Inhibitory Effects of N-[2-(4-acetyl-1-piperazinyl) phenyl]-2-(2-chlorophenoxy) acetamide on Osteoclast Differentiation In Vitro via the Downregulation of TRAF6
Abstract
:1. Introduction
2. Results
2.1. PPOA-N-Ac-2-Cl Inhibits RANKL-Induced Osteoclast Differentiation
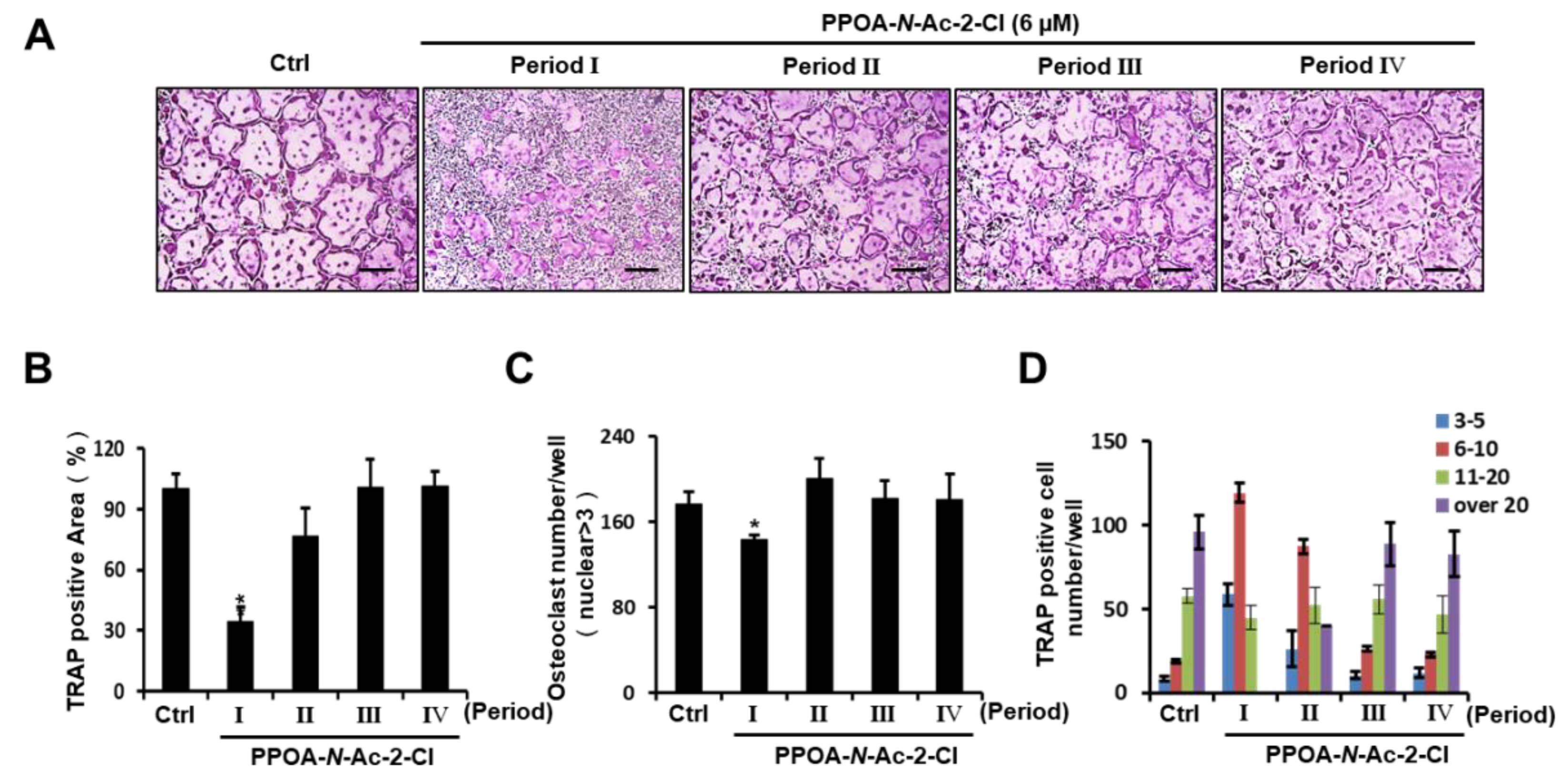
2.2. PPOA-N-Ac-2-Cl Inhibits Osteoclast Differentiation Only at the Early Stage
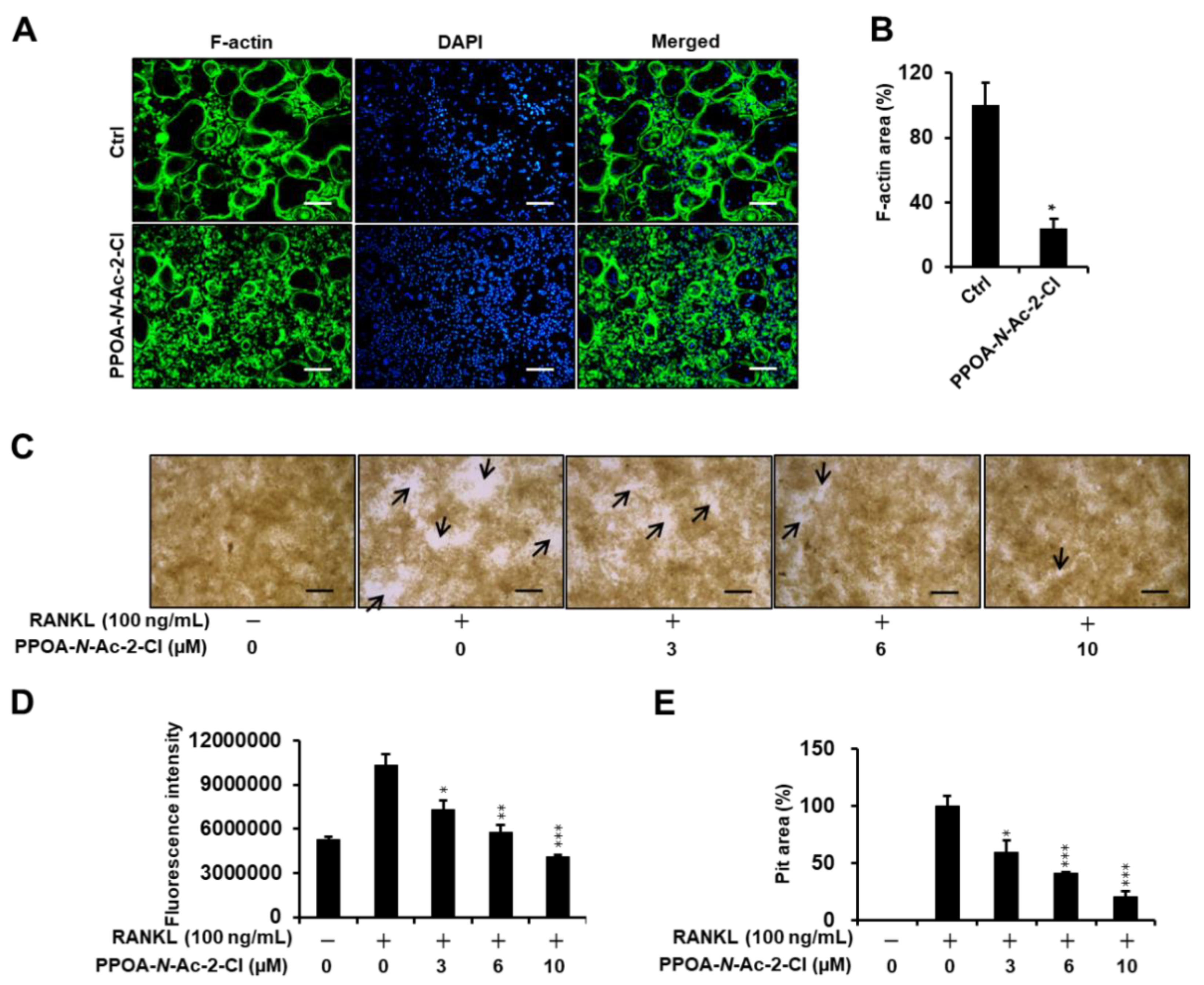
2.3. PPOA-N-Ac-2-Cl Inhibits Actin Ring Formation and Bone Resorption Activity
2.4. PPOA-N-Ac-2-Cl Downregulates Osteoclast Marker Genes Expression during Osteoclastogenesis
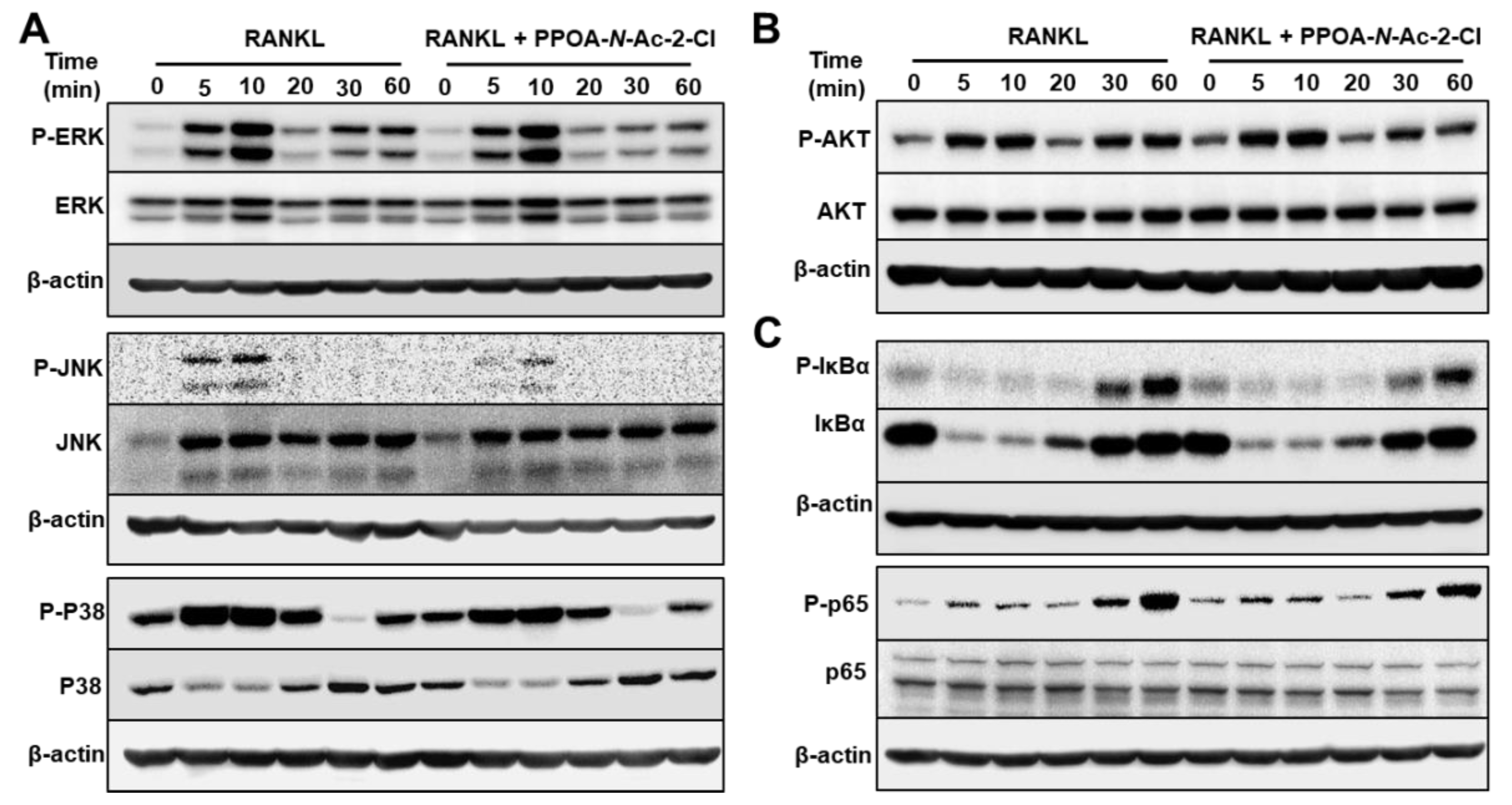
2.5. PPOA-N-Ac-2-Cl Suppresses the Phosphorylation of MAPK and NF-κB
2.6. PPOA-N-Ac-2-Cl Suppresses the Expression of TRAF6
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Bone Marrow-Derived Macrophage Isolation and Culture
4.3. Cell Viability Assay
4.4. F-actin Ring Formation Assay
4.5. Resorption Pit Assay
4.6. RNA Isolation and Quantitative Real-Time PCR
4.7. Western Blot Assays
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key triggers of osteoclast-related diseases and available strategies for targeted therapies: A review. Front. Med. 2017, 4, 234. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.B.; Lee, S.W.; Li, Y.J.; Kim, Y.A.; Han, S.Y.; Jhon, G.J.; Kim, H.H.; Lee, Z.H. Inhibition of osteoclast differentiation and bone resorption by a novel lysophosphatidylcholine derivative, scoh. Biochem. Pharmacol. 2004, 67, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Wachi, M.; Woo, J.T.; Kato, M.; Kasai, S.; Takahashi, F.; Lee, I.S.; Nagai, K. Fenton reaction is primarily involved in a mechanism of (-)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem. Biophys. Res. Commun. 2002, 292, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Matsubara, T.; Tsurukai, T.; Hata, K.; Nishimura, R.; Yoneda, T. Jnk/c-jun signaling mediates an anti-apoptotic effect of rankl in osteoclasts. J. Bone Miner. Res. 2008, 23, 907–914. [Google Scholar] [CrossRef]
- Hall, T.J.; Jeker, H.; Schaueblin, M. Wortmannin, a potent inhibitor of phosphatidylinositol 3-kinase, inhibits osteoclastic bone resorption in vitro. Calcif. Tissue Int. 1995, 56, 336–338. [Google Scholar] [CrossRef]
- Lee, S.E.; Woo, K.M.; Kim, S.Y.; Kim, H.M.; Kwack, K.; Lee, Z.H.; Kim, H.H. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone 2002, 30, 71–77. [Google Scholar] [CrossRef]
- Hu, B.; Sun, X.; Yang, Y.; Ying, Z.; Meng, J.; Zhou, C.; Jiang, G.; Li, S.; Wu, F.; Zhao, X.; et al. Tomatidine suppresses osteoclastogenesis and mitigates estrogen deficiency-induced bone mass loss by modulating traf6-mediated signaling. FASEB J. 2019, 33, 2574–2586. [Google Scholar] [CrossRef]
- Russell, R.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- McClung, M.; Harris, S.T.; Miller, P.D.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; Kendler, D.L.; Yuen, C.K.; Lewiecki, E.M. Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug holiday. Am. J. Med. 2013, 126, 13–20. [Google Scholar] [CrossRef]
- Solomon, D.H.; Rekedal, L.; Cadarette, S.M. Osteoporosis treatments and adverse events. Curr. Opin. Rheumatol. 2009, 21, 363–368. [Google Scholar] [CrossRef]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the american society for bone and mineral research. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.J.; McDonagh, T.E. Antitumor histone acetyl transferase inhibitors. US patent 20100168084, 1 July 2010. [Google Scholar]
- Miburn, M.; Millne, J. N-arylamethylbenzamide sirtuin modulators, and their therapeutic use. WO patent 2006094246, 8 September 2006. [Google Scholar]
- Lan, M.; Bai, Y. N-(2-(4-methylpiperazine-1-yl)-5-formylphenyl)amide compounds and medical application. CN patent 103058959, 24 April 2013. [Google Scholar]
- Acharya, P.; Dogo-Isonagie, C.; LaLonde, J.M.; Lam, S.N.; Leslie, G.J.; Louder, M.K.; Frye, L.L.; Debnath, A.K.; Greenwood, J.R.; Luongo, T.S.; et al. Structure-based identification and neutralization mechanism of tyrosine sulfate mimetics that inhibit hiv-1 entry. ACS Chem. Biol. 2011, 6, 1069–1077. [Google Scholar] [CrossRef]
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-based treatment strategies for osteoporosis: A literature review. Int J. Mol. Sci. 2019, 20, 2557. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Yang, Z.; Zheng, C.; Jing, J.; Chen, Y.; Ye, X.; Lian, X.; Qiu, W.; Yang, F.; et al. Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of nf-kappab ligand-induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+-nuclear factor of activated t-cells cytoplasmic 1 signaling pathways. J. Bone Miner. Res. 2012, 27, 1298–1308. [Google Scholar]
- Kim, J.H.; Kim, N. Regulation of nfatc1 in osteoclast differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Kim, G.K.; Maurizio, P.L.; Molnar, E.E.; Choi, Y. Traf6 autoubiquitination-independent activation of the nfkappab and mapk pathways in response to il-1 and rankl. PLoS ONE 2008, 3, e4064. [Google Scholar] [CrossRef]
- Kong, X.; Yang, Y.; Wu, W.; Wan, H.; Li, X.; Zhong, M.; Su, X.; Jia, S.; Lin, N. Triterpenoid saponin w3 from anemone flaccida suppresses osteoclast differentiation through inhibiting activation of mapks and nf-kappab pathways. Int. J. Biol. Sci. 2015, 11, 1204–1214. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Li, Z.; Ma, Y.; Zhang, L.; Zheng, C.; Qiu, W.; Wu, X.; Wang, X.; Li, H.; et al. Maslinic acid suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by regulating rankl-mediated nf-kappab and mapk signaling pathways. J. Bone Miner. Res. 2011, 26, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Arron, J.R. Traf6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. Bioessays 2003, 25, 1096–1105. [Google Scholar] [CrossRef]
- Dou, Y.; Tian, X.; Zhang, J.; Wang, Z.; Chen, G. Roles of traf6 in central nervous system. Curr Neuropharmacol 2018, 16, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Berthiaume, J.M.; Willis, M.S. Tumor necrosis factor receptor-associated factor 6 as a nuclear factor kappa b-modulating therapeutic target in cardiovascular diseases: At the heart of it all. Transl Res. 2018, 195, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, B.; Liang, C.; Li, Y.; Song, Y.H. Cytokine signaling in skeletal muscle wasting. Trends Endocrinol. Metab. 2016, 27, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, T.; Yan, X.; Liu, Z.; Yan, Y.; Yang, K.; Qi, J.; Zhou, H.; Qian, N.; Zhou, Q.; et al. A novel rhein derivative modulates bone formation and resorption and ameliorates estrogen-dependent bone loss. J. Bone Miner. Res. 2019, 34, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Hsia, Y.J.; Shih, K.C.; Chou, T.C. Fucoidan prevents rankl-stimulated osteoclastogenesis and lps-induced inflammatory bone loss via regulation of Akt/GSK3beta/PTEN/NFATc1 signaling pathway and calcineurin activity. Mar. Drugs 2019, 17, 345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, L.; Xu, K.; Jin, H.; Chen, K.; Wang, Z.; Liu, Q.; Cao, Z.; Kenny, J.; Liu, Y.; et al. Madecassoside inhibits estrogen deficiency-induced osteoporosis by suppressing rankl-induced osteoclastogenesis. J. Cell. Mol. Med. 2019, 23, 380–394. [Google Scholar] [CrossRef]
- Nagaoka, M.; Maeda, T.; Moriwaki, S.; Nomura, A.; Kato, Y. Petunidin, a b-ring 5′-O-methylated derivative of delphinidin, stimulates osteoblastogenesis and reduces srankl-induced bone loss. Int. J. Mol. Sci. 2019, 20, 2795. [Google Scholar] [CrossRef]
- Qin, S.; Ang, E.; Dai, L.; Yang, X.; Ye, D.; Chen, H.; Zhou, L.; Yang, M.; Teguh, D.; Tan, R.; et al. Natural germacrane sesquiterpenes inhibit osteoclast formation, bone resorption, rankl-induced nf-kappab activation, and ikappabalpha degradation. Int J. Mol. Sci. 2015, 16, 26599–26607. [Google Scholar] [CrossRef]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Sakurai, H.; Chiba, H.; Miyoshi, H.; Sugita, T.; Toriumi, W. Ikappab kinases phosphorylate nf-kappab p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 1999, 274, 30353–30356. [Google Scholar] [CrossRef]
- Takayanagi, H. The role of nfat in osteoclast formation. Ann. NY Acad Sci. 2007, 1116, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Gelb, B.D.; Shi, G.P.; Chapman, H.A.; Desnick, R.J. Pycnodysostosis, a lysosomal disease caused by cathepsin k deficiency. Science 1996, 273, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.H.; Ha Kim, J.; Choi, Y.; Kim, N. Nfatc1 induces osteoclast fusion via up-regulation of atp6v0d2 and the dendritic cell-specific transmembrane protein (dc-stamp). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Cackowski, F.C.; Anderson, J.L.; Patrene, K.D.; Choksi, R.J.; Shapiro, S.D.; Windle, J.J.; Blair, H.C.; Roodman, G.D. Osteoclasts are important for bone angiogenesis. Blood 2010, 115, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kim, K.J.; Kang, H.J.; Son, Y.J.; Choi, S.W.; Lee, M.J. The dual role of oat bran water extract in bone homeostasis through the regulation of osteoclastogenesis and osteoblast differentiation. Molecules 2018, 23, 3119. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Chen, Z.; Lee, J.; Lee, S.W.; Min, S.H.; Kim, N.D.; Lee, T.H. Inhibitory effects of 2n1hia (2-(3-(2-fluoro-4-methoxyphenyl)-6-oxo-1(6H)-pyridazinyl)-N-1H-indol-5-ylacetamide) on osteoclast differentiation via suppressing cathepsin k expression. Molecules 2018, 23, 3139. [Google Scholar] [CrossRef]
- Yun, I.G.; Ahn, S.H.; Yoon, W.J.; Kim, C.S.; Lim, Y.K.; Kook, J.K.; Jung, S.; Choi, C.H.; Lee, T.H. Litsea japonica leaf extract suppresses proinflammatory cytokine production in periodontal ligament fibroblasts stimulated with oral pathogenic bacteria or interleukin-1beta. Int. J. Mol. Sci. 2018, 19, 2494. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, J.K.; Kim, N.D.; Kim, S.H.; Lee, S.; Jung, S.; Chay, K.O.; Lee, T.H. Dpie [2-(1,2-diphenyl-1h-indol-3-yl)ethanamine] augments pro-inflammatory cytokine production in il-1beta-stimulated primary human oral cells. Int J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Cho, E.; Lee, J.K.; Lee, J.Y.; Chen, Z.; Ahn, S.H.; Kim, N.D.; Kook, M.S.; Min, S.H.; Park, B.J.; Lee, T.H. Bcpa {N,N′-1,4-butanediylbis[3-(2-chlorophenyl)acrylamide]} inhibits osteoclast differentiation through increased retention of peptidyl-prolyl cis-trans isomerase never in mitosis a-interacting 1. Int. J. Mol. Sci. 2018, 19, 3436. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Cho, E.; Lee, J.; Lee, S.; Lee, T.-H. Inhibitory Effects of N-[2-(4-acetyl-1-piperazinyl) phenyl]-2-(2-chlorophenoxy) acetamide on Osteoclast Differentiation In Vitro via the Downregulation of TRAF6. Int. J. Mol. Sci. 2019, 20, 5196. https://doi.org/10.3390/ijms20205196
Chen Z, Cho E, Lee J, Lee S, Lee T-H. Inhibitory Effects of N-[2-(4-acetyl-1-piperazinyl) phenyl]-2-(2-chlorophenoxy) acetamide on Osteoclast Differentiation In Vitro via the Downregulation of TRAF6. International Journal of Molecular Sciences. 2019; 20(20):5196. https://doi.org/10.3390/ijms20205196
Chicago/Turabian StyleChen, Zhihao, Eunjin Cho, Jinkyung Lee, Sunwoo Lee, and Tae-Hoon Lee. 2019. "Inhibitory Effects of N-[2-(4-acetyl-1-piperazinyl) phenyl]-2-(2-chlorophenoxy) acetamide on Osteoclast Differentiation In Vitro via the Downregulation of TRAF6" International Journal of Molecular Sciences 20, no. 20: 5196. https://doi.org/10.3390/ijms20205196




