsai, C.C.; Chen, M.H.; Ma, H.L.; Hung, S.C. Isolation and characterization of mesenchymal stromal cells from human anterior cruciate ligament. Cytotherapy 2008, 10, 806–814. [Google Scholar] [CrossRef]
- Cheng, M.T.; Yang, H.W.; Chen, T.H.; Lee, O.K. Isolation and characterization of multipotent stem cells from human cruciate ligaments. Cell Prolif. 2009, 42, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Ingham, S.M.; Mifune, Y.; Osawa, A.; Logar, A.; Usas, A.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Isolation and characterization of human anterior cruciate ligament-derived vascular stem cells. Stem Cells Dev. 2012, 21, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; John, T.; Conrad, C.; Lohan, A.; Hondke, S.; Ertel, W.; Kaps, C.; Endres, M.; Sittinger, M.; Ringe, J.; et al. Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation. Biomaterials 2011, 32, 4806–4815. [Google Scholar] [CrossRef] [PubMed]
- Lohan, A.; Kohl, B.; Meier, C.; Schulze-Tanzil, G. Tenogenesis of decellularized porcine achilles tendon matrix reseeded with human tenocytes in the nude mice xenograft model. Int. J. Mol. Sci. 2018, 19, 2059. [Google Scholar] [CrossRef] [PubMed]










| Target | Primary Antibody | Dilution | Secondary Antibody | Dilution |
|---|---|---|---|---|
| Aggrecan | mouse antihuman R&D systems, Minneapolis, USA | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| CD44 | mouse-antihuman, Cell signalling Technology, Danvers, USA | 1:50 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| Collagen type I | goat anti human, Abcam, Cambridge, UK | 1:50 | donkey anti goat, Alexa Fluor 488, Invitrogen, Carlsbad, USA | 1:200 |
| Collagen type II | rabbit anti human, Acris Laboratories, Hiddenhausen, Germany | 1:50 | donkey anti rabbit, Alexa Fluor 488, Invitrogen | 1:200 |
| Collagen type III | mouse anti human Acris Laboratories, Hiddenhausen, Germany | 1:30 | donkey-anti-mouse cyanine-3 (cy3), Invitrogen | 1:200 |
| Decorin | rabbit anti human, Acris Laboratories, Hiddenhausen, Germany | 1:30 | donkey anti rabbit, Alexa Fluor 488, Invitrogen | 1:200 |
| Elastin | mouse anti human Acris Laboratories, Hiddenhausen, Germany | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| Fibronectin | mouse-antihuman, Dianova, Hamburg, Germany | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| β1-integrin | mouse-antihuman, Merck-Millipore | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| Lubricin | rabbit-antihuman, Abcam, Cambridge, UK | 1:30 | donkey anti rabbit, Alexa Fluor 488, Invitrogen | 1:200 |
| αSMA | mouse-antihuman, Sigma-Aldrich | 1:50 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| Tenascin C | mouse-antihuman, GeneTex Inc. Biozol, Eching, Germany | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| VEGF | mouse-antihuman, R&D Systems | 1:30 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| Vimentin | mouse-antihuman, Dako Cytomation, Hamburg, Germany | 1:50 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| Vinculin | mouse-antihuman 1:50, Sigma-Aldrich | 1:50 | donkey-anti-mouse cy-3, Invitrogen | 1:200 |
| Gene Symbol | Species | Gene Name | Amplicon Length (bp **) | Assay ID *** |
|---|---|---|---|---|
| COLA1 | homo sapiens | type I collagen | 66 | Hs00164004_m1 |
| DCN | homo sapiens | decorin | 77 | Hs00370384_m1 |
| PRG4 | homo sapiens | lubricin | 65 | Hs00195140_m1 |
| SCXB | homo sapiens | scleraxis homolog B | 63 | Hs03054634_g1 |
| TNC | homo sapiens | tenascin C | 61 | Hs01115665_m1 |
| HPRT | homo sapiens | hypoxanthine-guanine phosphoribosyltransferase | 100 | Hs99999909_m1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, S.; Gögele, C.; Ondruschka, B.; Hammer, N.; Kohl, B.; Schulze-Tanzil, G. Migrating Myofibroblastic Iliotibial Band-Derived Fibroblasts Represent a Promising Cell Source for Ligament Reconstruction. Int. J. Mol. Sci. 2019, 20, 1972. https://doi.org/10.3390/ijms20081972
Schwarz S, Gögele C, Ondruschka B, Hammer N, Kohl B, Schulze-Tanzil G. Migrating Myofibroblastic Iliotibial Band-Derived Fibroblasts Represent a Promising Cell Source for Ligament Reconstruction. International Journal of Molecular Sciences. 2019; 20(8):1972. https://doi.org/10.3390/ijms20081972
Chicago/Turabian StyleSchwarz, Silke, Clemens Gögele, Benjamin Ondruschka, Niels Hammer, Benjamin Kohl, and Gundula Schulze-Tanzil. 2019. "Migrating Myofibroblastic Iliotibial Band-Derived Fibroblasts Represent a Promising Cell Source for Ligament Reconstruction" International Journal of Molecular Sciences 20, no. 8: 1972. https://doi.org/10.3390/ijms20081972