A Dual Molecular Biointerface Combining RGD and KRSR Sequences Improves Osteoblastic Functions by Synergizing Integrin and Cell-Membrane Proteoglycan Binding
Abstract
:1. Introduction
2. Results and Discussion
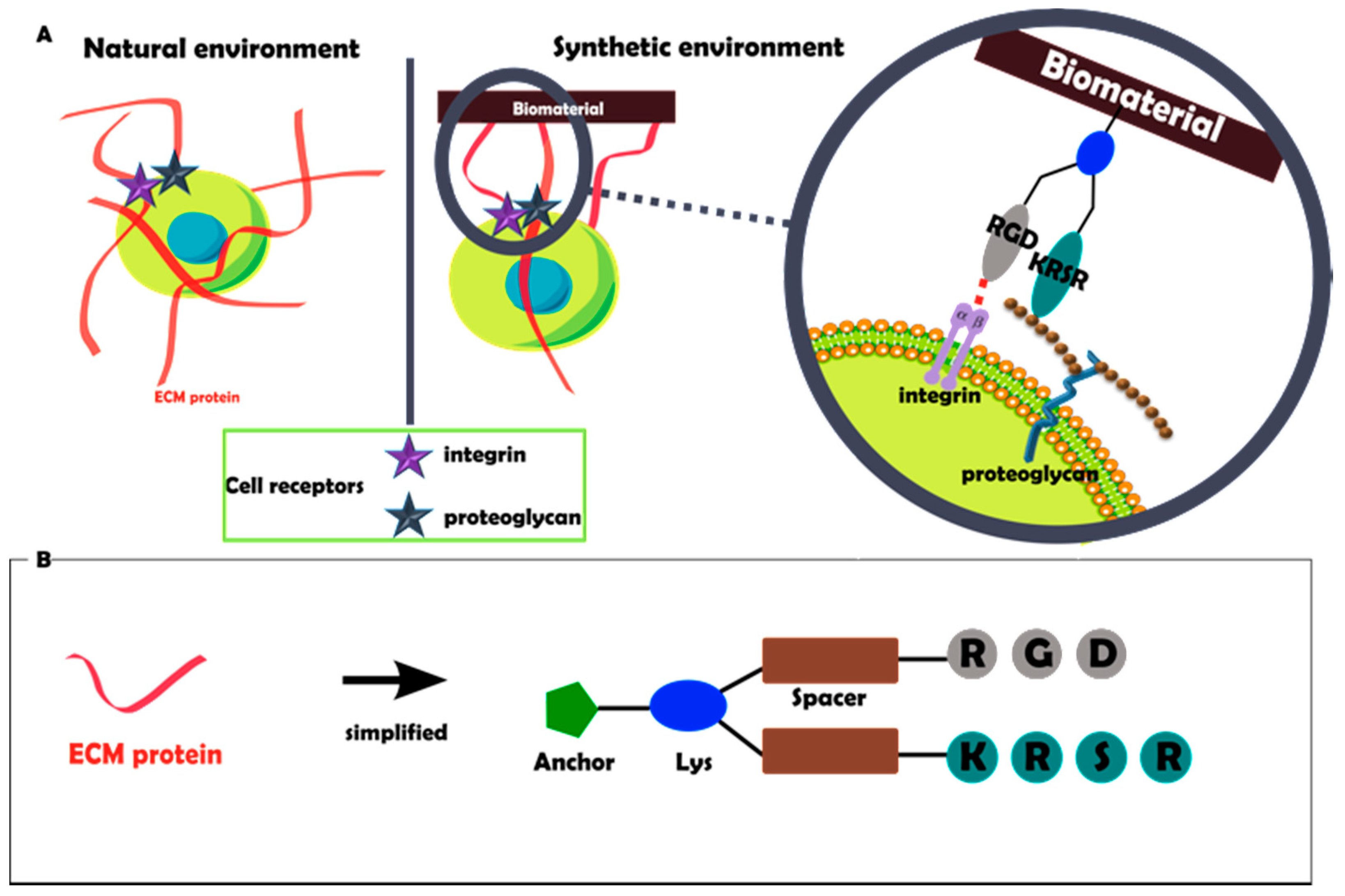
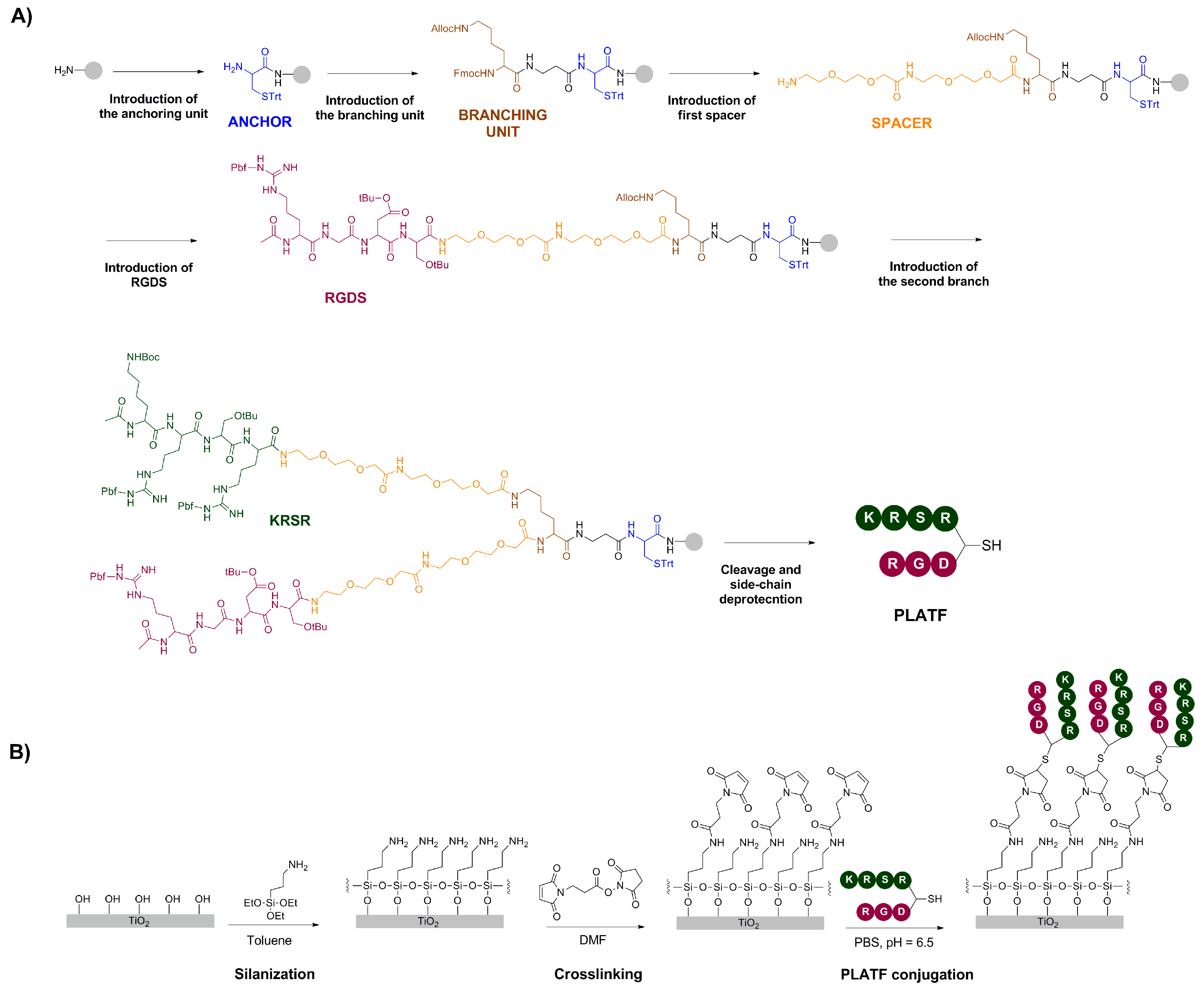
2.1. Design and Synthesis of the Molecular Biointerface
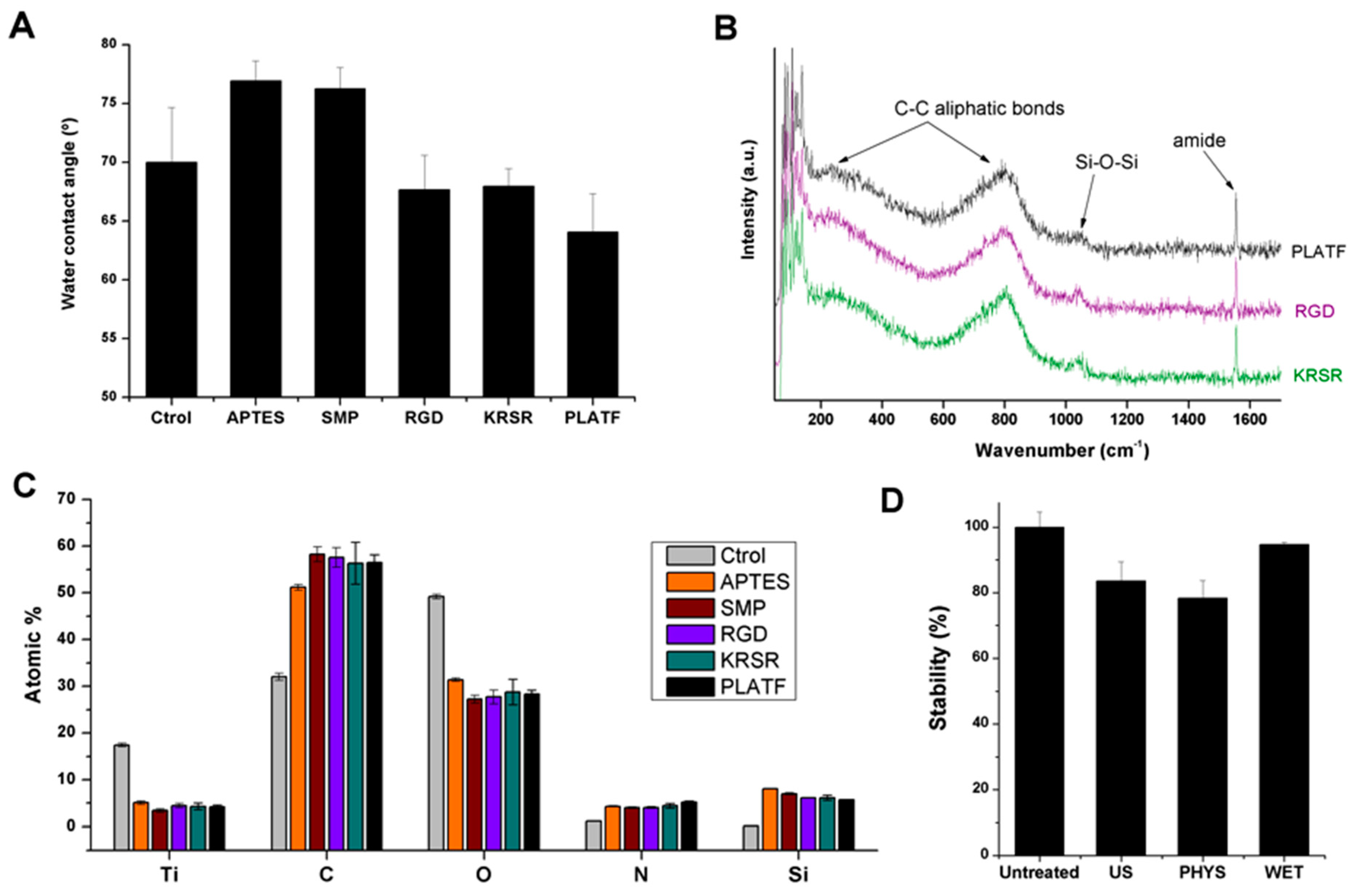
2.2. Physicochemical characterization of the biointerfaces
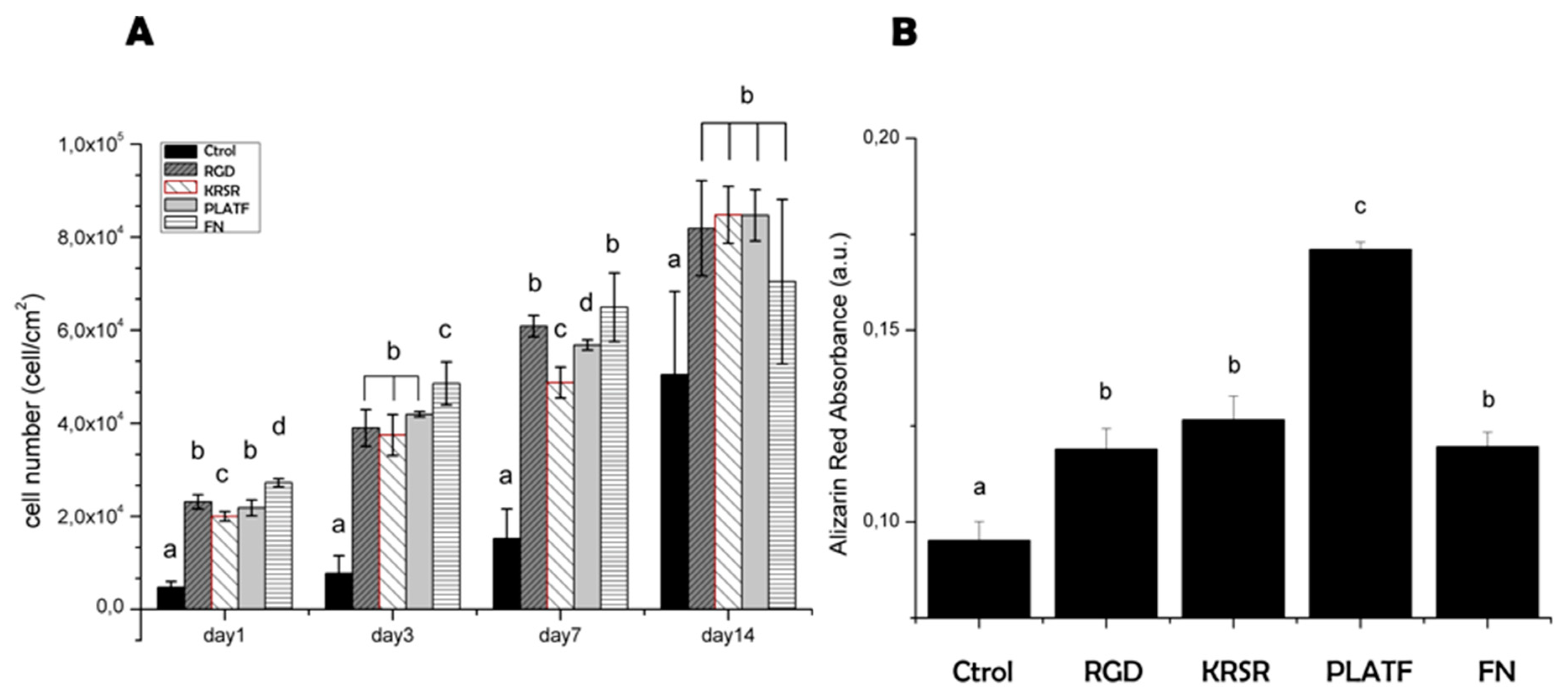
2.3. Biological Characterization of the Biointerfaces
3. Materials and Methods
3.1. Production of Biomolecular Interfaces
3.1.1. Synthesis of Biomolecular Coatings
3.1.2. Production of Titanium Samples
3.1.3. Biomolecule Grafting
3.2. Physicochemical Characterization
3.2.1. Contact Angle Measurements
3.2.2. Raman Spectroscopy
3.2.3. X-ray Photoelectron Spectroscopy (XPS)
3.2.4. Stability Treatments
3.3. Biological Characterization
3.3.1. Cell Culture
3.3.2. Immunofluorescence Analysis: Cell Adhesion and Spreading
3.3.3. Cell Proliferation and Mineralization
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Shekaran, A.; Garcia, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. Part A 2011, 96, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Tejero, R.; Anitua, E.; Orive, G. Toward the biomimetic implant surface: Biopolymers on titanium-based implants for bone regeneration. Prog. Polym. Sci. 2014, 39, 1406–1447. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliver. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium coatings and surface modifications: Toward clinically useful bioactive implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Rodda, A.E.; Meagher, L.; Nisbet, D.R.; Forsythe, J.S. Specific control of cell–material interactions: Targeting cell receptors using ligand-functionalized polymer substrates. Prog. Polym. Sci. 2014, 39, 1312–1347. [Google Scholar] [CrossRef]
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: A systematic review. BMC Med. 2016, 14, 103. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Fan, Y.; Li, X. The use of bioactive peptides to modify materials for bone tissue repair. Regen. Biomater. 2017, 4, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Mas-Moruno, C.; Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Kapp, T.G.; Kessler, H. αvβ3- or α5β1-integrin-selective peptidomimetics for surface coating. Angew. Chem. Int. Ed. 2016, 55, 7048–7067. [Google Scholar] [CrossRef]
- Williams, D.F. The role of short synthetic adhesion peptides in regenerative medicine; the debate. Biomaterials 2011, 32, 4195–4197. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C. Surface functionalization of biomaterials for bone tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Elsevier, Woodhead Publishing: Sawston, UK, 2018; pp. 73–100. [Google Scholar]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional coatings and nanotopographies: Toward cell instructive and antibacterial implants. Adv. Healthcare Mater. 2018, 8, e1801103. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M.; Beck-Sickinger, A.G. Multifunctional biomaterial coatings: Synthetic challenges and biological activity. Biol. Chem. 2017, 398, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Thombare, V.J.; Hutton, C.A.; O’Connor, A.J.; Qiao, G.G.; Heath, D.E. Beyond RGD; nanoclusters of syndecan- and integrin-binding ligands synergistically enhance cell/material interactions. Biomaterials 2018, 187, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.-J.; Mas-Moruno, C.; et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef] [PubMed]
- Dee, K.C.; Andersen, T.T.; Bizios, R. Design and function of novel osteoblast-adhesive peptides for chemical modification of biomaterials. J. Biomed. Mater. Res. 1998, 40, 371–377. [Google Scholar] [CrossRef]
- Hasenbein, M.E.; Andersen, T.T.; Bizios, R. Micropatterned surfaces modified with select peptides promote exclusive interactions with osteoblasts. Biomaterials 2002, 23, 3937–3942. [Google Scholar] [CrossRef]
- Schuler, M.; Hamilton, D.W.; Kunzler, T.P.; Sprecher, C.M.; de Wild, M.; Brunette, D.M.; Textor, M.; Tosatti, S.G.P. Comparison of the response of cultured osteoblasts and osteoblasts outgrown from rat calvarial bone chips to nonfouling KRSR and FHRRIKA-peptide modified rough titanium surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 517–527. [Google Scholar] [CrossRef]
- Dettin, M.; Conconi, M.T.; Gambaretto, R.; Pasquato, A.; Folin, M.; Di Bello, C.; Parnigotto, P.P. Novel osteoblast-adhesive peptides for dental / orthopedic biomaterials. J. Biomed. Mater. Res. 2002, 60, 466–471. [Google Scholar] [CrossRef]
- Balasundaram, G.; Webster, T.J. Increased osteoblast adhesion on nanograined Ti modified with KRSR. J. Biomed. Mater. Res. Part A 2007, 80, 602–611. [Google Scholar] [CrossRef]
- Sun, S.; Yu, W.; Zhang, Y.; Zhang, F. Increased preosteoblast adhesion and osteogenic gene expression on TiO2 nanotubes modified with KRSR. J. Mater. Sci. Mater. Med. 2013, 24, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Healy, K.E. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol. Prog. 1999, 15, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M.; Hassert, R.; John, T.; Braun, K.; Wießler, M.; Abel, B.; Beck-Sickinger, A.G. Multifunctional coating improves cell adhesion on titanium by using cooperatively acting peptides. Angew. Chem. Int. Ed. 2016, 55, 4826–4830. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk-Biegun, M.K.; Werten, M.W.T.; Posadowska, U.; Storm, I.M.; de Wolf, F.A.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Cohen Stuart, M.A.; Kamperman, M. Nanofibrillar hydrogel scaffolds from recombinant protein-based polymers with integrin- and proteoglycan-binding domains. J. Biomed. Mater. Res. Part A 2016, 104, 3082–3092. [Google Scholar] [CrossRef]
- Bell, B.F.; Schuler, M.; Tosatti, S.; Textor, M.; Schwartz, Z.; Boyan, B.D. Osteoblast response to titanium surfaces functionalized with extracellular matrix peptide biomimetics. Clin. Oral Implants Res. 2011, 22, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Broggini, N.; Tosatti, S.; Ferguson, S.J.; Schuler, M.; Textor, M.; Bornstein, M.M.; Bosshardt, D.D.; Buser, D. Evaluation of chemically modified SLA implants (modSLA) biofunctionalized with integrin (RGD)- and heparin (KRSR)-binding peptides. J. Biomed. Mater. Res. Part A 2012, 100, 703–711. [Google Scholar] [CrossRef]
- Kim, J.W.; Ki, C.S.; Park, Y.H.; Kim, H.J.; Um, I.C. Effect of RGDS and KRSR peptides immobilized on silk fibroin nanofibrous mats for cell adhesion and proliferation. Macromol. Res. 2010, 18, 442–448. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Fraioli, R.; Albericio, F.; Manero, J.M.; Gil, F.J. Novel peptide-based platform for the dual presentation of biologically active peptide motifs on biomaterials. ACS Appl. Mater. Interfaces 2014, 6, 6525–6536. [Google Scholar] [CrossRef]
- Herranz-Diez, C.; Mas-Moruno, C.; Neubauer, S.; Kessler, H.; Gil, F.J.; Pegueroles, M.; Guillem-Marti, J. Tuning mesenchymal stem cell response onto Titanium-Niobium-Hafnium alloy by recombinant fibronectin fragments. ACS Appl. Mater. Interfaces 2016, 8, 2517–2525. [Google Scholar] [CrossRef]
- Fraioli, R.; Dashnyam, K.; Kim, J.-H.; Perez, R.A.; Kim, H.-W.; Gil, J.; Ginebra, M.-P.; Manero, J.M.; Mas-Moruno, C. Surface guidance of stem cell behavior: Chemically tailored co-presentation of integrin-binding peptides stimulates osteogenic differentiation in vitro and bone formation in vivo. Acta Biomater. 2016, 43, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogués, M.; Velasco, F.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: The blending of cell integration and bacterial inhibition properties on the surface of biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 21618–21630. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogués, M.; Buxadera-Palomero, J.; Ginebra, M.-P.; Manero, J.M.; Gil, F.; Mas-Moruno, C. All-in-one trifunctional strategy: A cell adhesive, bacteriostatic and bactericidal coating for titanium implants. Colloids Surf. B Biointerfaces 2018, 169, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Dorfner, P.M.; Manzenrieder, F.; Neubauer, S.; Reuning, U.; Burgkart, R.; Kessler, H. Behavior of primary human osteoblasts on trimmed and sandblasted Ti6Al4V surfaces functionalized with integrin avb3-selective cyclic RGD peptides. J. Biomed. Mater. Res. Part A 2013, 101, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Rechenmacher, F.; Neubauer, S.; Mas-Moruno, C.; Dorfner, P.M.; Polleux, J.; Guasch, J.; Conings, B.; Boyen, H.G.; Bochen, A.; Sobahi, T.R.; et al. A molecular toolkit for the functionalization of titanium-based biomaterials that selectively control integrin-mediated cell adhesion. Chemistry 2013, 19, 9218–9223. [Google Scholar] [CrossRef]
- Pallarola, D.; Bochen, A.; Boehm, H.; Rechenmacher, F.; Sobahi, T.R.; Spatz, J.P.; Kessler, H. Interface immobilization chemistry of cRGD-based peptides regulates integrin mediated cell adhesion. Adv. Funct. Mater. 2014, 24, 943–956. [Google Scholar] [CrossRef]
- Xiao, S.J.; Textor, M.; Spencer, N.D.; Wieland, M.; Keller, B.; Sigrist, H. Immobilization of the cell-adhesive peptide Arg-Gly-Asp-Cys (RGDC) on titanium surfaces by covalent chemical attachment. J. Mater. Sci. Mater. Med. 1997, 8, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.J.; Textor, M.; Spencer, N.D. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir 1998, 14, 5507–5516. [Google Scholar] [CrossRef]
- Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Manero, J.M.; Gil, J.; Kessler, H.; Mas-Moruno, C. Mimicking bone extracellular matrix: Integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation, and differentiation on titanium. Colloids Surf. B 2015, 128, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [Green Version]
- Tuma, R. Raman spectroscopy of proteins: From peptides to large assemblies. J. Raman Spectrosc. 2005, 36, 307–319. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Lee, J.-Y.; Choo, J.-E.; Choi, Y.-S.; Lee, K.-Y.; Min, D.-S.; Pi, S.-H.; Seol, Y.-J.; Lee, S.-J.; Jo, I.-H.; Chung, C.-P.; et al. Characterization of the surface immobilized synthetic heparin binding domain derived from human fibroblast growth factor-2 and its effect on osteoblast differentiation. J. Biomed. Mater. Res. Part A 2007, 83, 970–979. [Google Scholar] [CrossRef]
- Cavalcanti-Adam, E.A.; Shapiro, I.M.; Composto, R.J.; Macarak, E.J.; Adams, C.S. RGD Peptides immobilized on a mechanically deformable surface promote osteoblast differentiation. J. Bone Miner. Res. 2002, 17, 2130–2140. [Google Scholar] [CrossRef]

| Code | tR (min) a | Purity (%) a | m/z calcd. | [M + H]+ |
|---|---|---|---|---|
| PLATF | 4.253 | 97 | 1926.98 | 1927.13 |
| RGD | 5.790 | >99 | 810.35 | 811.49 |
| KRSR | 5.348 | >99 | 922.50 | 923.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyos-Nogués, M.; Falgueras-Batlle, E.; Ginebra, M.-P.; Manero, J.M.; Gil, J.; Mas-Moruno, C. A Dual Molecular Biointerface Combining RGD and KRSR Sequences Improves Osteoblastic Functions by Synergizing Integrin and Cell-Membrane Proteoglycan Binding. Int. J. Mol. Sci. 2019, 20, 1429. https://doi.org/10.3390/ijms20061429
Hoyos-Nogués M, Falgueras-Batlle E, Ginebra M-P, Manero JM, Gil J, Mas-Moruno C. A Dual Molecular Biointerface Combining RGD and KRSR Sequences Improves Osteoblastic Functions by Synergizing Integrin and Cell-Membrane Proteoglycan Binding. International Journal of Molecular Sciences. 2019; 20(6):1429. https://doi.org/10.3390/ijms20061429
Chicago/Turabian StyleHoyos-Nogués, Mireia, Elena Falgueras-Batlle, Maria-Pau Ginebra, José María Manero, Javier Gil, and Carlos Mas-Moruno. 2019. "A Dual Molecular Biointerface Combining RGD and KRSR Sequences Improves Osteoblastic Functions by Synergizing Integrin and Cell-Membrane Proteoglycan Binding" International Journal of Molecular Sciences 20, no. 6: 1429. https://doi.org/10.3390/ijms20061429






