The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn
Abstract
:1. Introduction
2. Results and Discussion
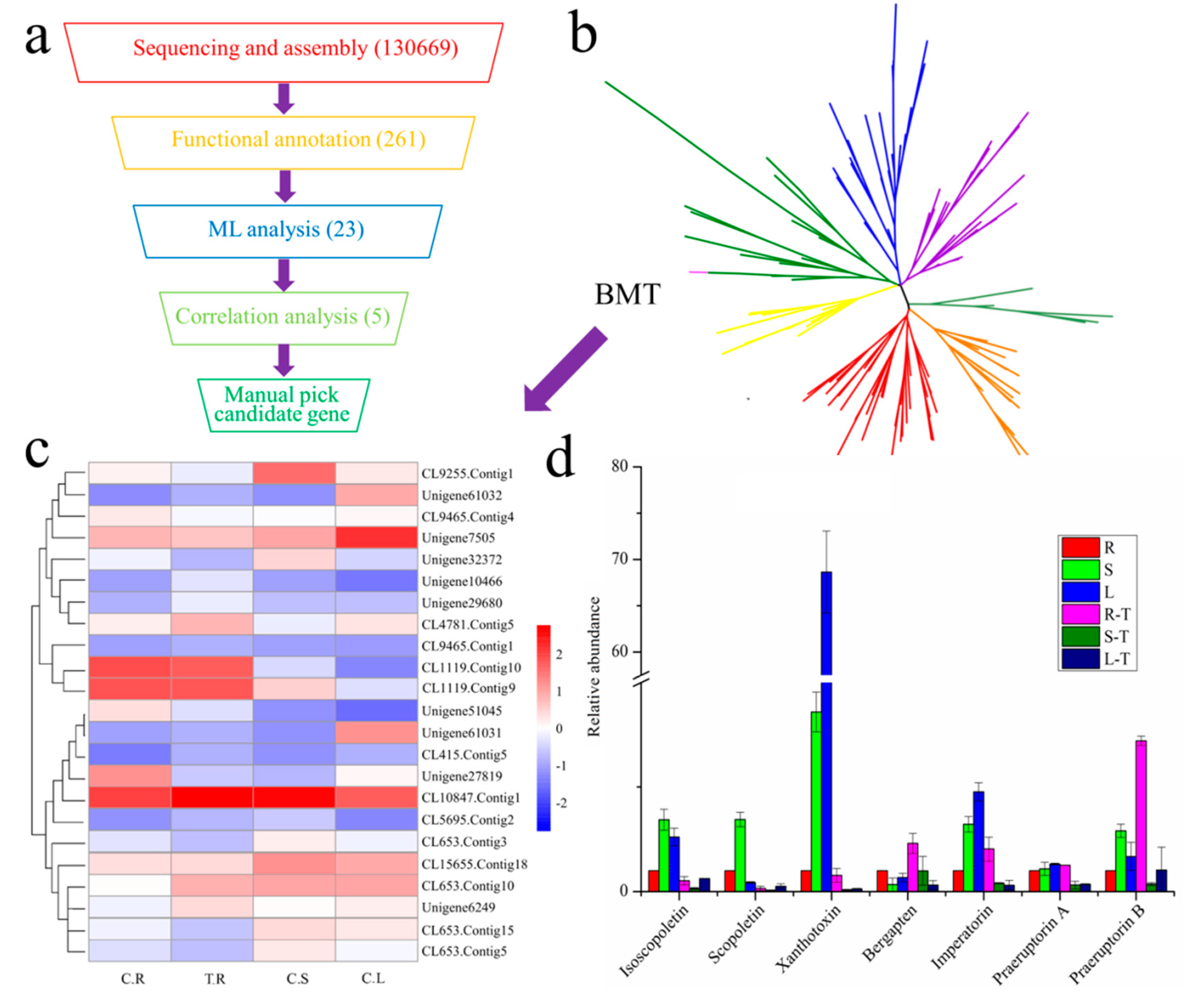
2.1. Candidate Gene Mining with Transcriptome Sequencing and Phytochemical Analysis
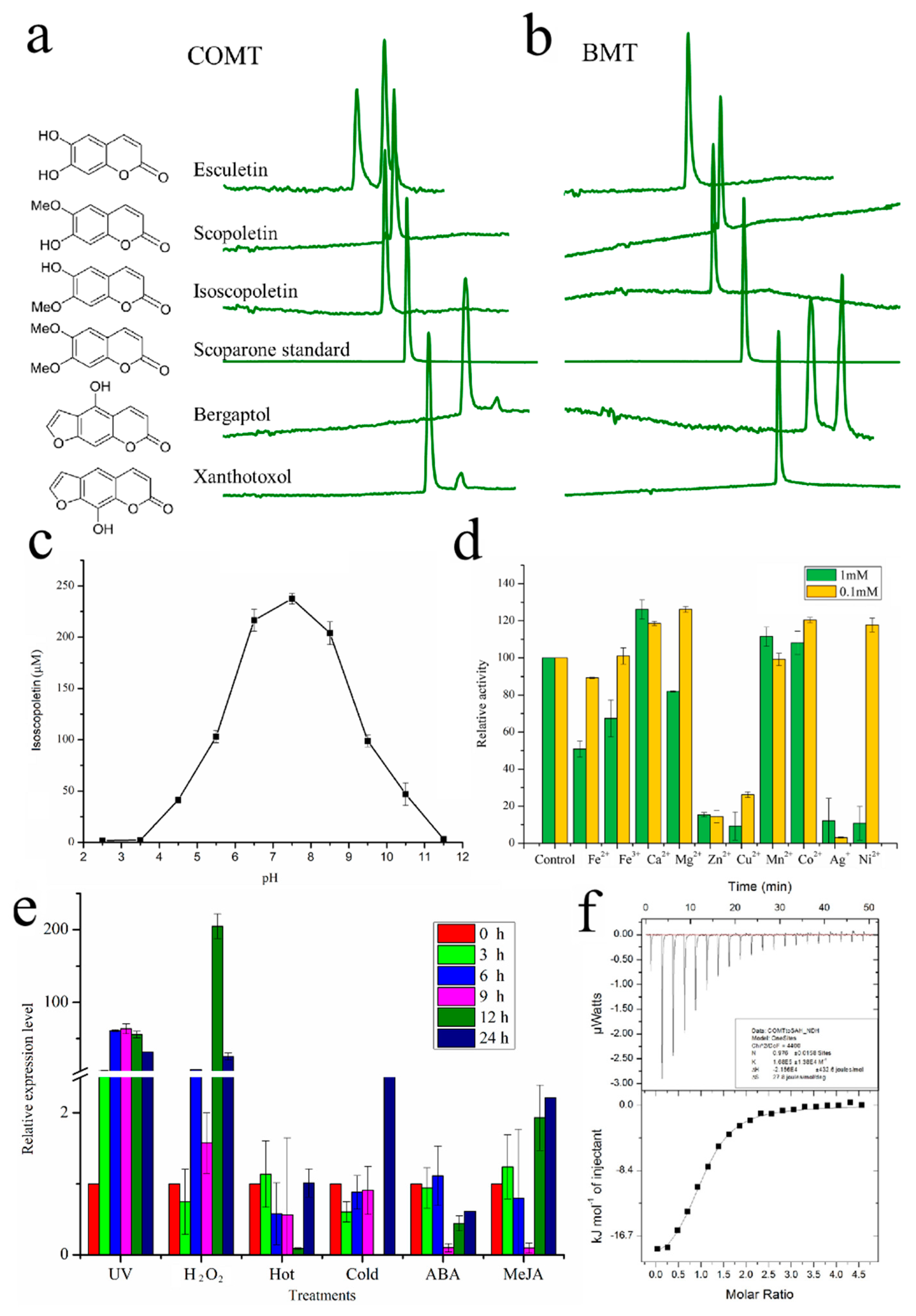
2.2. Functional Characterization of COMT-S
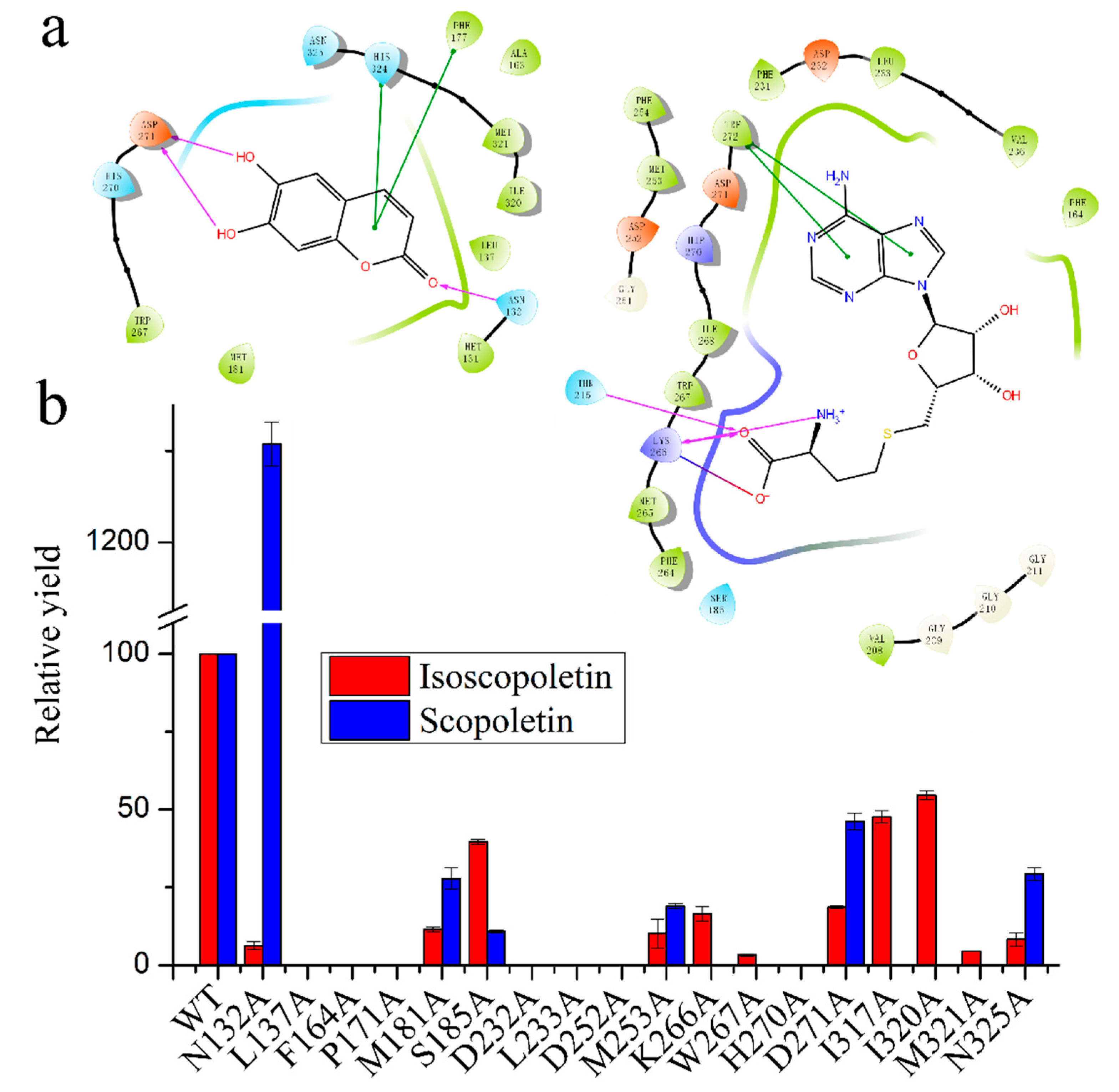
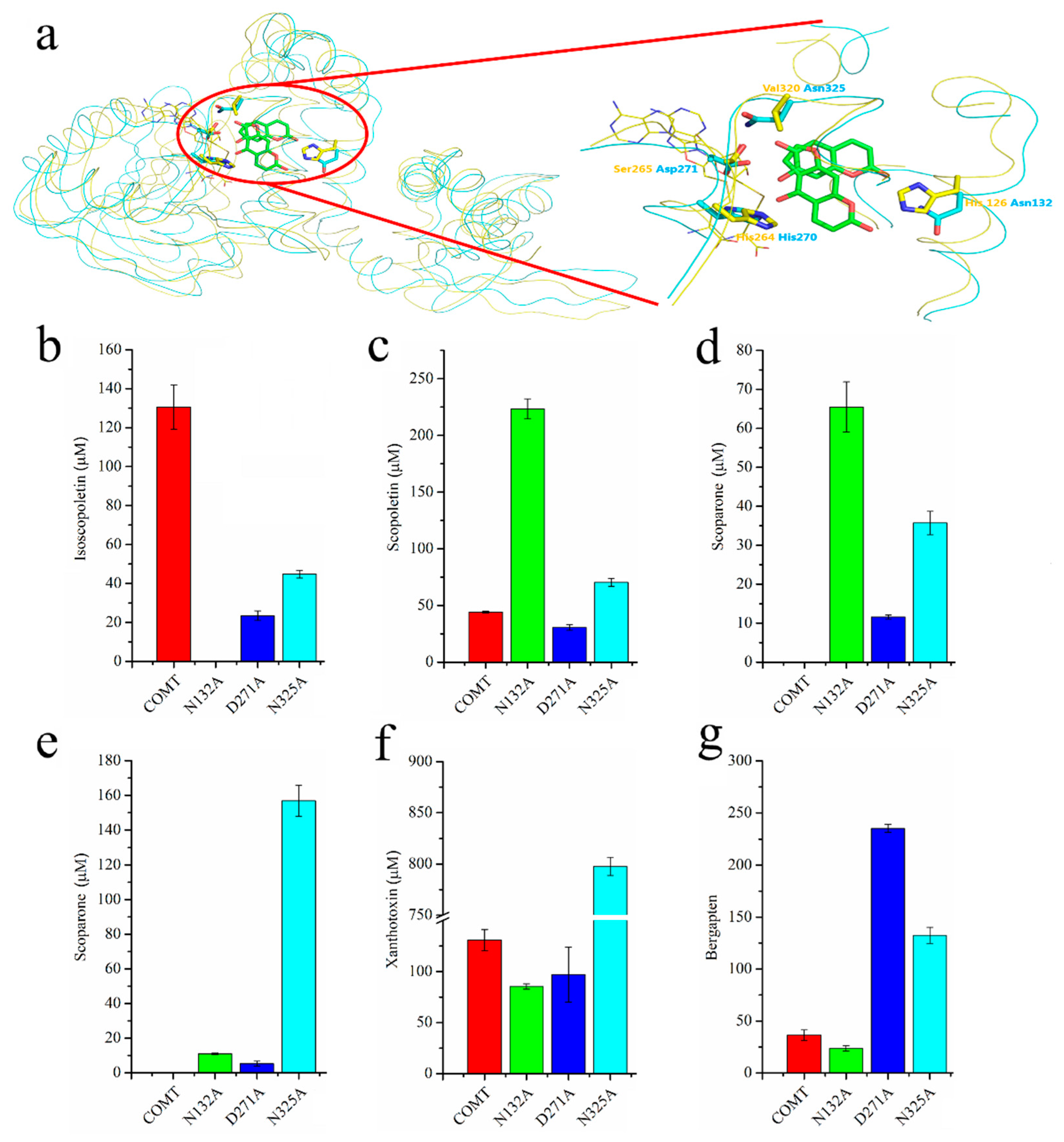
2.3. Docking of COMT-S to Identify the Key Residues Involved in SAH/Esculetin Binding
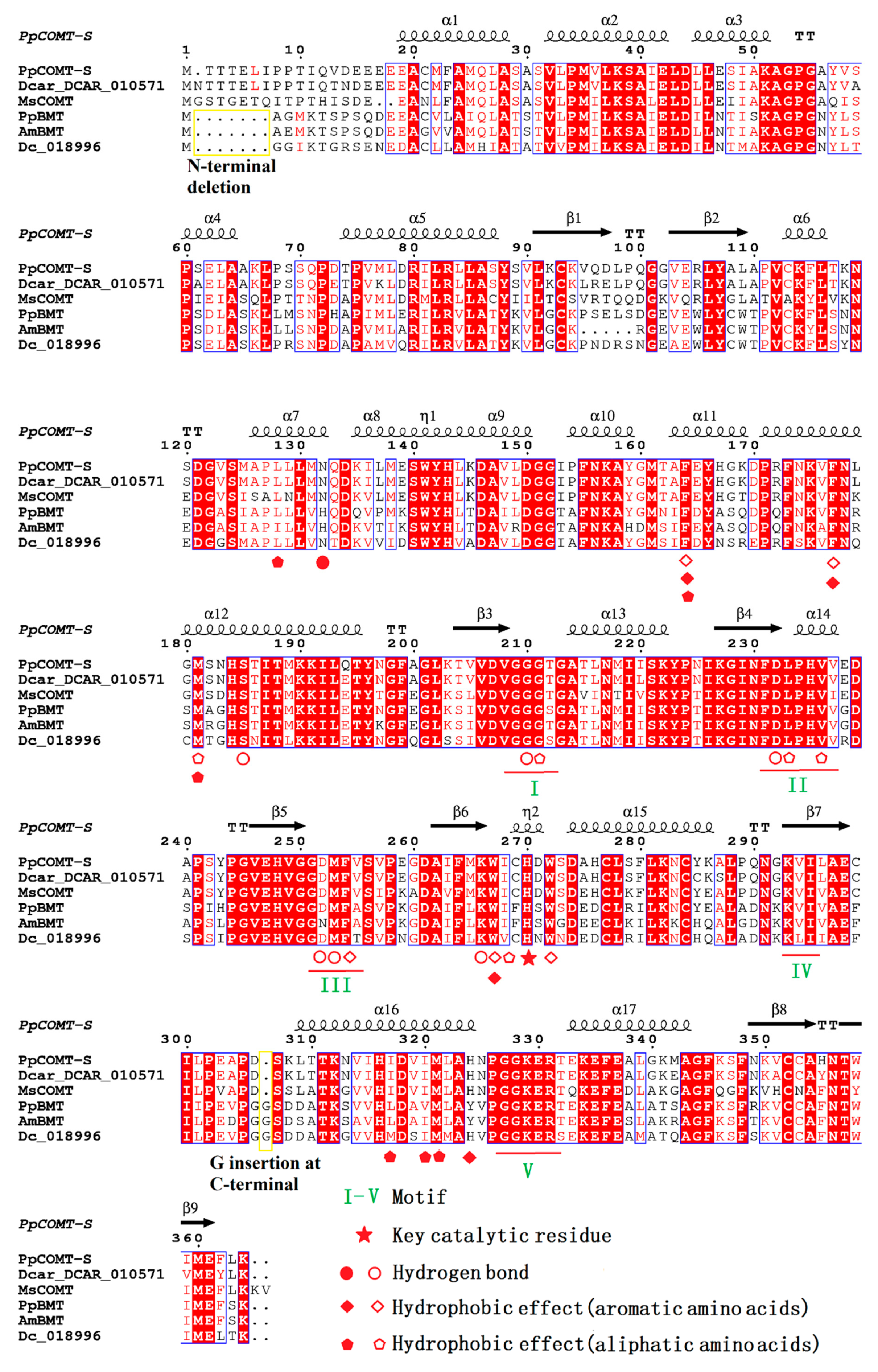
2.4. Sequence Alignment and Biochemical Analysis to Identify the Key Residues Involved in Substrate Heterozygosity
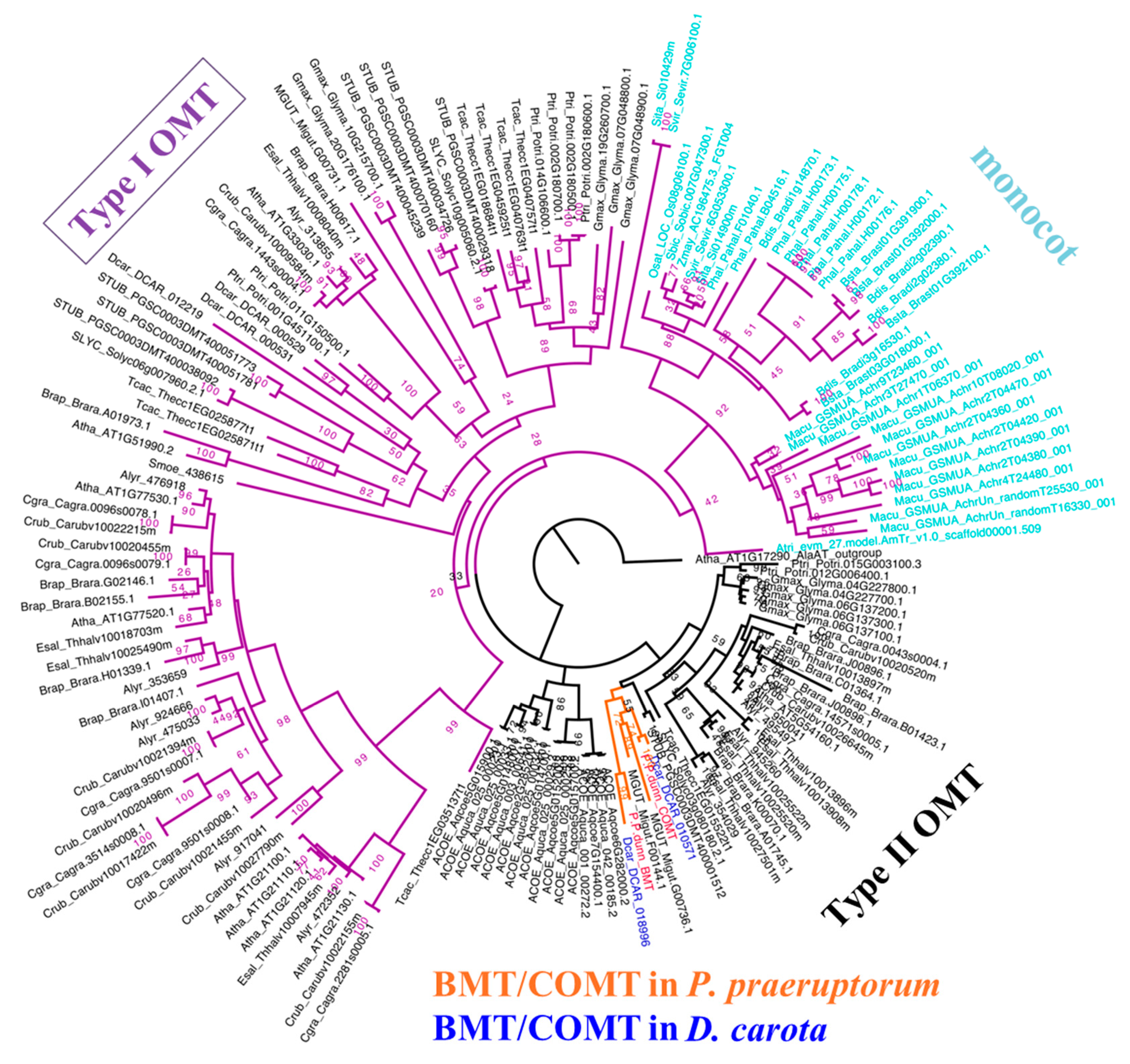
2.5. BMT Was Evolved as a Special Enzyme from COMT-S by Gene Duplication
3. Materials and Methods
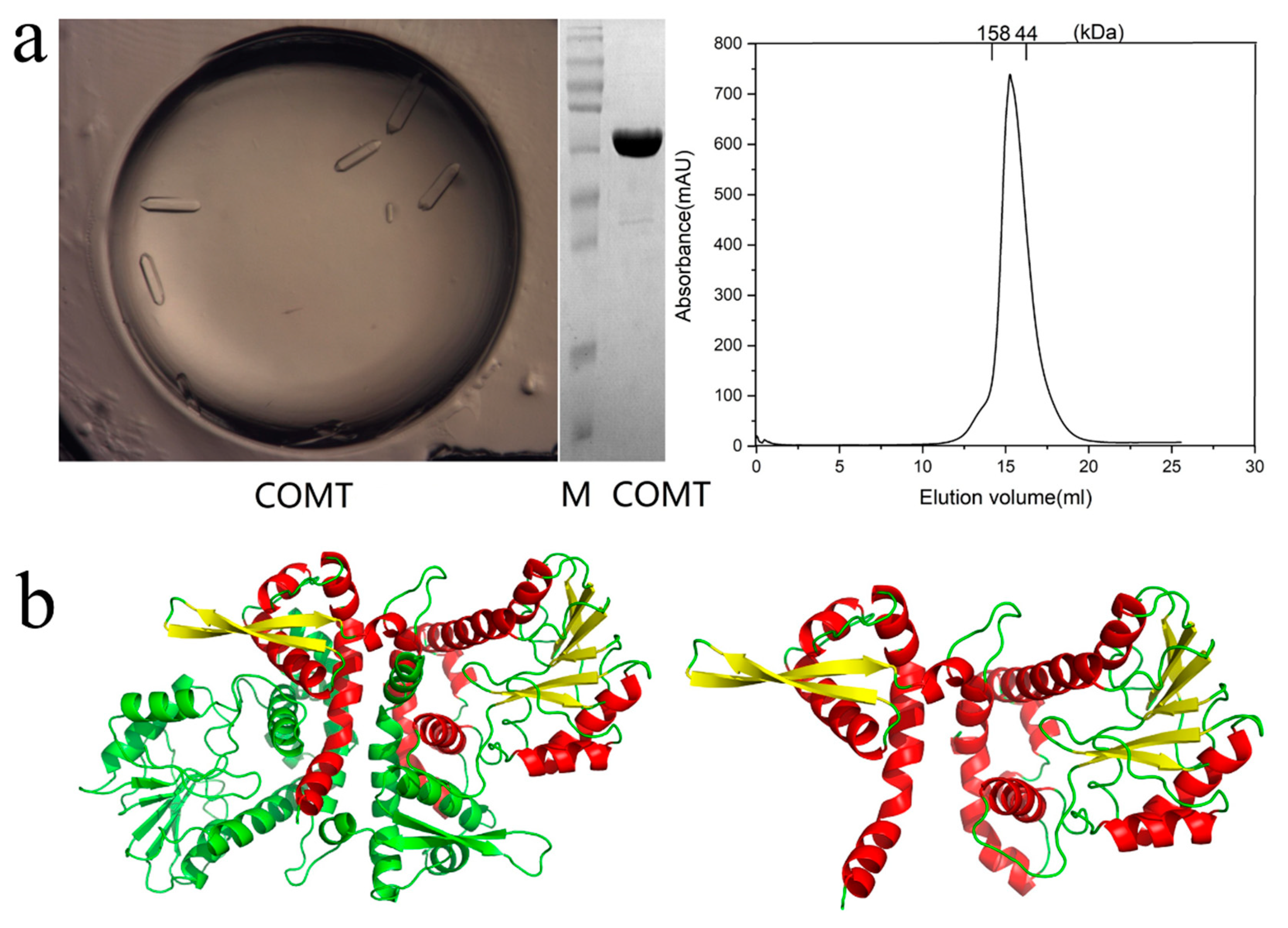
3.1. Protein Expression and Purification
3.2. Crystallization and Structure Determination
3.3. Enzymatic Activity Assays of PpBMT and COMT-S
3.4. HPLC/Electrospray-Ionization Quadrupole Time-of-Flight Mass Spectrometry (Q-TOF MS) Analysis of Coumarin Compounds Measurement
3.5. Q-PCR Analysis and Induction of COMT-S Expression by Elicitors
3.6. Transcriptome Sequence, Assembly, Bioinformatics Analysis, and Docking
3.7. Isothermal Titration Calorimetry
3.8. Evolutionary Analysis of BMT and COMT-S
3.9. Statistical Analysis and the Preparation of Graphs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OMTs | O-methyltransferases |
| COMT | caffeic acid O-methyltransferase |
| BMT | Bergaptol O-methyltransferase |
| HPLC | High Performance Liquid Chromatography |
| SD | Standard Deviation |
| PDB | Protein Data Bank |
| SAM | S-adenosyl-l-methionine |
| ESC | esculetin |
| SAH | S-adenosyl-l-homocysteine |
| NCBI | National Center for Biotechnology Information |
References
- Kumar, A.; Maurya, R.A.; Sharma, S.; Ahmad, P.; Singh, A.; Bhatia, G.; Srivastava, A.K. Pyranocoumarins: A new class of anti-hyperglycemic and anti-dyslipidemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 6447–6451. [Google Scholar] [CrossRef]
- Yu, P.J.; Jin, H.; Zhang, J.Y.; Wang, G.F.; Li, J.R.; Zhu, Z.G.; Tian, Y.X.; Wu, S.Y.; Xu, W.; Zhang, J.J. Pyranocoumarins isolated from Peucedanum praeruptorum Dunn suppress lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-κB and STAT3 activation. Inflammation 2012, 35, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Galanie, S.; Thodey, K.; Trenchard, I.J.; Interrante, M.F.; Smolke, C.D. Complete biosynthesis of opioids in yeast. Science 2015, 349, 1095–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef]
- Qu, Y.; Easson, M.L.; Froese, J.; Simionescu, R.; Hudlicky, T.; de Luca, V. Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc. Natl. Acad. Sci. USA 2015, 112, 6224–6229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Shen, X.; Yuan, Q.; Yan, Y. Microbial biosynthesis of the anticoagulant precursor 4-hydroxycoumarin. Nat. Commun. 2013, 4, 2603. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhou, D.; Kandavelu, P.; Zhang, H.; Yuan, Q.; Wang, B.C.; Rose, J.; Yan, Y. Structural Insights into Substrate Specificity of Feruloyl-CoA 6’-Hydroxylase from Arabidopsis thaliana. Sci. Rep. 2015, 5, 10355. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, N.; Zeng, Z.; Xu, S.; Huang, C.; Wang, W.; Liu, T.; Luo, J.; Kong, L. Cloning, Functional Characterization, and Catalytic Mechanism of a Bergaptol O-Methyltransferase from Peucedanum praeruptorum Dunn. Front. Plant Sci. 2016, 7, 722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, T.; Luo, J.; Zhang, Q.; Xu, S.; Han, C.; Xu, J.; Chen, M.; Chen, Y.; Kong, L. Integration of a decrescent transcriptome and metabolomics dataset of Peucedanum praeruptorum to investigate the CYP450 and MDR genes involved in coumarins biosynthesis and transport. Front. Plant. Sci. 2015, 6, 996. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytother. Res. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Zubieta, C.; Kota, P.; Ferrer, J.L.; Dixon, R.A.; Noel, J.P. Structural basis for the modulation of lignin monomer methylation by caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase. Plant Cell 2002, 14, 1265–1277. [Google Scholar] [CrossRef]
- Robin, A.Y.; Giustini, C.; Graindorge, M.; Matringe, M.; Dumas, R. Crystal structure of norcoclaurine-6-O-methyltransferase a key rate-limiting step in the synthesis of benzylisoquinoline alkaloids. Plant J. 2016, 87, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Hehmann, M.; Lukacin, R.; Ekiert, H.; Matern, U. Furanocoumarin biosynthesis in Ammi majus L. Cloning of bergaptol O-methyltransferase. Eur. J. Biochem. 2004, 271, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.X.; Liu, X.M.; Ge, C.H.; Gao, L.; Peng, X.M.; Zhao, P.P.; Yan, M. The Effects of Galangin on a Mouse Model of Vitiligo Induced by Hydroquinone. Phytother. Res. 2014, 28, 1533. [Google Scholar] [CrossRef] [PubMed]
- Parisa, S. Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: A review. J. Ethnopharmacol. 2014, 156, 235–270. [Google Scholar]
- Hou, Z.; Luo, J.; Wang, J.; Kong, L. Separation of minor coumarins from Peucedanum praeruptorum using HSCCC and preparative HPLC guided by HPLC/MS. Sep. Purif. Technol. 2010, 75, 132–137. [Google Scholar] [CrossRef]
- Lv, H.; Luo, J.; Wang, X.; Kong, L. Application of UPLC-Quadrupole-TOF-MS Coupled with Recycling Preparative HPLC in Isolation and Preparation of Coumarin Isomers with Similar Polarity from Peucedanum praeruptorum. Chromatographia 2012, 76, 141–148. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Sharma, S.K.; Garrett, J.M.; Brown, S.A. Separation of the S-adenosylmethionine: 5-and 8-hydroxyfuranocoumarin O-methyltransferases of Ruta graveolens L. by general ligand affinity chromatography. Z. Naturforsch. C 1979, 34, 387–391. [Google Scholar] [CrossRef]
- Mcneely, W.; Kl, D.C.N. 5-Methoxypsoralen. A review of its effects in psoriasis and vitiligo. Drugs 1998, 56, 667. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Zhang, B.; Ma, J.; Hettenhausen, C.; Cao, G.; Sun, G.; Wu, J.; Wu, J. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp. Bot. 2014, 65, 4305–4315. [Google Scholar] [Green Version]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review Scopoletin—A Coumarin Phytoalexin with Medicinal Properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Ishikawa, A.; Kuma, T.; Sasaki, H.; Sasaki, N.; Ozeki, Y.; Kobayashi, N.; Kitamura, Y. Constitutive expression of bergaptol O-methyltransferase in Glehnia littoralis cell cultures. Plant Cell Rep. 2009, 28, 257–265. [Google Scholar] [CrossRef]
- Iorizzo, M.; Ellison, S.; Senalik, D.; Zeng, P.; Satapoomin, P.; Huang, J.; Bowman, M.; Iovene, M.; Sanseverino, W.; Cavagnaro, P. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat. Genet. 2016, 48, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Yao, R.; Zhao, Y.; Liu, T.; Huang, C.; Xu, S.; Sui, Z.; Luo, J.; Kong, L. Identification and functional characterization of a p-coumaroyl CoA 2′-hydroxylase involved in the biosynthesis of coumarin skeleton from Peucedanum praeruptorum Dunn. Plant Mol. Biol. 2017, 95, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Minor, W.; Cymborowski, M.; Otwinowski, Z.; Chruszcz, M. HKL-3000: The integration of data reduction and structure solution–from diffraction images to an initial model in minutes. Acta Crystallogr. D 2006, 62, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 2011, 67, 235–242. [Google Scholar] [PubMed] [Green Version]
- Adams, P.D.; Grosse-Kunstleve, R.W.; Hung, L.W.; Ioerger, T.R.; McCoy, A.J.; Moriarty, N.W.; Read, R.J.; Sacchettini, J.C.; Sauter, N.K.; Terwilliger, T.C. PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr. D 2002, 58, 1948–1954. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, J.; Xu, S.; Wang, W.; Liu, T.; Han, C.; Chen, Y.; Kong, L. Selection of Reference Genes for Gene Expression Normalization in Peucedanum praeruptorum Dunn under Abiotic Stresses, Hormone Treatments and Different Tissues. PLoS ONE 2016, 11, e0152356. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an induced fit receptor structure in virtual screening. Chem. Biol. Drug Des. 2006, 67, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinf. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, N.; Sui, Z.; Huang, C.; Zeng, Z.; Kong, L. The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn. Int. J. Mol. Sci. 2019, 20, 1533. https://doi.org/10.3390/ijms20071533
Zhao Y, Wang N, Sui Z, Huang C, Zeng Z, Kong L. The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn. International Journal of Molecular Sciences. 2019; 20(7):1533. https://doi.org/10.3390/ijms20071533
Chicago/Turabian StyleZhao, Yucheng, Nana Wang, Ziwei Sui, Chuanlong Huang, Zhixiong Zeng, and Lingyi Kong. 2019. "The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn" International Journal of Molecular Sciences 20, no. 7: 1533. https://doi.org/10.3390/ijms20071533




