Mechanisms Underlying Bone Loss Associated with Gut Inflammation
Abstract
:1. Introduction
2. Main Risk Factors of Bone Deterioration in Patients with GI Disease
2.1. Genetic Factors
2.1.1. IL-1β
2.1.2. RANKL/OPG
2.1.3. IL-6
2.1.4. Vitamin D Receptor (VDR)
2.2. Microbiota
2.3. Vitamin D Homeostasis
2.4. Medication
3. Animal Models of Gut Inflammation-Induced Bone Loss
3.1. Models Based on Genetic Modification
3.1.1. HLA-B27 Transgenic (B27-Tg) Rats
3.1.2. VDR−/− Mice
3.1.3. IL-10 Deficiency
3.1.4. IL-2 Deficiency
3.1.5. Tnf ΔARE Mice
3.1.6. gp130ΔSTAT/ΔSTAT and gp130Y757F/Y757F Mice
3.1.7. Hematopoietic Cell-Specific STAT3-KO Mice (STAT3-CFF; Tie2Cre+-Stat3fl/fl)
3.1.8. A20 (Tumor Necrosis Factor α-Induced Protein3; TNFAIP3) Deficiency
3.1.9. IKK2caIEC Mice
3.1.10. FXR−/− Mice
3.1.11. Mdr2−/− Mice
3.2. Models Based on Dysbiosis-Associated Gut Inflammation
3.2.1. Germ-Free (GF) Models
3.2.2. Antibiotics Treatment Models
3.3. Chemical Irritant-Induced Model
3.3.1. Trinitrobenzene Sulfonic Acid (TNBS)
3.3.2. Dextran Sulfate Sodium (DSS)
3.4. Immune Cell-Transfer Induced Model (CD45RB Model)
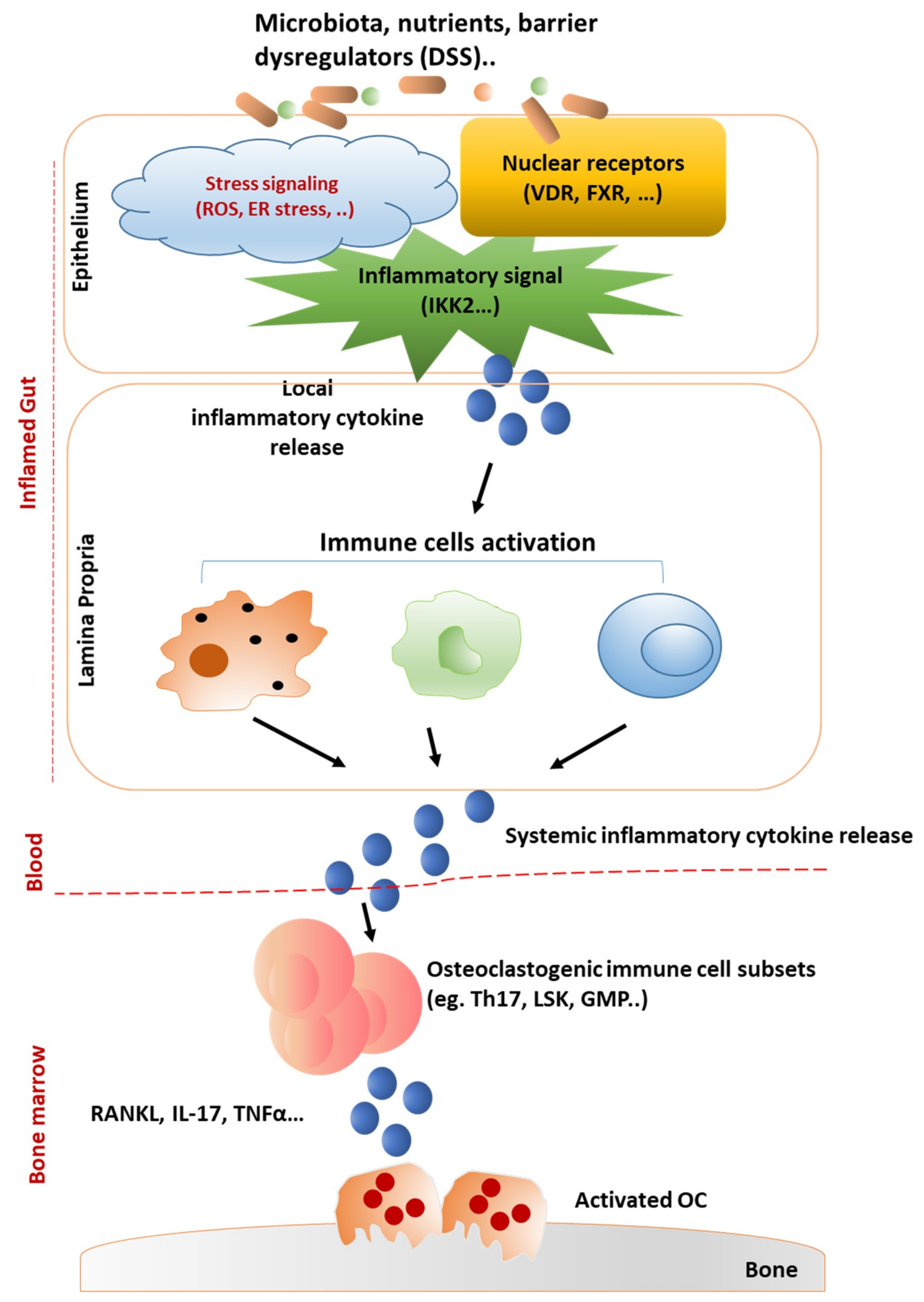
4. Networks of Gut-Residing Factors Regulating Bone Marrow Microenvironment and Bone Loss
4.1. Intestinal Barrier-Regulated Bone Loss
4.2. Effect of Gut-Derived Cytokines on Bone Marrow Microenvironment
5. Closing Remarks
Funding
Conflicts of Interest
Abbreviations
| GALT | Gut-Associated Lymphoid Tissue |
| GI | Gastrointestinal |
| IBD | Inflammatory Bowel Disease |
| UC | Ulcerative Colitis |
| CD | Crohn’s Disease |
| GM | Gut Microbiota |
| OP | Osteoporosis |
| BMD | Bone Mass/Mineral Density |
| OC | Osteoclast |
| OB | Osteoblast |
| IL | Interleukin |
| RANKL | Receptor Activator of Nuclear Factor Kappa-Β Ligand |
| OPG | Osteoprotegerin |
| VDR | Vitamin D Receptor |
| PTH | Parathyroid Hormone |
| OCN | Osteocalcin |
| PINP | Procollagen type I N-terminal Propeptide |
| BW | Body Weight |
| CTX-I | Type I Collagen C-telopeptide |
| BM | Bone Marrow |
| OC.N. | Osteoclast Number |
| ALP | Alkaline Phosphatase |
| n.s. | No Significant Difference |
| BV/TV | Bone Volume/Total Volume |
| N.A. | Not Available |
| BV | Bone Volume |
| Tb.Th. | Trabecular Thickness |
| Tb.N. | Trabecular No. |
| ROS | Reactive Oxygen Species |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of activated B cells |
| IEC | Intestinal Epithelial Cell |
| ILC | Innate Lymphoid Cells |
| FXR | Farnesoid X Receptor |
| Mdr-2 | Multidrug Resistance-2 |
| GF | Germ Free |
| ConD-GF | Conventionalized Germ Free |
| IGF | Insulin-like Growth Factor |
| TJ | Tight Junction |
| SCFA | Short Chain Fatty Acid |
| TNBS | Trinitrobenzene Sulfonic Acid4 |
| BFR | Bone Formation Rate |
| BS | Bone Surface |
| OS | Osteocyte Surface |
| Ct.Ar. | Cortical Bone Area |
| Ct.Th. | Cortical Thickness |
| Ct.Ar/Tt.Ar | Cortical Bone Fraction |
| DSS | Dextran Sulfate Sodium |
| Conn.D | Connective Density |
| Tb.Sp. | Trabecular Space |
| Tg | Transgenic |
| SpA | Spondyloarthritis |
| STAT | Signal Transducer and Activator of Transcription |
| PSC | Primary Sclerosing Cholangitis |
| OVX | Ovariectomy |
| CONV-R | Conventionally Raised |
| GIP | Glucose-dependent Insulinotropic Polypeptide |
| BMMSC | Bone Marrow Mesenchymal Stem Cells |
| ER | Endoplasmic Reticulum |
| GWAS | Genome-wide association studies |
| UPR | Unfolded Proteins |
| TUDCA | Tauroursodeoxycholic Acid |
References
- Forchielli, M.L.; Walker, W.A. The role of gut-associated lymphoid tissues and mucosal defence. Br. J. Nutr. 2005, 93, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, C.; Scholmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T. Recent Research on Joint Pain and Arthritis in Patients With Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2017, 13, 688–690. [Google Scholar]
- Kelsall, B.L. Innate and adaptive mechanisms to control [corrected] pathological intestinal inflammation. J. Pathol. 2008, 214, 242–259. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 198–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, T.; Lam, D.; Bronze, M.S.; Humphrey, M.B. Osteoporosis in inflammatory bowel disease. Am. J. Med. 2009, 122, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Szafors, P.; Che, H.; Barnetche, T.; Morel, J.; Gaujoux-Viala, C.; Combe, B.; Lukas, C. Risk of fracture and low bone mineral density in adults with inflammatory bowel diseases. A systematic literature review with meta-analysis. Osteoporos. Int. 2018, 29, 2389–2397. [Google Scholar] [CrossRef]
- Lima, C.A.; Lyra, A.C.; Mendes, C.M.C.; Lopes, M.B.; Coqueiro, F.G.; Rocha, R.; Santana, G.O. Bone mineral density and inflammatory bowel disease severity. Braz. J. Med. Biol. Res. 2017, 50, 6374. [Google Scholar] [CrossRef]
- Bourikas, L.A.; Papadakis, K.A. Musculoskeletal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 191–524. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Hudec, S.M.; Camacho, P.M. Secondary causes of osteoporosis. Endocr. Pract. 2013, 19, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colia, R.; Corrado, A.; Cantatore, F.P. Rheumatologic and extraintestinal manifestations of inflammatory bowel diseases. Ann. Med. 2016, 48, 577–585. [Google Scholar] [CrossRef]
- Yamamoto-Furusho, J.K.; Santiago-Hernandez, J.J.; Perez-Hernandez, N.; Ramirez-Fuentes, S.; Fragoso, J.M.; Vargas-Alarcon, G. Interleukin 1 beta (IL-1B) and IL-1 antagonist receptor (IL-1RN) gene polymorphisms are associated with the genetic susceptibility and steroid dependence in patients with ulcerative colitis. J. Clin. Gastroenterol. 2011, 45, 531–535. [Google Scholar] [CrossRef]
- Vounotrypidis, P.; Kouklakis, G.; Anagnostopoulos, K.; Zezos, P.; Polychronidis, A.; Maltezos, E.; Efremidou, E.; Pitiakoudis, M.; Lyratzopoulos, N. Interleukin-1 associations in inflammatory bowel disease and the enteropathic seronegative spondylarthritis. Autoimmun. Highlights 2013, 4, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [Green Version]
- Nemetz, A.; Toth, M.; Garcia-Gonzalez, M.A.; Zagoni, T.; Feher, J.; Pena, A.S.; Tulassay, Z. Allelic variation at the interleukin 1beta gene is associated with decreased bone mass in patients with inflammatory bowel diseases. Gut 2001, 49, 644–649. [Google Scholar] [CrossRef] [Green Version]
- Hugle, B.; Speth, F.; Haas, J.P. Inflammatory bowel disease following anti-interleukin-1-treatment in systemic juvenile idiopathic arthritis. Pediatric Rheumatol. Online J. 2017, 15, 16. [Google Scholar] [CrossRef] [Green Version]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Ludwiczek, O.; Gabriel, M.; Obrist, P.; Wolf, A.M.; Tilg, H. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 2005, 54, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Taranta, A.; Fortunati, D.; Longo, M.; Rucci, N.; Iacomino, E.; Aliberti, F.; Facciuto, E.; Migliaccio, S.; Bardella, M.T.; Dubini, A.; et al. Imbalance of osteoclastogenesis-regulating factors in patients with celiac disease. J. Bone Miner. Res. 2004, 19, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Horwood, N.J.; Elliott, J.; Martin, T.J.; Gillespie, M.T. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J. Immunol. 2001, 166, 491–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krela-Kazmierczak, I.; Kaczmarek-Rys, M.; Szymczak, A.; Michalak, M.; Skrzypczak-Zielinska, M.; Drweska-Matelska, N.; Marcinkowska, M.; Eder, P.; Lykowska-Szuber, L.; Wysocka, E.; et al. Bone Metabolism and the c.−223C > T Polymorphism in the 5’UTR Region of the Osteoprotegerin Gene in Patients with Inflammatory Bowel Disease. Calcif. Tissue Int. 2016, 99, 616–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasciano, A.C.; Blutt, S.E.; Estes, M.K.; Mecsas, J. Induced Differentiation of M Cell-like Cells in Human Stem Cell-derived Ileal Enteroid Monolayers. J. Vis. Exp. 2019, 149, 59894. [Google Scholar] [CrossRef]
- Schulte, C.M.; Dignass, A.U.; Goebell, H.; Roher, H.D.; Schulte, K.M. Genetic factors determine extent of bone loss in inflammatory bowel disease. Gastroenterology 2000, 119, 909–920. [Google Scholar] [CrossRef]
- Todhunter, C.E.; Sutherland-Craggs, A.; Bartram, S.A.; Donaldson, P.T.; Daly, A.K.; Francis, R.M.; Mansfield, J.C.; Thompson, N.P. Influence of IL-6, COL1A1, and VDR gene polymorphisms on bone mineral density in Crohn’s disease. Gut 2005, 54, 1579–1584. [Google Scholar] [CrossRef]
- Szymczak-Tomczak, A.; Krela-Kazmierczak, I.; Kaczmarek-Rys, M.; Hryhorowicz, S.; Stawczyk-Eder, K.; Szalata, M.; Skrzypczak-Zielinska, M.; Lykowska-Szuber, L.; Eder, P.; Michalak, M.; et al. Vitamin D receptor (VDR) TaqI polymorphism, vitamin D and bone mineral density in patients with inflammatory bowel diseases. Adv. Clin. Exp. Med. 2019, 28, 955–960. [Google Scholar] [CrossRef]
- Ahmad, I.; Jafar, T.; Mahdi, F.; Ameta, K.; Arshad, M.; Das, S.K.; Waliullah, S.; Rizvi, I.; Mahdi, A.A. Association of vitamin D receptor gene polymorphism (TaqI and Apa1) with bone mineral density in North Indian postmenopausal women. Gene 2018, 659, 123–127. [Google Scholar] [CrossRef]
- Morrison, N.A.; Qi, J.C.; Tokita, A.; Kelly, P.J.; Crofts, L.; Nguyen, T.V.; Sambrook, P.N.; Eisman, J.A. Prediction of bone density from vitamin D receptor alleles. Nature 1994, 367, 284. [Google Scholar] [CrossRef]
- Noble, C.L.; McCullough, J.; Ho, W.; Lees, C.W.; Nimmo, E.; Drummond, H.; Bear, S.; Hannan, J.; Millar, C.; Ralston, S.H.; et al. Low body mass not vitamin D receptor polymorphisms predict osteoporosis in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2008, 27, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson, C.; Sjogren, K. Effects of the gut microbiota on bone mass. Trends Endocrinol. Metab. 2015, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Arvikar, S.L.; Fisher, M.C. Inflammatory bowel disease associated arthropathy. Curr. Rev. Musculoskelet. Med. 2011, 4, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakenhoff, L.K.; van der Heijde, D.M.; Hommes, D.W.; Huizinga, T.W.; Fidder, H.H. The joint-gut axis in inflammatory bowel diseases. J. Crohn’s Colitis 2010, 4, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, S.; Colombel, J.F.; Feagan, B.G.; Reich, K.; Deodhar, A.A.; McInnes, I.B.; Porter, B.; Das Gupta, A.; Pricop, L.; Fox, T.; et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: A retrospective analysis of pooled data from 21 clinical trials. Ann. Rheum. Dis. 2019, 78, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Muniz Pedrogo, D.A.; Chen, J.; Hillmann, B.; Jeraldo, P.; Al-Ghalith, G.; Taneja, V.; Davis, J.M.; Knights, D.; Nelson, H.; Faubion, W.A.; et al. An Increased Abundance of Clostridiaceae Characterizes Arthritis in Inflammatory Bowel Disease and Rheumatoid Arthritis: A Cross-sectional Study. Inflamm. Bowel Dis. 2019, 25, 902–913. [Google Scholar] [CrossRef]
- Breban, M.; Tap, J.; Leboime, A.; Said-Nahal, R.; Langella, P.; Chiocchia, G.; Furet, J.P.; Sokol, H. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 1614–1622. [Google Scholar] [CrossRef]
- Hwang, C.; Ross, V.; Mahadevan, U. Micronutrient deficiencies in inflammatory bowel disease: From A to zinc. Inflamm. Bowel Dis. 2012, 18, 1961–1981. [Google Scholar] [CrossRef]
- Demay, M.B. Mechanism of vitamin D receptor action. Ann. NY. Acad. Sci. 2006, 1068, 204–213. [Google Scholar] [CrossRef]
- Abreu-Delgado, Y.; Isidro, R.A.; Torres, E.A.; Gonzalez, A.; Cruz, M.L.; Isidro, A.A.; Gonzalez-Keelan, C.I.; Medero, P.; Appleyard, C.B. Serum vitamin D and colonic vitamin D receptor in inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 3581–3591. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Golan, M.A.; Annunziata, M.L.; Du, J.; Dougherty, U.; Kong, J.; Musch, M.; Huang, Y.; Pekow, J.; et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J. Clin. Investig. 2013, 123, 3983–3996. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Du, J.; Zhang, Z.; Liu, T.; Shi, Y.; Ge, X.; Li, Y.C. MicroRNA-346 mediates tumor necrosis factor alpha-induced downregulation of gut epithelial vitamin D receptor in inflammatory bowel diseases. Inflamm. Bowel Dis. 2014, 20, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappa, H.M.; Gordon, C.M.; Saslowsky, T.M.; Zholudev, A.; Horr, B.; Shih, M.C.; Grand, R.J. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics 2006, 118, 1950–1961. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.D.; Wadhera, V.; Leach, S.T.; Woodhead, H.J.; Lemberg, D.A.; Mendoza-Cruz, A.C.; Day, A.S. Vitamin D deficiency in children with inflammatory bowel disease. Dig. Dis. Sci. 2011, 56, 830–836. [Google Scholar] [CrossRef]
- Sentongo, T.A.; Semaeo, E.J.; Stettler, N.; Piccoli, D.A.; Stallings, V.A.; Zemel, B.S. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am. J. Clin. Nutr. 2002, 76, 1077–1081. [Google Scholar] [CrossRef] [Green Version]
- Laakso, S.; Valta, H.; Verkasalo, M.; Toiviainen-Salo, S.; Viljakainen, H.; Makitie, O. Impaired bone health in inflammatory bowel disease: A case-control study in 80 pediatric patients. Calcif. Tissue Int. 2012, 91, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Harpavat, M.; Greenspan, S.L.; O’Brien, C.; Chang, C.C.; Bowen, A.; Keljo, D.J. Altered bone mass in children at diagnosis of Crohn disease: A pilot study. J. Pediatric Gastroenterol. Nutr. 2005, 40, 295–300. [Google Scholar] [CrossRef]
- Pappa, H.M.; Grand, R.J.; Gordon, C.M. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm. Bowel Dis. 2006, 12, 1162–1174. [Google Scholar] [CrossRef]
- Del Pinto, R.; Ferri, C.; Cominelli, F. Vitamin D Axis in Inflammatory Bowel Diseases: Role, Current Uses and Future Perspectives. Int. J. Mol. Sci. 2017, 11, 2360. [Google Scholar] [CrossRef] [Green Version]
- Neurath, M. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.P.; Leufkens, H.G.; Cooper, C. The epidemiology of corticosteroid-induced osteoporosis: A meta-analysis. Osteoporos. Int. 2002, 13, 777–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, L.; Humphrey, M.B. Glucocorticoid-Induced Osteoporosis. N. Engl. J. Med. 2018, 379, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Pukajlo-Marczyk, A.; Jakubowska, A.; Bargenda-Lange, A.; Kilis-Pstrusinska, K.; Zwolinska, D. Assessment of the Concentration of Bone Metabolism Markers: Sclerostin and FGF-23 in Children with Idiopathic Nephrotic Syndrome Treated with Glucocorticosteroids. Dis. Markers 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Delucchi, A.; Toro, L.; Alzamora, R.; Barrientos, V.; Gonzalez, M.; Andaur, R.; Leon, P.; Villanueva, F.; Galindo, M.; Las Heras, F.; et al. Glucocorticoids Decrease Longitudinal Bone Growth in Pediatric Kidney Transplant Recipients by Stimulating the FGF23/FGFR3 Signaling Pathway. J. Bone Miner. Res. 2019, 34. [Google Scholar] [CrossRef]
- El-Hodhod, M.A.; Hamdy, A.M.; Abbas, A.A.; Moftah, S.G.; Ramadan, A.A. Fibroblast growth factor 23 contributes to diminished bone mineral density in childhood inflammatory bowel disease. BMC Gastroenterol. 2012, 12, 44. [Google Scholar] [CrossRef] [Green Version]
- Taurog, J.D.; Maika, S.D.; Satumtira, N.; Dorris, M.L.; McLean, I.L.; Yanagisawa, H.; Sayad, A.; Stagg, A.J.; Fox, G.M.; Le O’Brien, A.; et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol. Rev. 1999, 169, 209–223. [Google Scholar]
- Rauner, M.; Stupphann, D.; Haas, M.; Fert, I.; Glatigny, S.; Sipos, W.; Breban, M.; Pietschmann, P. The HLA-B27 transgenic rat, a model of spondyloarthritis, has decreased bone mineral density and increased RANKL to osteoprotegerin mRNA ratio. J. Rheumatol. 2009, 36, 120–126. [Google Scholar] [CrossRef]
- Rauner, M.; Thiele, S.; Fert, I.; Araujo, L.M.; Layh-Schmitt, G.; Colbert, R.A.; Hofbauer, C.; Bernhardt, R.; Burki, A.; Schwiedrzik, J.; et al. Loss of bone strength in HLA-B27 transgenic rats is characterized by a high bone turnover and is mainly osteoclast-driven. Bone 2015, 75, 183–191. [Google Scholar] [CrossRef]
- Papet, I.; El Yousfi, M.; Godin, J.P.; Mermoud, A.F.; Davicco, M.J.; Coxam, V.; Breuille, D.; Obled, C. HLA-B27 rats develop osteopaenia through increased bone resorption without any change in bone formation. J. Musculoskelet. Neuronal Interact. 2008, 8, 251–256. [Google Scholar]
- Ansalone, C.; Utriainen, L.; Milling, S.; Goodyear, C.S. Role of Gut Inflammation in Altering the Monocyte Compartment and Its Osteoclastogenic Potential in HLA-B27-Transgenic Rats. Arthritis Rheumatol. 2017, 69, 1807–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amling, M.; Priemel, M.; Holzmann, T.; Chapin, K.; Rueger, J.M.; Baron, R.; Demay, M.B. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: Formal histomorphometric and biomechanical analyses. Endocrinology 1999, 140, 4982–4987. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, R.; Nakaya, Y.; Katsumata, S.; Kajita, Y.; Uehara, M.; Tanaka, S.; Sakai, A.; Kato, S.; Nakamura, T.; Suzuki, K. Dietary calcium and phosphorus ratio regulates bone mineralization and turnover in vitamin D receptor knockout mice by affecting intestinal calcium and phosphorus absorption. J. Bone Miner. Res. 2003, 18, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Fleet, J.C. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology 2009, 136, 1317–1327. [Google Scholar] [CrossRef] [Green Version]
- Kallay, E.; Pietschmann, P.; Toyokuni, S.; Bajna, E.; Hahn, P.; Mazzucco, K.; Bieglmayer, C.; Kato, S.; Cross, H.S. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis 2001, 22, 1429–1435. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yamaori, S.; Tanabe, T.; Johnson, C.H.; Krausz, K.W.; Kato, S.; Gonzalez, F.J. Implication of intestinal VDR deficiency in inflammatory bowel disease. Biochim. Biophys. Acta 2013, 1830, 2118–2128. [Google Scholar] [CrossRef] [Green Version]
- Lieben, L.; Masuyama, R.; Torrekens, S.; Van Looveren, R.; Schrooten, J.; Baatsen, P.; Lafage-Proust, M.H.; Dresselaers, T.; Feng, J.Q.; Bonewald, L.F.; et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J. Clin. Investig. 2012, 122, 1803–1815. [Google Scholar] [CrossRef]
- Dresner-Pollak, R.; Gelb, N.; Rachmilewitz, D.; Karmeli, F.; Weinreb, M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology 2004, 127, 792–801. [Google Scholar] [CrossRef]
- Ciucci, T.; Ibanez, L.; Boucoiran, A.; Birgy-Barelli, E.; Pene, J.; Abou-Ezzi, G.; Arab, N.; Rouleau, M.; Hebuterne, X.; Yssel, H.; et al. Bone marrow Th17 TNFalpha cells induce osteoclast differentiation, and link bone destruction to IBD. Gut 2015, 64, 1072–1081. [Google Scholar] [CrossRef]
- Thurston, R.D.; Larmonier, C.B.; Majewski, P.M.; Ramalingam, R.; Midura-Kiela, M.; Laubitz, D.; Vandewalle, A.; Besselsen, D.G.; Muhlbauer, M.; Jobin, C.; et al. Tumor necrosis factor and interferon-gamma down-regulate Klotho in mice with colitis. Gastroenterology 2010, 138, 1384–1394. [Google Scholar] [CrossRef] [Green Version]
- Sadlack, B.; Merz, H.; Schorle, H.; Schimpl, A.; Feller, A.C.; Horak, I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 1993, 75, 253–261. [Google Scholar] [CrossRef]
- Ashcroft, A.J.; Cruickshank, S.M.; Croucher, P.I.; Perry, M.J.; Rollinson, S.; Lippitt, J.M.; Child, J.A.; Dunstan, C.; Felsburg, P.J.; Morgan, G.J.; et al. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity 2003, 19, 849–861. [Google Scholar] [CrossRef]
- Kontoyiannis, D.; Pasparakis, M.; Pizarro, T.T.; Cominelli, F.; Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: Implications for joint and gut-associated immunopathologies. Immunity 1999, 10, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Ernst, M.; Inglese, M.; Waring, P.; Campbell, I.K.; Bao, S.; Clay, F.J.; Alexander, W.S.; Wicks, I.P.; Tarlinton, D.M.; Novak, U.; et al. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J. Exp. Med. 2001, 194, 189–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, N.A.; Jenkins, B.J.; Quinn, J.M.; Nakamura, A.; Glatt, M.; Gillespie, M.T.; Ernst, M.; Martin, T.J. Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J. Clin. Investig. 2004, 113, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Welte, T.; Zhang, S.S.; Wang, T.; Zhang, Z.; Hesslein, D.G.; Yin, Z.; Kano, A.; Iwamoto, Y.; Li, E.; Craft, J.E.; et al. STAT3 deletion during hematopoiesis causes Crohn’s disease-like pathogenesis and lethality: A critical role of STAT3 in innate immunity. Proc. Natl. Acad. Sci. USA. 2003, 100, 1879–1884. [Google Scholar] [CrossRef] [Green Version]
- Mantel, C.; Messina-Graham, S.; Moh, A.; Cooper, S.; Hangoc, G.; Fu, X.Y.; Broxmeyer, H.E. Mouse hematopoietic cell-targeted STAT3 deletion: Stem/progenitor cell defects, mitochondrial dysfunction, ROS overproduction, and a rapid aging-like phenotype. Blood 2012, 120, 2589–2599. [Google Scholar] [CrossRef] [Green Version]
- Matmati, M.; Jacques, P.; Maelfait, J.; Verheugen, E.; Kool, M.; Sze, M.; Geboes, L.; Louagie, E.; Mc Guire, C.; Vereecke, L.; et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat. Genet. 2011, 43, 908–912. [Google Scholar] [CrossRef]
- Vereecke, L.; Vieira-Silva, S.; Billiet, T.; van Es, J.H.; Mc Guire, C.; Slowicka, K.; Sze, M.; van den Born, M.; De Hertogh, G.; Clevers, H.; et al. A20 controls intestinal homeostasis through cell-specific activities. Nat. Commun. 2014, 5, 5103. [Google Scholar] [CrossRef] [Green Version]
- Ke, K.; Chen, T.H.; Arra, M.; Mbalaviele, G.; Swarnkar, G.; Abu-Amer, Y. Attenuation of NF-kappaB in Intestinal Epithelial Cells Is Sufficient to Mitigate the Bone Loss Comorbidity of Experimental Mouse Colitis. J. Bone Miner. Res. 2019, 34, 1880–1893. [Google Scholar] [CrossRef]
- Guma, M.; Stepniak, D.; Shaked, H.; Spehlmann, M.E.; Shenouda, S.; Cheroutre, H.; Vicente-Suarez, I.; Eckmann, L.; Kagnoff, M.F.; Karin, M.; et al. Constitutive intestinal NF-kappaB does not trigger destructive inflammation unless accompanied by MAPK activation. J. Exp. Med. 2011, 208, 1889–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlantis, K.; Wullaert, A.; Sasaki, Y.; Schmidt-Supprian, M.; Rajewsky, K.; Roskams, T.; Pasparakis, M. Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J. Clin. Investig. 2011, 121, 2781–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maran, R.R.; Thomas, A.; Roth, M.; Sheng, Z.; Esterly, N.; Pinson, D.; Gao, X.; Zhang, Y.; Ganapathy, V.; Gonzalez, F.J.; et al. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J. Pharm. Exp. Ther. 2009, 328, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.W.; An, J.H.; Park, H.; Yang, J.Y.; Choi, H.J.; Kim, S.W.; Park, Y.J.; Kim, S.Y.; Yim, M.; Baek, W.Y.; et al. Positive regulation of osteogenesis by bile acid through FXR. J. Bone Miner. Res. 2013, 28, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Kang, J.H.; Sim, J.S.; Kim, J.W.; Koh, J.T.; Shin, C.S.; Lim, H.; Yim, M. The farnesoid X receptor negatively regulates osteoclastogenesis in bone remodeling and pathological bone loss. Oncotarget 2017, 8, 76558–76573. [Google Scholar] [CrossRef] [Green Version]
- Liao, L.; Schneider, K.M.; Galvez, E.J.C.; Frissen, M.; Marschall, H.U.; Su, H.; Hatting, M.; Wahlstrom, A.; Haybaeck, J.; Puchas, P.; et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut 2019, 68, 1477–1492. [Google Scholar] [CrossRef]
- Schmidt, T.; Schwinge, D.; Rolvien, T.; Jeschke, A.; Schmidt, C.; Neven, M.; Butscheidt, S.; Kriz, M.; Kunzmann, L.; Mussawy, H.; et al. Th17 cell frequency is associated with low bone mass in primary sclerosing cholangitis. J. Hepatol. 2019, 70, 941–953. [Google Scholar] [CrossRef]
- Sjogren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Backhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef] [Green Version]
- Novince, C.M.; Whittow, C.R.; Aartun, J.D.; Hathaway, J.D.; Poulides, N.; Chavez, M.B.; Steinkamp, H.M.; Kirkwood, K.A.; Huang, E.; Westwater, C.; et al. Commensal Gut Microbiota Immunomodulatory Actions in Bone Marrow and Liver have Catabolic Effects on Skeletal Homeostasis in Health. Sci. Rep. 2017, 7, 5747. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA. 2016, 113, 7554–7563. [Google Scholar] [CrossRef] [Green Version]
- Metzger, C.E.; Narayanan, A.; Zawieja, D.C.; Bloomfield, S.A. Inflammatory Bowel Disease in a Rodent Model Alters Osteocyte Protein Levels Controlling Bone Turnover. J. Bone Miner. Res. 2017, 32, 802–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, V.M.; Ramalingam, R.; Larmonier, C.B.; Thurston, R.D.; Laubitz, D.; Midura-Kiela, M.T.; McFadden, R.M.; Kuro, O.M.; Kiela, P.R.; Ghishan, F.K.; et al. Post-translational loss of renal TRPV5 calcium channel expression, Ca(2+) wasting, and bone loss in experimental colitis. Gastroenterology 2013, 145, 613–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, L.; Senagore, P.; Young, V.B.; McCabe, L.R. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Ebersole, J.L. A novel murine model for chronic inflammatory alveolar bone loss. J. Periodontal. Res. 2010, 45, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, R.; Raehtz, S.; Parameswaran, N.; McCabe, L.R. Intestinal inflammation without weight loss decreases bone density and growth. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, 1149–1157. [Google Scholar] [CrossRef] [Green Version]
- Byrne, F.R.; Morony, S.; Warmington, K.; Geng, Z.; Brown, H.L.; Flores, S.A.; Fiorino, M.; Yin, S.L.; Hill, D.; Porkess, V.; et al. CD4 + CD45RBHi T cell transfer induced colitis in mice is accompanied by osteopenia which is treatable with recombinant human osteoprotegerin. Gut 2005, 54, 78–86. [Google Scholar] [CrossRef]
- Ibanez, L.; Abou-Ezzi, G.; Ciucci, T.; Amiot, V.; Belaid, N.; Obino, D.; Mansour, A.; Rouleau, M.; Wakkach, A.; Blin-Wakkach, C.; et al. Inflammatory Osteoclasts Prime TNFalpha-Producing CD4(+) T Cells and Express CX3 CR1. J. Bone Miner. Res. 2016, 31, 1899–1908. [Google Scholar] [CrossRef]
- Brown, M.A.; Kenna, T.; Wordsworth, B.P. Genetics of ankylosing spondylitis--insights into pathogenesis. Nat. Rev. Rheumatol. 2016, 12, 81–91. [Google Scholar] [CrossRef]
- Ossum, A.M.; Palm, O.; Lunder, A.K.; Cvancarova, M.; Banitalebi, H.; Negard, A.; Hoie, O.; Henriksen, M.; Moum, B.A.; Hoivik, M.L.; et al. Ankylosing Spondylitis and Axial Spondyloarthritis in Patients With Long-term Inflammatory Bowel Disease: Results From 20 Years of Follow-up in the IBSEN Study. J. Crohn’s Colitis 2018, 12, 96–104. [Google Scholar] [CrossRef]
- Olivieri, I.; Cantini, F.; Castiglione, F.; Felice, C.; Gionchetti, P.; Orlando, A.; Salvarani, C.; Scarpa, R.; Vecchi, M.; Armuzzi, A.; et al. Italian Expert Panel on the management of patients with coexisting spondyloarthritis and inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 822–830. [Google Scholar] [CrossRef]
- Salvarani, C.; Fries, W. Clinical features and epidemiology of spondyloarthritides associated with inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.R.; Wordsworth, B.P.; Jewell, D.P. Peripheral arthropathies in inflammatory bowel disease: Their articular distribution and natural history. Gut 1998, 42, 387–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragoulis, G.E.; Liava, C.; Daoussis, D.; Akriviadis, E.; Garyfallos, A.; Dimitroulas, T. Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment. World J. Gastroenterol. 2019, 25, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Taurog, J.D.; Richardson, J.A.; Croft, J.T.; Simmons, W.A.; Zhou, M.; Fernandez-Sueiro, J.L.; Balish, E.; Hammer, R.E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 1994, 180, 2359–2364. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yoshizawa, T.; Fukuda, T.; Shirode-Fukuda, Y.; Yu, T.; Sekine, K.; Sato, T.; Kawano, H.; Aihara, K.; Nakamichi, Y.; et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology 2013, 154, 1008–1020. [Google Scholar] [CrossRef] [Green Version]
- Nakamichi, Y.; Udagawa, N.; Suda, T.; Takahashi, N. Mechanisms involved in bone resorption regulated by vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 177, 70–76. [Google Scholar] [CrossRef]
- Evans, K.E.; Fox, S.W. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.G.; Sugiyama, E.; Shinoda, K.; Taki, H.; Hounoki, H.; Abdel-Aziz, H.O.; Maruyama, M.; Kobayashi, M.; Ogawa, H.; Miyahara, T.; et al. Interleukin-10 inhibits RANKL-mediated expression of NFATc1 in part via suppression of c-Fos and c-Jun in RAW264.7 cells and mouse bone marrow cells. Bone 2007, 41, 592–602. [Google Scholar] [CrossRef]
- Nelson, B.H. IL-2, regulatory T cells, and tolerance. J. Immunol. 2004, 172, 3983–3988. [Google Scholar] [CrossRef] [Green Version]
- Ward, N.C.; Yu, A.; Moro, A.; Ban, Y.; Chen, X.; Hsiung, S.; Keegan, J.; Arbanas, J.M.; Loubeau, M.; Thankappan, A.; et al. IL-2/CD25: A Long-Acting Fusion Protein That Promotes Immune Tolerance by Selectively Targeting the IL-2 Receptor on Regulatory T Cells. J. Immunol. 2018, 201, 2579–2592. [Google Scholar] [CrossRef] [Green Version]
- Josien, R.; Li, H.L.; Ingulli, E.; Sarma, S.; Wong, B.R.; Vologodskaia, M.; Steinman, R.M.; Choi, Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J. Exp. Med. 2000, 191, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naka, T.; Kishimoto, T. Joint disease caused by defective gp130-mediated STAT signaling. Arthritis Res 2002, 4, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.J.; Roberts, A.W.; Greenhill, C.J.; Najdovska, M.; Lundgren-May, T.; Robb, L.; Grail, D.; Ernst, M. Pathologic consequences of STAT3 hyperactivation by IL-6 and IL-11 during hematopoiesis and lymphopoiesis. Blood 2007, 109, 2380–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellmark, P.; Ingvarsson, J.; Carlsson, A.; Lundin, B.S.; Wingren, C.; Borrebaeck, C.A. Identification of protein expression signatures associated with Helicobacter pylori infection and gastric adenocarcinoma using recombinant antibody microarrays. Mol. Cell. Proteom. 2006, 5, 1638–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.M.; Putoczki, T.L.; Ernst, M. STAT3-Activating Cytokines: A Therapeutic Opportunity for Inflammatory Bowel Disease? J. Interferon Cytokine Res. 2015, 35, 340–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Welte, T.; Troiano, N.; Maher, S.E.; Fu, X.Y.; Bothwell, A.L. Osteoporosis with increased osteoclastogenesis in hematopoietic cell-specific STAT3-deficient mice. Biochem. Biophys. Res. Commun. 2005, 328, 800–807. [Google Scholar] [CrossRef]
- Zaidi, D.; Wine, E. Regulation of Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-kappabeta) in Inflammatory Bowel Diseases. Front. Pediatr. 2018, 6, 317. [Google Scholar] [CrossRef]
- Abu-Amer, Y. NF-kappaB signaling and bone resorption. Osteoporos. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.G.; Boone, D.L.; Chai, S.; Libby, S.L.; Chien, M.; Lodolce, J.P.; Ma, A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 2000, 289, 2350–2354. [Google Scholar] [CrossRef]
- Han, C.Y. Update on FXR Biology: Promising Therapeutic Target? Int. J. Mol. Sci. 2018, 19, 2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popp, A.W.; Isenegger, J.; Buergi, E.M.; Buergi, U.; Lippuner, K. Glucocorticosteroid-induced spinal osteoporosis: Scientific update on pathophysiology and treatment. Eur. Spine J. 2006, 15, 1035–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parseus, A.; Sommer, N.; Sommer, F.; Caesar, R.; Molinaro, A.; Stahlman, M.; Greiner, T.U.; Perkins, R.; Backhed, F. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, P.; Hochrath, K.; Horvath, A.; Chen, P.; Seebauer, C.T.; Llorente, C.; Wang, L.; Alnouti, Y.; Fouts, D.E.; Starkel, P.; et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 2018, 67, 2150–2166. [Google Scholar] [CrossRef]
- Inagaki, T.; Moschetta, A.; Lee, Y.K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. USA. 2006, 103, 3920–3925. [Google Scholar] [CrossRef] [Green Version]
- Borst, P.; Elferink, R.O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef] [Green Version]
- Ruetz, S.; Gros, P. Phosphatidylcholine translocase: A physiological role for the mdr2 gene. Cell 1994, 77, 1071–1081. [Google Scholar] [CrossRef]
- Fickert, P.; Pollheimer, M.J.; Beuers, U.; Lackner, C.; Hirschfield, G.; Housset, C.; Keitel, V.; Schramm, C.; Marschall, H.U.; Karlsen, T.H.; et al. Characterization of animal models for primary sclerosing cholangitis (PSC). J. Hepatol. 2014, 60, 1290–1303. [Google Scholar] [CrossRef] [Green Version]
- Giordano, D.M.; Pinto, C.; Maroni, L.; Benedetti, A.; Marzioni, M. Inflammation and the Gut-Liver Axis in the Pathophysiology of Cholangiopathies. Int. J. Mol. Sci. 2018, 19, 3003. [Google Scholar] [CrossRef] [Green Version]
- Angulo, P.; Grandison, G.A.; Fong, D.G.; Keach, J.C.; Lindor, K.D.; Bjornsson, E.; Koch, A. Bone disease in patients with primary sclerosing cholangitis. Gastroenterology 2011, 140, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Katt, J.; Schwinge, D.; Schoknecht, T.; Quaas, A.; Sobottka, I.; Burandt, E.; Becker, C.; Neurath, M.F.; Lohse, A.W.; Herkel, J.; et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 2013, 58, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macpherson, A.J.; Harris, N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004, 4, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; McCoy, K.D.; Macpherson, A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 2007, 19, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methe, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollag, R.J.; Zhong, Q.; Ding, K.H.; Phillips, P.; Zhong, L.; Qin, F.; Cranford, J.; Mulloy, A.L.; Cameron, R.; Isales, C.M.; et al. Glucose-dependent insulinotropic peptide is an integrative hormone with osteotropic effects. Mol. Cell. Endocrinol. 2001, 177, 35–41. [Google Scholar] [CrossRef]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic, L. Reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson, C.; Engdahl, C.; Fak, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Moverare-Skrtic, S.; Islander, U.; Sjogren, K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 2014, 9, 92368. [Google Scholar] [CrossRef]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.L.; Moniz, C.; Chambers, T.J.; Chow, J.W. Colitis causes bone loss in rats through suppression of bone formation. Gastroenterology 1996, 111, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Terzoudis, S.; Zavos, C.; Koutroubakis, I.E. The bone and fat connection in inflammatory bowel diseases. Inflamm. Bowel Dis. 2014, 20, 2207–2217. [Google Scholar] [CrossRef]
- Bryant, R.V.; Schultz, C.G.; Ooi, S.; Goess, C.; Costello, S.P.; Vincent, A.D.; Schoeman, S.N.; Lim, A.; Bartholomeusz, F.D.; Travis, S.P.L.; et al. Obesity in Inflammatory Bowel Disease: Gains in Adiposity despite High Prevalence of Myopenia and Osteopenia. Nutrients 2018, 10, 1192. [Google Scholar] [CrossRef] [Green Version]
- Kredel, L.I.; Siegmund, B. Adipose-tissue and intestinal inflammation—Visceral obesity and creeping fat. Front. Immunol. 2014, 5, 462. [Google Scholar] [CrossRef] [Green Version]
- Hamdani, G.; Gabet, Y.; Rachmilewitz, D.; Karmeli, F.; Bab, I.; Dresner-Pollak, R. Dextran sodium sulfate-induced colitis causes rapid bone loss in mice. Bone 2008, 43, 945–950. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, L.; Ma, Y.; Shuai, Y.; Li, L.; Luo, K.; Liu, W.; Jin, Y. Autophagy Maintains the Function of Bone Marrow Mesenchymal Stem Cells to Prevent Estrogen Deficiency-Induced Osteoporosis. Theranostics 2017, 7, 4498–4516. [Google Scholar] [CrossRef]
- Ostanin, D.V.; Bao, J.; Koboziev, I.; Gray, L.; Robinson-Jackson, S.A.; Kosloski-Davidson, M.; Price, V.H.; Grisham, M.B. T cell transfer model of chronic colitis: Concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Dahan, S.; Roth-Walter, F.; Arnaboldi, P.; Agarwal, S.; Mayer, L. Epithelia: Lymphocyte interactions in the gut. Immunol. Rev. 2007, 215, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeissig, S.; Burgel, N.; Gunzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D.; et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Rios-Arce, N.D.; Collins, F.L.; Schepper, J.D.; Steury, M.D.; Raehtz, S.; Mallin, H.; Schoenherr, D.T.; Parameswaran, N.; McCabe, L.R. Epithelial Barrier Function in Gut-Bone Signaling. Adv. Exp. Med. Biol. 2017, 1033, 151–183. [Google Scholar] [PubMed] [Green Version]
- Kurl, S.; Heinonen, K.; Lansimies, E.; Launiala, K. Determinants of bone mineral density in prematurely born children aged 6-7 years. Acta Paediatr. 1998, 87, 650–653. [Google Scholar] [CrossRef]
- Takada, M.; Shimada, M.; Hosono, S.; Tauchi, M.; Minato, M.; Takahashi, S.; Okuni, M.; Takeuchi, S. Trace elements and mineral requirements for very low birth weight infants in rickets of prematurity. Early Hum. Dev. 1992, 29, 333–338. [Google Scholar] [CrossRef]
- Halpern, M.D.; Denning, P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Al-Sadi, R.; Guo, S.; Ye, D.; Rawat, M.; Ma, T.Y. TNF-alpha Modulation of Intestinal Tight Junction Permeability Is Mediated by NIK/IKK-alpha Axis Activation of the Canonical NF-kappaB Pathway. Am. J. Pathol. 2016, 186, 1151–1165. [Google Scholar] [CrossRef] [Green Version]
- Al-Sadi, R.M.; Ma, T.Y. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J. Immunol. 2007, 178, 4641–4649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sadi, R.; Ye, D.; Said, H.M.; Ma, T.Y. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am. J. Pathol. 2010, 177, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleynen, I.; Boucher, G.; Jostins, L.; Schumm, L.P.; Zeissig, S.; Ahmad, T.; Andersen, V.; Andrews, J.M.; Annese, V.; Brand, S.; et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: A genetic association study. Lancet 2016, 387, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Kaser, A.; Lee, A.H.; Franke, A.; Glickman, J.N.; Zeissig, S.; Tilg, H.; Nieuwenhuis, E.E.; Higgins, D.E.; Schreiber, S.; Glimcher, L.H.; et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008, 134, 743–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.S.; Chen, Y.; Fan, L.; Xi, Q.L.; Wu, G.H.; Li, X.X.; Yuan, T.L.; He, S.Q.; Yu, Y.; Shao, M.L.; et al. The Endoplasmic Reticulum Stress Sensor IRE1alpha in Intestinal Epithelial Cells Is Essential for Protecting against Colitis. J. Biol. Chem. 2015, 290, 15327–15336. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.D.; Kaczmarek, A.; Krysko, O.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. ER stress-induced inflammation: Does it aid or impede disease progression? Trends Mol. Med. 2012, 18, 589–598. [Google Scholar] [CrossRef]
- Navid, F.; Colbert, R.A. Causes and consequences of endoplasmic reticulum stress in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 25–40. [Google Scholar] [CrossRef]
- Rahmati, M.; Moosavi, M.A.; McDermott, M.F. ER Stress: A Therapeutic Target in Rheumatoid Arthritis? Trends Pharmacol. Sci. 2018, 39, 610–623. [Google Scholar] [CrossRef]
- Scheiber, A.L.; Guess, A.J.; Kaito, T.; Abzug, J.M.; Enomoto-Iwamoto, M.; Leikin, S.; Iwamoto, M.; Otsuru, S. Endoplasmic reticulum stress is induced in growth plate hypertrophic chondrocytes in G610C mouse model of osteogenesis imperfecta. Biochem. Biophys. Res. Commun. 2019, 509, 235–240. [Google Scholar] [CrossRef]
- Yoo, S.A.; You, S.; Yoon, H.J.; Kim, D.H.; Kim, H.S.; Lee, K.; Ahn, J.H.; Hwang, D.; Lee, A.S.; Kim, K.J.; et al. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J. Exp. Med. 2012, 209, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.M.; Mahomed, N.; Gandhi, R.; Keystone, E.C.; Berger, S.A. TNFalpha modulates protein degradation pathways in rheumatoid arthritis synovial fibroblasts. Arthritis Res. Ther. 2012, 14, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laukens, D.; Devisscher, L.; Van den Bossche, L.; Hindryckx, P.; Vandenbroucke, R.E.; Vandewynckel, Y.P.; Cuvelier, C.; Brinkman, B.M.; Libert, C.; Vandenabeele, P.; et al. Tauroursodeoxycholic acid inhibits experimental colitis by preventing early intestinal epithelial cell death. Lab. Invest. 2014, 94, 1419–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bossche, L.; Hindryckx, P.; Devisscher, L.; Devriese, S.; Van Welden, S.; Holvoet, T.; Vilchez-Vargas, R.; Vital, M.; Pieper, D.H.; Vanden Bussche, J.; et al. Ursodeoxycholic Acid and Its Taurine- or Glycine-Conjugated Species Reduce Colitogenic Dysbiosis and Equally Suppress Experimental Colitis in Mice. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhao, J.; Gui, W.; Sun, D.; Dai, H.; Xiao, L.; Chu, H.; Du, F.; Zhu, Q.; Schnabl, B.; et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br. J. Pharmacol. 2018, 175, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Arai, Y.; Park, E.M.; Park, S.; Bello, A.; Han, I.B.; Lee, S.H. Osteogenic Potential of Tauroursodeoxycholic Acid as an Alternative to rhBMP-2 in a Mouse Spinal Fusion Model. Tissue Eng. Part A 2018, 24, 407–417. [Google Scholar] [CrossRef]
- Arai, Y.; Choi, B.; Kim, B.J.; Rim, W.; Park, S.; Park, H.; Ahn, J.; Lee, S.H. Tauroursodeoxycholic acid (TUDCA) counters osteoarthritis by regulating intracellular cholesterol levels and membrane fluidity of degenerated chondrocytes. Biomater. Sci. 2019, 7, 3178–3189. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Rioux, J.D.; Xavier, R.J.; Taylor, K.D.; Silverberg, M.S.; Goyette, P.; Huett, A.; Green, T.; Kuballa, P.; Barmada, M.M.; Datta, L.W.; et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 2007, 39, 596–604. [Google Scholar] [CrossRef]
- Cadwell, K.; Liu, J.Y.; Brown, S.L.; Miyoshi, H.; Loh, J.; Lennerz, J.K.; Kishi, C.; Kc, W.; Carrero, J.A.; Hunt, S.; et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008, 456, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adolph, T.E.; Tomczak, M.F.; Niederreiter, L.; Ko, H.J.; Bock, J.; Martinez-Naves, E.; Glickman, J.N.; Tschurtschenthaler, M.; Hartwig, J.; Hosomi, S.; et al. Paneth cells as a site of origin for intestinal inflammation. Nature 2013, 503, 272–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, N.Y.; Chen, C.W.; Kagwiria, R.; Liang, R.; Beyer, C.; Distler, A.; Luther, J.; Engelke, K.; Schett, G.; Distler, J.H. Inactivation of autophagy ameliorates glucocorticoid-induced and ovariectomy-induced bone loss. Ann. Rheum. Dis. 2016, 75, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qi, M.; An, Y.; Zhang, L.; Yang, R.; Doro, D.H.; Liu, W.; Jin, Y. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell 2018, 17, 12709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, P.; Gao, F.; Niu, D.; Sun, X.; Song, Q.; Guo, C.; Liang, Y.; Sun, W. The Role of Autophagy in Chondrocyte Metabolism and Osteoarthritis: A Comprehensive Research Review. Biomed. Res. Int. 2019, 2019, 5171602. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.; Kim, S.; Goldshteyn, V.; Kim, T.; Park, N.H.; Wang, C.Y.; Kim, R.H. Beclin1 Modulates Bone Homeostasis by Regulating Osteoclast and Chondrocyte Differentiation. J. Bone Miner. Res. 2019, 34, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xiao, Y. The Autophagy in Osteoimmonology: Self-Eating, Maintenance, and Beyond. Front. Endocrinol. 2019, 10, 490. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Q.; Wang, F. Mesenchymal stem cells for the treatment of ulcerative colitis: A systematic review and meta-analysis of experimental and clinical studies. Stem Cell Res. Ther. 2019, 10, 266. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, F.A.; Wyzga, N.; Hyams, J.S.; Gronowicz, G.A. Effect of Crohn’s disease on bone metabolism in vitro: A role for interleukin-6. J. Bone Miner. Res. 2002, 17, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.M.; Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, K.L.; Zheng, L.B.; Kanazawa, Y.; Shih, D.Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Trottier, M.D.; Irwin, R.; Li, Y.; McCabe, L.R.; Fraker, P.J. Enhanced production of early lineages of monocytic and granulocytic cells in mice with colitis. Proc. Natl. Acad. Sci. USA. 2012, 109, 16594–16599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desalegn, G.; Pabst, O. Inflammation triggers immediate rather than progressive changes in monocyte differentiation in the small intestine. Nat. Commun. 2019, 10, 3229. [Google Scholar] [CrossRef]
- Oostlander, A.E.; Everts, V.; Schoenmaker, T.; Bravenboer, N.; van Vliet, S.J.; van Bodegraven, A.A.; Lips, P.; de Vries, T.J. T cell-mediated increased osteoclast formation from peripheral blood as a mechanism for Crohn’s disease-associated bone loss. J. Cell. Biochem. 2012, 113, 260–268. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson, C.; Nigro, G.; Boneca, I.G.; Backhed, F.; Sansonetti, P.; Sjogren, K. Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cell. Immunol. 2017, 317, 55–58. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links Between the Microbiome and Bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.L.; Rios-Arce, N.D.; Atkinson, S.; Bierhalter, H.; Schoenherr, D.; Bazil, J.N.; McCabe, L.R.; Parameswaran, N. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol. Rep. 2017, 5, 13263. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.; Lee, T.; Young, V.B.; Parameswaran, N.; McCabe, L.R. Colitis-induced bone loss is gender dependent and associated with increased inflammation. Inflamm. Bowel Dis. 2013, 19, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Thayu, M.; Shults, J.; Burnham, J.M.; Zemel, B.S.; Baldassano, R.N.; Leonard, M.B. Gender differences in body composition deficits at diagnosis in children and adolescents with Crohn’s disease. Inflamm. Bowel Dis. 2007, 13, 1121–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Models Based on Genetic Modification | ||||
| Animal Strains | GI Phenotype | Skeletal Phenotype | Systemic and Other Organ Phenotype | Reference |
| HLA-B27 Tg rats | IBD dysbiosis | Spondylarthritis; RANKL/OPG ↑ osteoclastogenesis ↑ | circulating monocytes ↑ serum OCN, PINP: n.s. | [57,58,59,60,61] |
| VDR−/− mice | normal intestine; colonic 8-OHdG ↑ Ca2+ absorption ↑ | rickets, osteomalacia; femur BMD and MAR ↓ | BW↓ Serum OCN, 25-OH-D3, Ca2+ ↓ 1,25(OH)2D3 and PTH ↑ | [62,63,64,65] |
| Vdr(ΔIEpC) mice | intestinal Ca2+ absorption ↓ | osteopenia; bone turnover ↑; BV/TV, Ct.Th., Tb.N., Tb.Th. ↓; mineralization and skeletal Ca2+ ↓; RANKL/OPG ↑ | Serum PTH, 1,25(OH)2D3, CTX, and OCN ↑ | [66,67] |
| IL-10−/− mice | colitis | bone mass, Tb.Th. and Tb.N. ↓ bone formation ↓ %Th17-TNFα+ in BM ↑ | serum OCN ↓ renal Klotho ↓ | [68,69,70] |
| IL-2−/− mice | colitis; colonic dendritic cells, macrophages, and antigen-presenting CD4+T cells ↑ | osteopenia; bone formation ↓, OC.N.↑, BM monocytes ↑, RANKL, OPG ↑ | serum RANKL, OPG, MCP-1, IL-6, TNFα, and interferon-gamma (IFNγ) ↑ | [71,72] |
| TnfΔARE mice | CD-like IBD | spontaneous polyarthritis | serum TNF ↑ | [73] |
| gp130 ΔSTAT/ΔSTAT mice | ulceration | chronic synovitis; degraded articular cartilage; chondrocyte differentiation ↓ trabecular BV/TV n.s. | N/A | [74,75] |
| gp130 Y757F/Y757F mice | gastric cancer | BV/TV ↓ bone turnover ↑ | N/A | [75] |
| STAT3-CFF (Tie2Cre+-Stat3fl/fl) mice | CD-like IBD, immune cell infiltration TNFα ↑, INFγ ↑ | BV, Tb. Th., and Tb.N. ↓ OC.N. ↑ mitochondrial dysfunction in stem/progenitor cells and ROS ↑ | N/A | [76,77] |
| A20myel-KO mice | less stable microbiota | severe polyarthritis macrophage NF-κB activity and TNF ↑ osteoclastogenesis ↑ | serum inflammatory cytokines ↑ Spleen and inguinal lymph nodes Th17 ↑ | [78,79] |
| IKK2caIEC | mild gut inflammation at 8–10 weeks, inflammatory cytokines in IECs and ILC3 ↑ | BV/TV ↓ Osteoclastogenesis ↑ | serum inflammatory cytokines and CTX ↑ | [80,81,82] |
| FXR−/− mice | barrier dysfunction apoptotic goblet cells | BFR, Tb. BV. and Tb. Th. ↓ OB differentiation ↓ osteoclast generation ↑ | N/A | [83,84,85] |
| mdr2−/− mice | barrier dysfunction; dysbiosis | osteopenia trabecular bone mass ↓ | N/A | [86,87] |
| Models Based on Dysbiosis-Associated Gut Inflammation | ||||
| Animal Strains | GI Phenotype | Skeletal Phenotype | Systemic and Other Organ Phenotype | Reference |
| GF mice | serotonin ↑ CD4+ T cells ↓ TNFα ↓ | BV/TV, Tb.N. and BFR ↑; Tb.Sp. and OC.N. ↓ BM-CD4+T cells, Th17, TNFα and IL-6 ↓ BM-OC precursor ↓OC fusion ↓ | serum OCN, CTX, Ca2+, serotonin and TNF ↓ IGF ↑ | [88,89] |
| ConvD-GF | compared with GF mice: colon Rankl, TNFα, IL-1β ↑ cecal SCFA ↑ | compared with GF mice: femur length↑ Periosteal area ↑ endosteal area ↑ IGF-1 and RANKL in BM ↑ | serum CTX-I, PINP: n.s. serum IGF-1 ↑ muscle IGF-1 ↓ fat pad ↑ | [90] |
| Models Based on Chemical Induction | ||||
| Animal Strains | GI Phenotype | Skeletal Phenotype | Systemic and Other Organ Phenotype | Reference |
| TNBS | crypt loss, cellularity, and edema | BV/TV, BFR and osteoid surface ↓ OC.S ↑ % of TNFα+, IL-6+, RANKL+, and OPG+ osteocytes ↓ % of sclerostin+ and IGF+ osteocyte ↑ | N/A | [91] |
| TNBS | Severe colitis; colon TNF, IFNγ, IL-17, IL-1β ↑ | cortical bone fraction ↓ BV/TV and Tb.Th ↓ | serum OCN and RANKL ↓ serum tDPD, TNFα and IL-6 ↑ renal Ca2+ reabsorption ↓ urinal Ca2+ excretion ↑ renal TRPV5 and Klotho ↓ | [70,92] |
| DSS | mucosal inflammation and ulceration | BMD, Tb.Th., MAR, and Ct.BMD ↓ OB.Ar ↓ OC.Ar ↑ Growth plate height and cartilage gene ↓ | Femoral fat pads ↑ Liver mass ↓ serum TNFα ↑ IGF-1 ↓ | [93] |
| DSS | colonic length ↓ | Alveolar bone loss ↑ | Liver cystine ↓ | [94] |
| DSS | lymphocyte aggregates ↑; colon TNFα, IFNγ, IL-6 and IL-22 ↑ | Trabecular BV/TV, Tb.N., Tb.Th. ↓ Tb.Sp. ↑ growth plate thickness and ColX ↓ BFR and osteoblast surface (OB.S) ↓; TNFα ↑ | inguinal fat mass ↓ retroperitoneal fat mass↑ serum OCN ↓ | [95] |
| Adoptive Transfer of Effector Immune Cells to Immunodeficient Mice | ||||
| Animal Strains | GI Phenotype | Skeletal Phenotype | Systemic and Other Organ Phenotype | Reference |
| CD4+IL-10−/−Rag1−/− transfer | Severe colitis; epithelial injury; colon TNF, IFNγ, IL-17, IL-1β ↑ | Ct.Ar/Tt.Ar ↓ BV/TV, Conn.D, Tb.N, and Tb.Th. ↓ | serum tDPD and TNFα ↑ serum RANKL ↓ renal Ca2+ reabsorption and TRPV5 ↓ urinal Ca2+ excretion ↑ | [92] |
| CD4+CD45RB-scid/scid- transfer | Colitis; immune cell infiltration in colon | Osteopenia BMD and osteoblast number (OB.N) ↓ TNFα ↑ | BW ↓ SAA and WBC ↑ RBC, haemoglobin, and haematocrit ↓ serum PTH, ALP ↓ TRAP ↑ | [96] |
| CD4+CD45RB-Rag1−/− transfer | IBD | %CX3CR1+OC ↑ OC from IBD mice induce TNFα-producing CD4+T cells | BW ↓ | [97] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, K.; Arra, M.; Abu-Amer, Y. Mechanisms Underlying Bone Loss Associated with Gut Inflammation. Int. J. Mol. Sci. 2019, 20, 6323. https://doi.org/10.3390/ijms20246323
Ke K, Arra M, Abu-Amer Y. Mechanisms Underlying Bone Loss Associated with Gut Inflammation. International Journal of Molecular Sciences. 2019; 20(24):6323. https://doi.org/10.3390/ijms20246323
Chicago/Turabian StyleKe, Ke, Manoj Arra, and Yousef Abu-Amer. 2019. "Mechanisms Underlying Bone Loss Associated with Gut Inflammation" International Journal of Molecular Sciences 20, no. 24: 6323. https://doi.org/10.3390/ijms20246323




