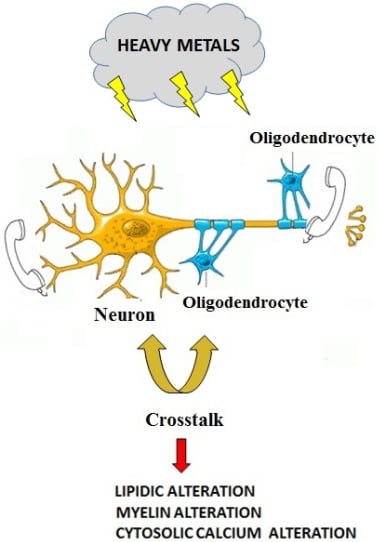
Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction
Abstract
:1. Introduction
2. Results
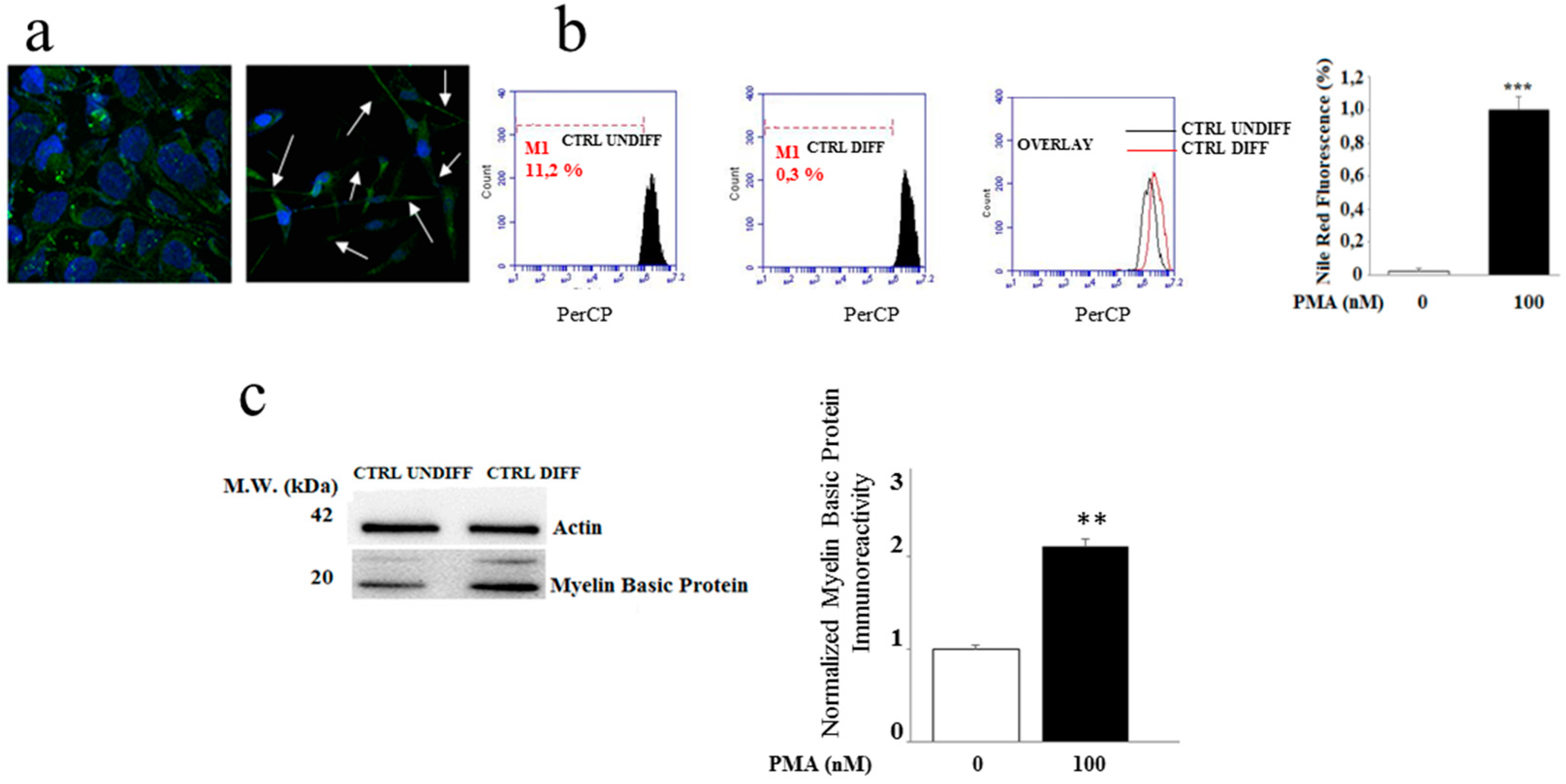
2.1. Differentiation of the MO3.13 Cell Line
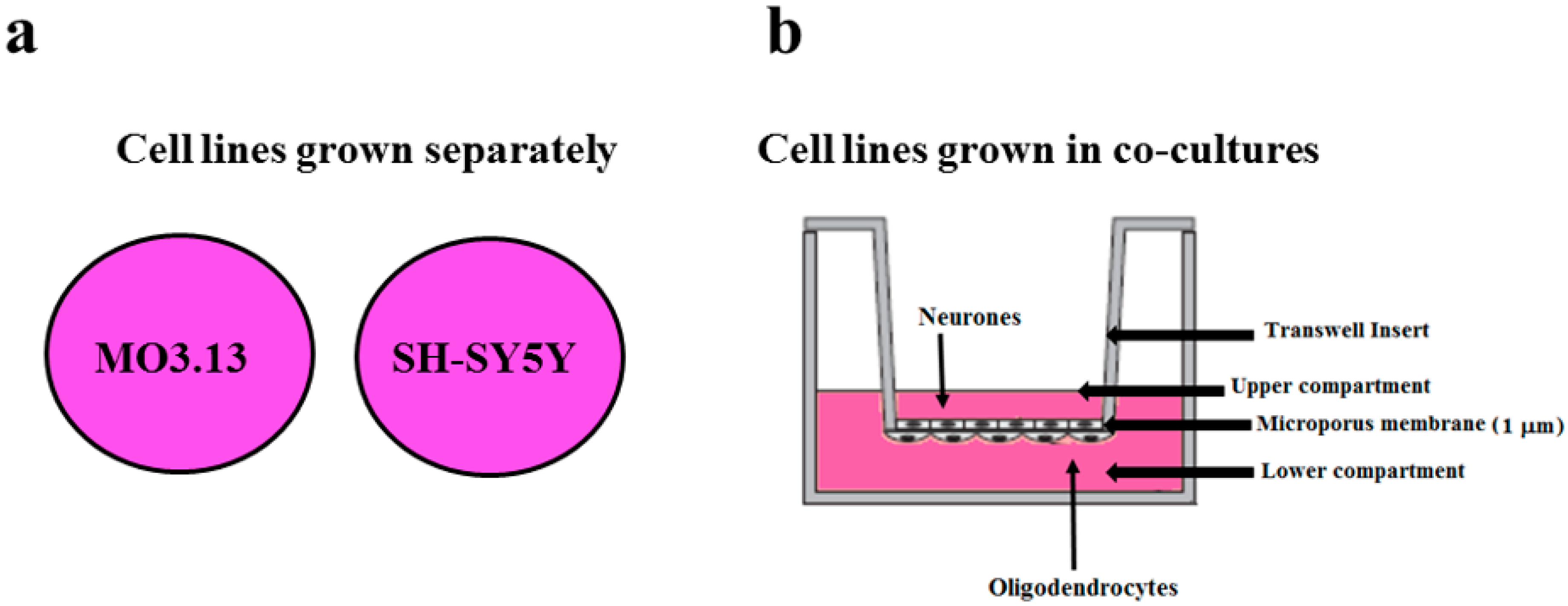
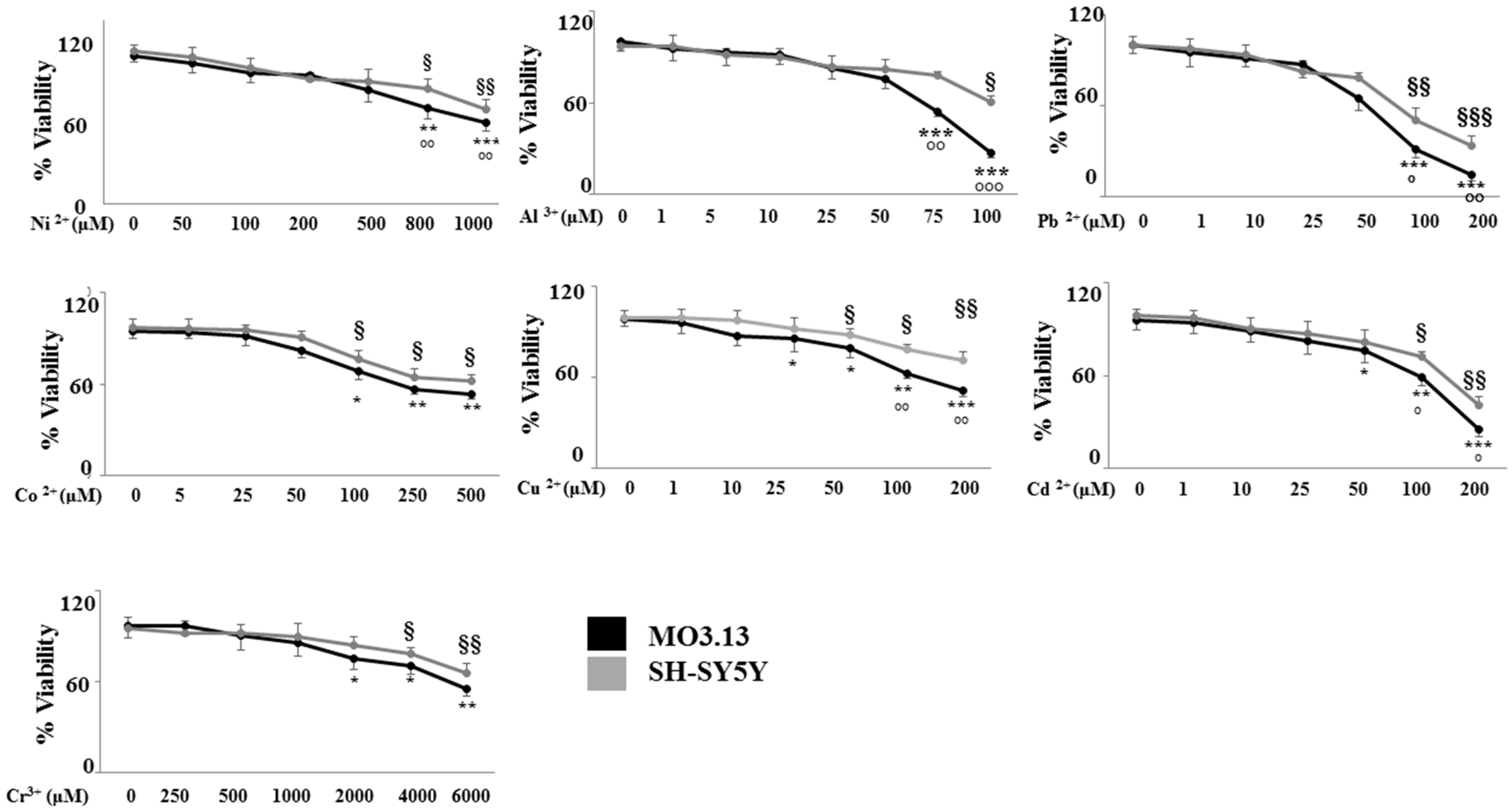
2.2. The Effect of Heavy Metals on the Viability of Oligodendrocytes and Neurons Grown Either Separately or in Co-Culture
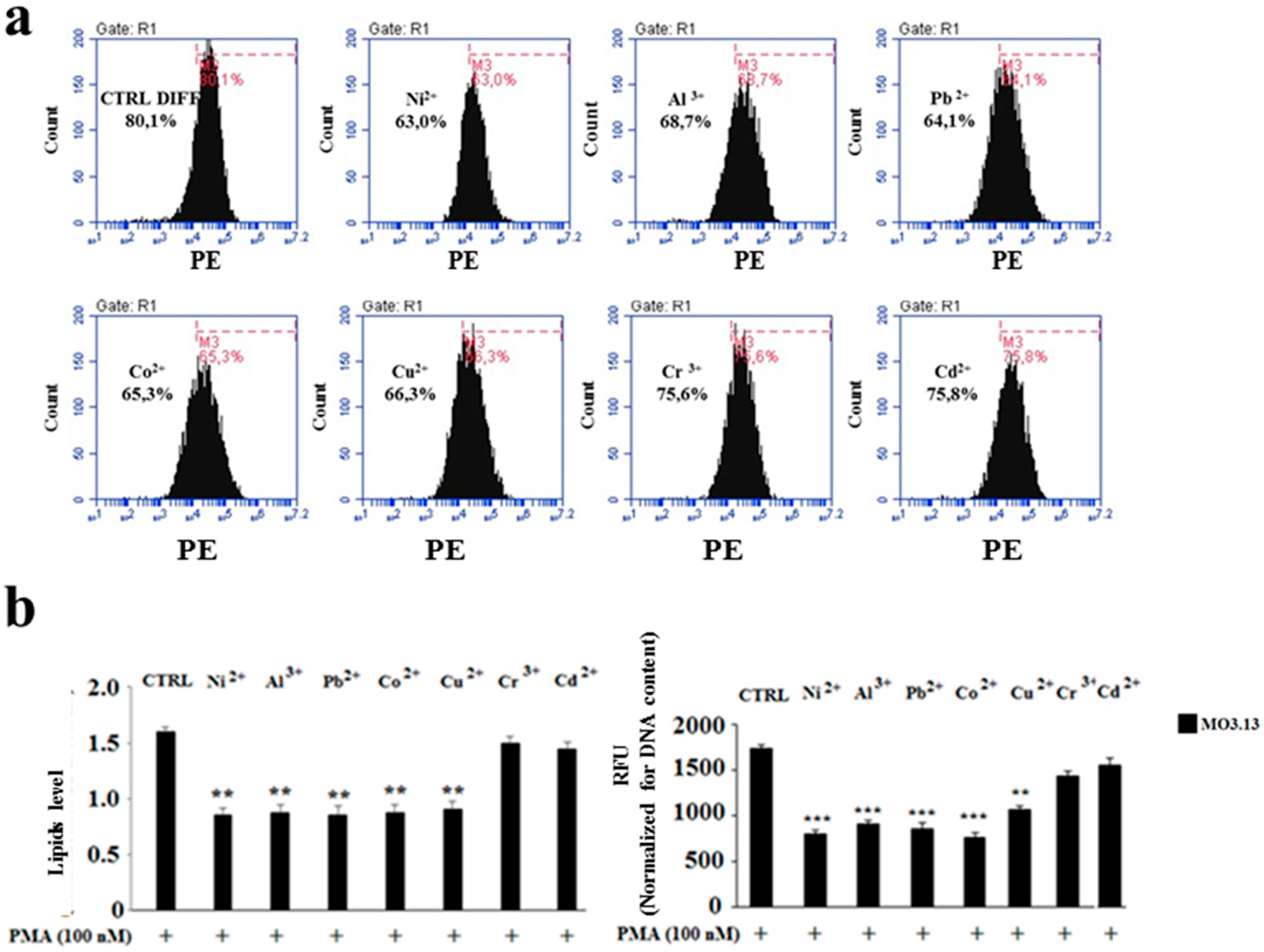
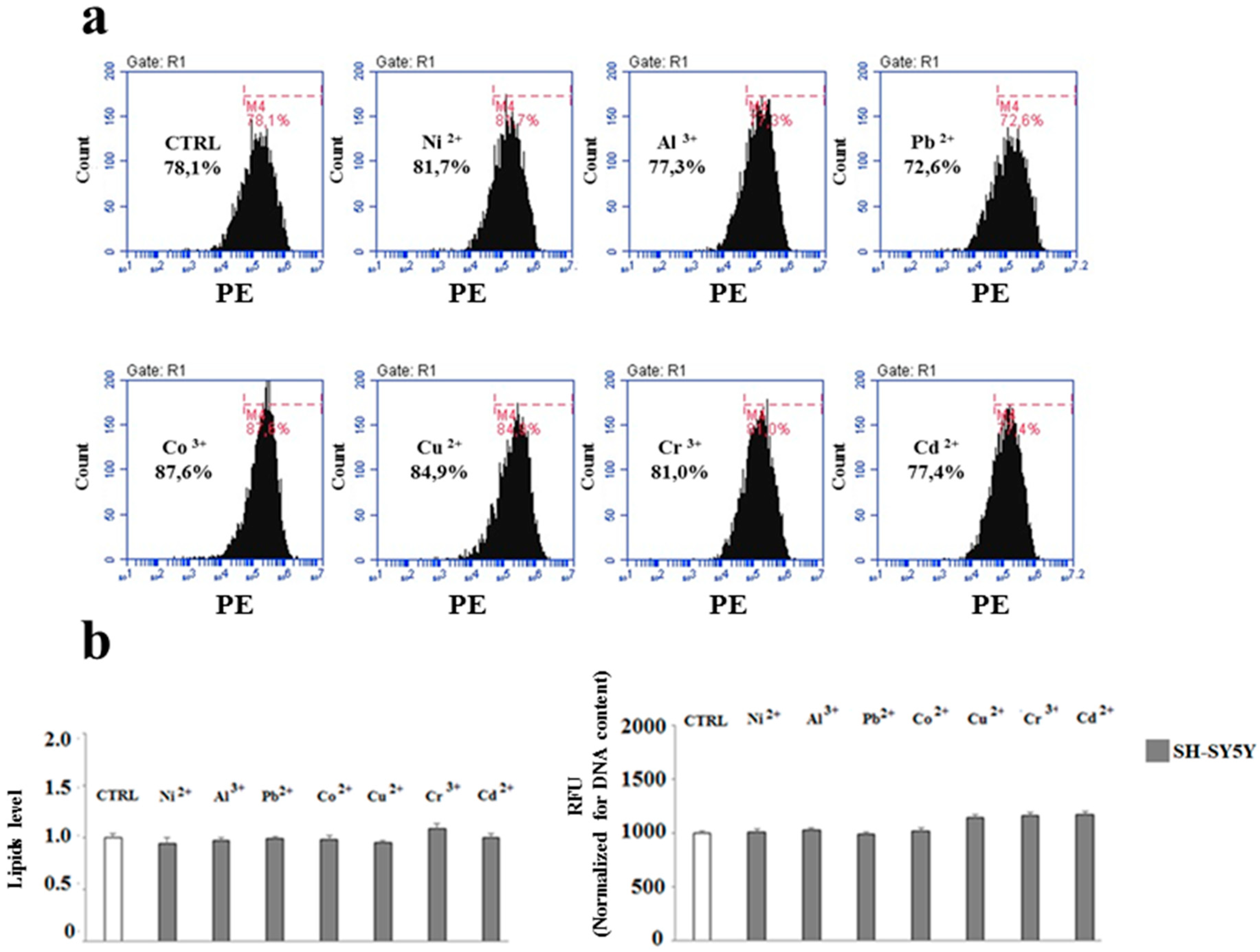
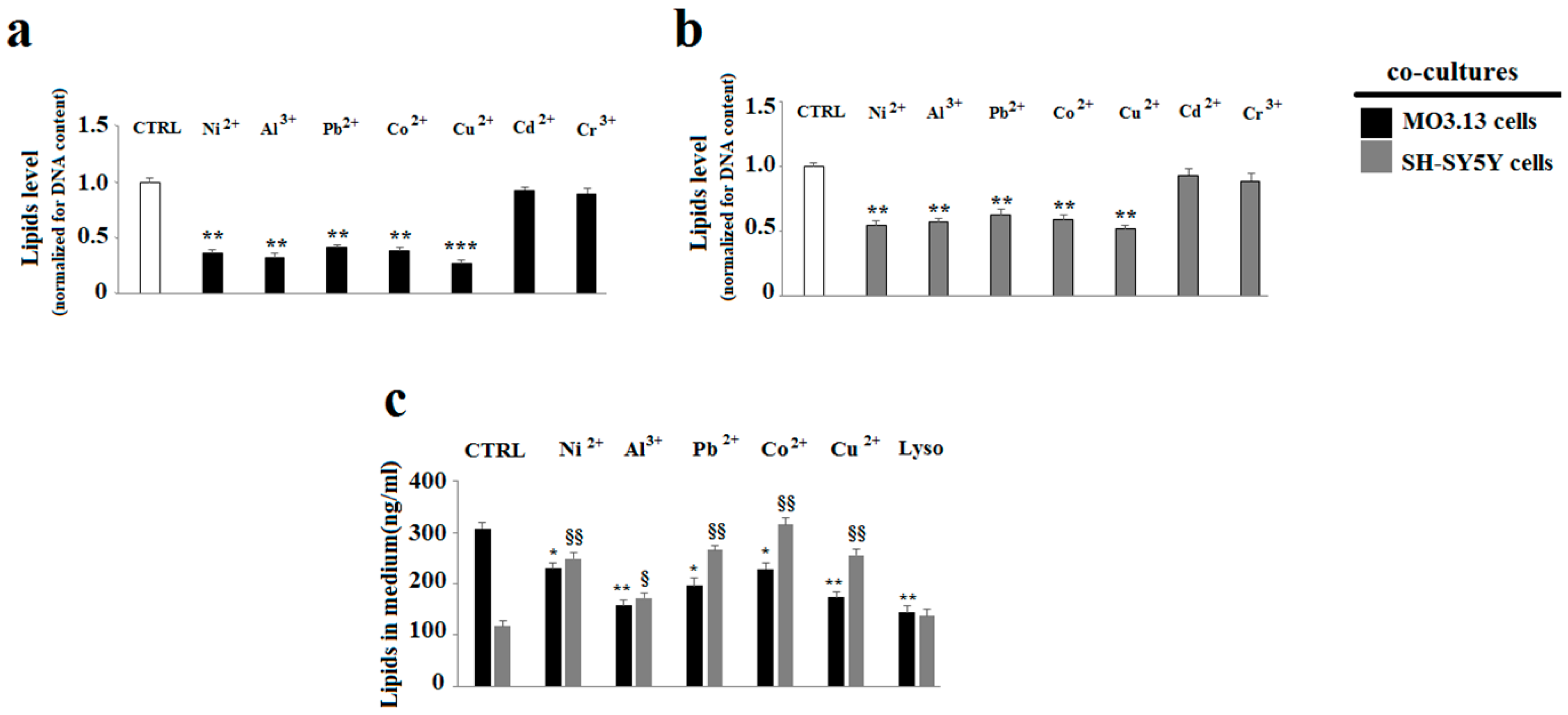
2.3. The Effect of Heavy Metals on the Lipid Component in Cells Grown Alone or in Co-Culture
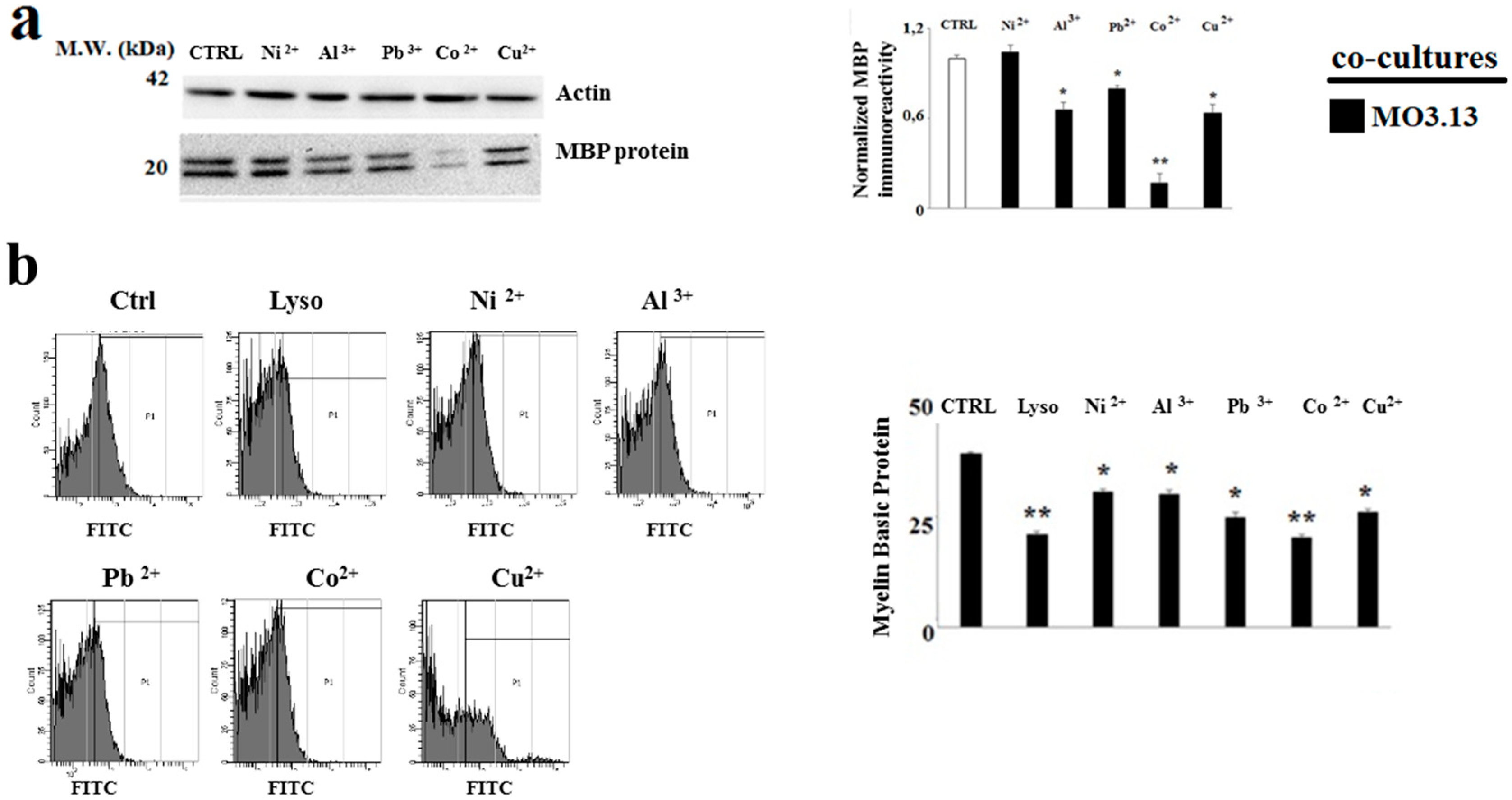
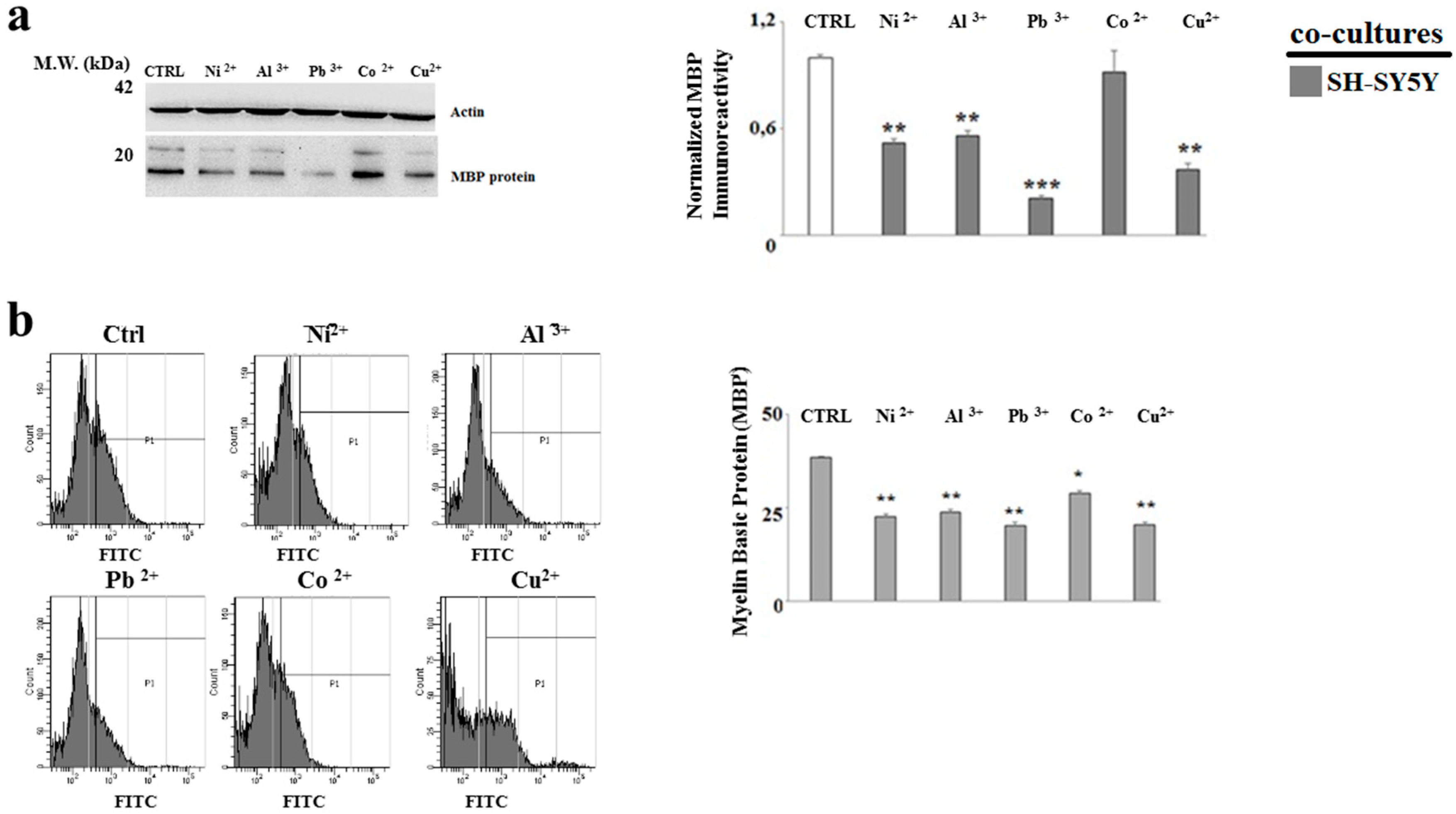
2.4. The Treatment with Heavy Metals Reduces the MBP Expression in Oligodendrocytes and Neurons Grown in Co-Culture
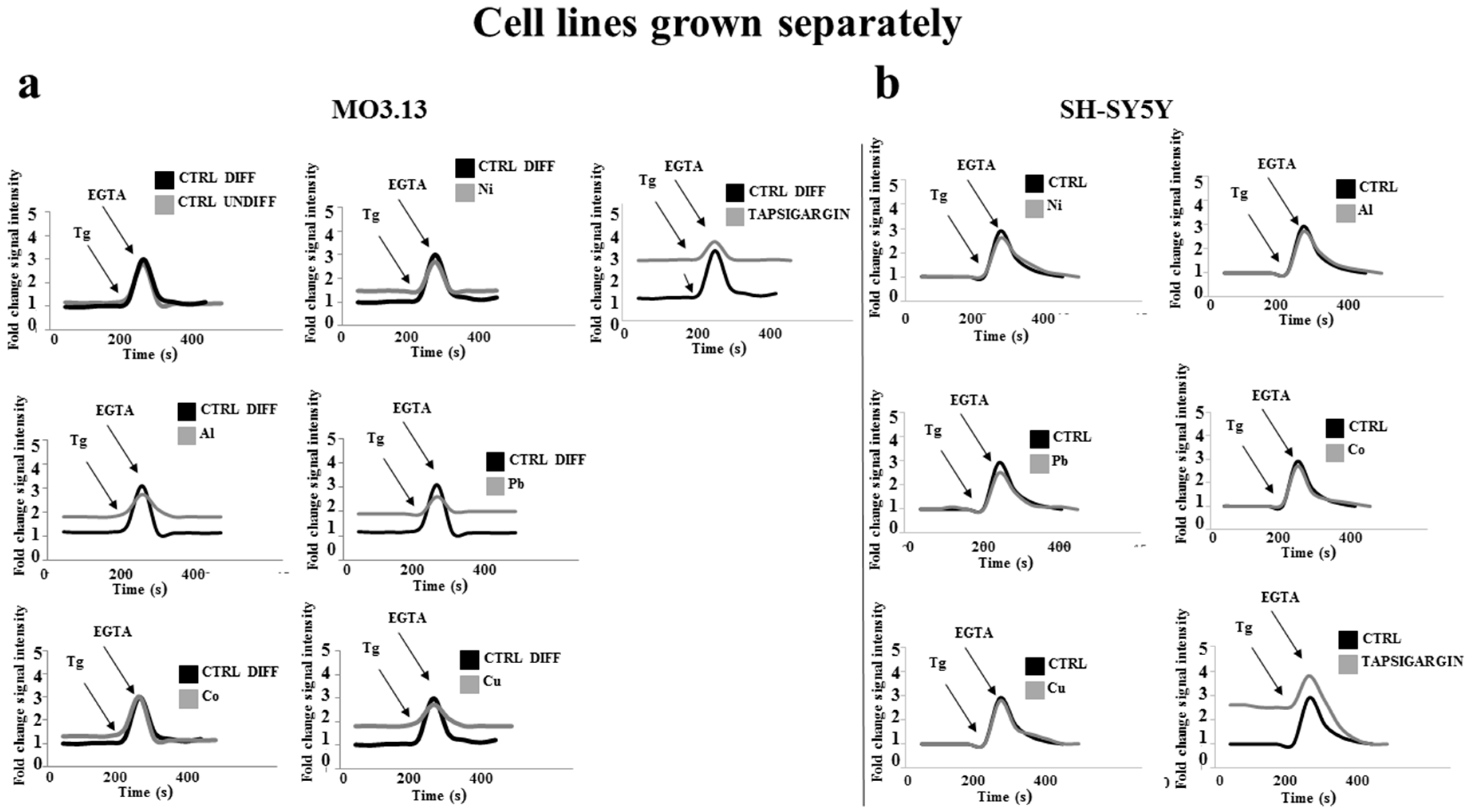
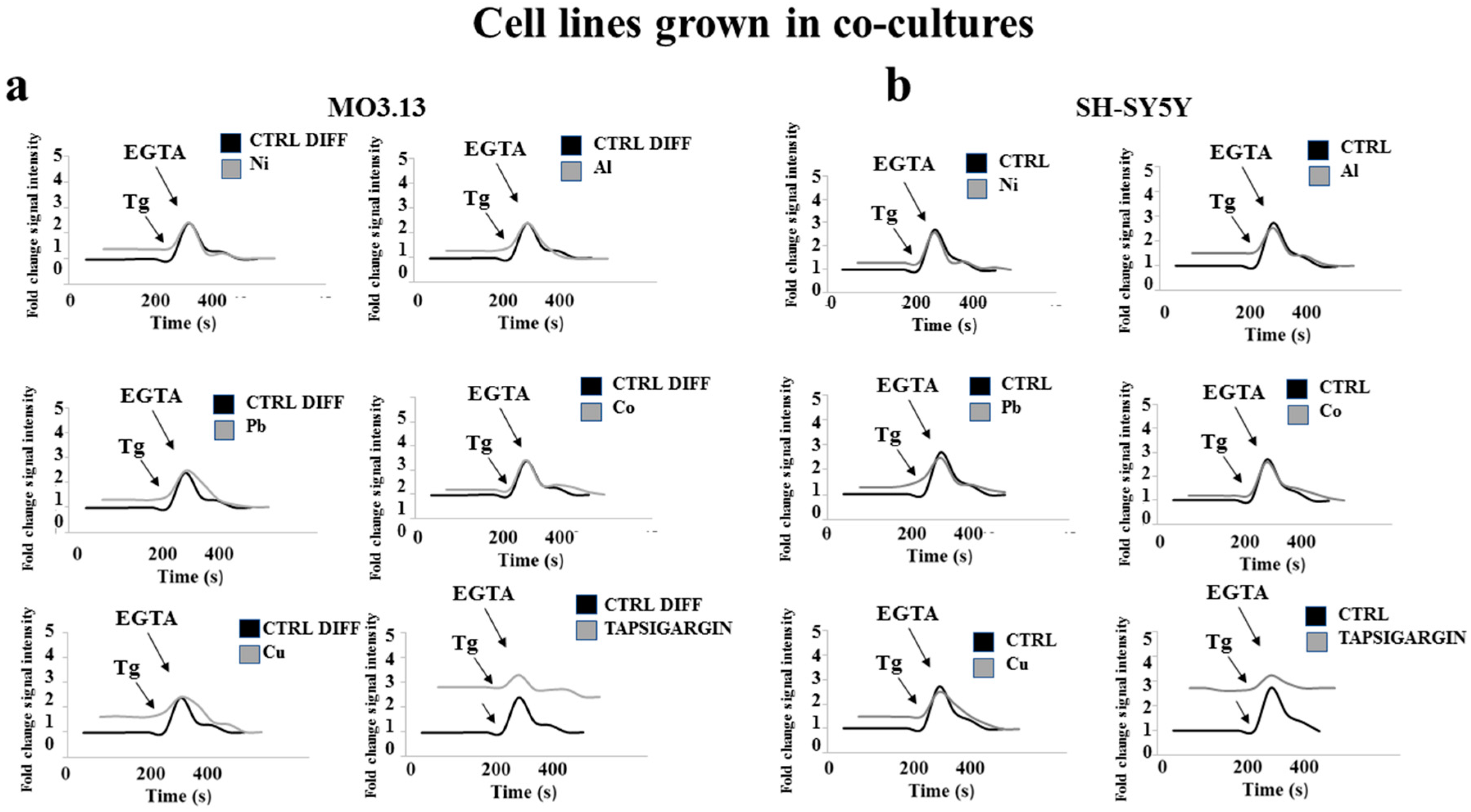
2.5. Heavy Metal-Dependent Dysregulation of Cytosolic Calcium
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Cultures
4.3. Cell Differentiation
4.4. Lipid Extraction: Flow Cytometric and Spectrometric Quantification
4.5. Phenotypic and Metabolic Characteristics of Mature Oligodendrocytes Measurement by Immunofluorescence
4.6. Cell Growth Assays
4.7. Intracellular Calcium Measurements
4.8. Measurement of the Expression of the Myelin Basic Protein (MBP) through Flow Cytometric Investigation
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reeder, R.J.; Schoonen, M.A.A.; Lanzirotti, A. Metal Speciation and its Role in Bioaccessibility and Bioavailability. Rev. Miner. Geochem. 2006, 64, 59–113. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Erust, C.; Ozdemiroglu, S.; Fonti, V.; Beolchini, F. Metal removal and stabilization by chemical and biotechnological processes. J. Clean. Prod. 2015, 86, 24–36. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci. World J. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O. A New Strategy for Heavy Metal Polluted Environments. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Maretta, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; Bosco, F.; Mollace, R.; Scarano, F.; Palma, E.; Scicchitano, M.; et al. Ethanol-induced cardiomyocyte toxicity implicit autophagy and NFkBtranscription factor. Pharmacol. Res. 2018, 133, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Pokorska-Niewiada, K.; Rajkowska-Myśliwiec, M.; Protasowicki, M. Acute Lethal Toxicity of Heavy Metals to the Seeds of Plants of High Importance to Humans. Bull. Environ. Contam. Toxicol. 2018, 101, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Hyder, O.; Chung, M.; Cosgrove, D.; Herman, J.M.; Li, Z.; Firoozmand, A.; Gurakar, A.; Koteish, A.; Pawlik, T.M. Cadmium exposure and liver disease among US adults. J. Gastrointest. Surg. 2013, 17, 1265–1273. [Google Scholar] [CrossRef]
- Mudgal, V.; Madaan, N.; Mudgal, A.; Singh, R.; Mishra, S. Effect of toxic metals on human health. Open Nutraceut. J. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef] [PubMed]
- Wegener, A.; Deboux, C.; Bachelin, C.; Frah, M.; Kerninon, C.; Seilhean, D.; Weider, M.; Wegner, M.; Nait-Oumesmar, B. Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain 2015, 138, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, H.; Martin, E.; Hassani, H.; Clavairoly, A.; Maire, C.L.; Viadieu, A.; Kerninon, C.; Delmasure, A.; Frah, M.; Weber, M.; et al. Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J. Neurosci. 2013, 33, 9752–9768. [Google Scholar] [CrossRef] [PubMed]
- Bachelin, C.; Zujovic, V.; Buchet, D.; Mallet, J.; Baron-Van Evercooren, A. Ectopic expression of polysialylated neural cell adhesion molecule in adult macaque Schwann cells promotes their migration and remyelination potential in the central nervous system. Brain 2010, 133, 406–420. [Google Scholar] [CrossRef]
- Czopka, T.; French-Constant, C.F.; Lyons, D.A. Individual Oligodendrocytes Have Only a Few Hours in which to Generate New Myelin Sheaths In Vivo. Dev. Cell 2013, 25, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Patzig, J.; Jahn, O.; Tenzer, S.; Wichert, S.P.; de Monasterio-Schrader, P.; Rosfa, S.; Kuharev, J.; Yan, K.; Bormuth, I.; Bremer, J. Quantitative and integrative proteome analysis of peripheral nerve myelin identifies novel myelin proteins and candidate neuropathy loci. J. Neurosci. 2011, 31, 16369–16386. [Google Scholar] [CrossRef]
- Clemente, D.; Ortega, M.C.; Melero-Jerez, C.; De Castro, F. The effect of glia-glia interactions onoligodendrocyte precursor cell biology during development and in demyelinating diseases. Front. Cell. Neurosci. 2013, 7, 268. [Google Scholar] [CrossRef]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2016, 8, a020453. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Tomkova, S.; Misuth, M.; Lenkavska, L.; Miskovsky, P.; Huntosova, V. In vitro identification of mitochondrial oxidative stress production by time-resolved fluorescence imaging of glioma cells. BBA Mol. Cell Res. 2018, 1865, 616–628. [Google Scholar] [CrossRef]
- Mitew, S.; Gobius, O.; Fenlon, L.R.; McDougall, S.J.; Hawkes, D.; Xing, Y.L.; Bujalka, H.; Gundlach, A.L.; Richards, L.J.; Kilpatrick, T.J.; et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 2018, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Bercury, K.K.; Macklin, W.B. Dynamics and mechanisms of CNS myelination. Dev. Cell 2015, 32, 447–458. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Carresi, C.; Scarano, F.; Bosco, F.; Nucera, S.; Ruga, S.; Zito, M.C.; et al. The “frail” brain blood barrier in neurodegenerative diseases: Role of early disruption of endothelial cell-to-cell connections. Int. J. Mol. Sci. 2018, 19, 2693. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. The Role of Endothelial Dysfunction in Peripheral Blood Nerve Barrier: Molecular Mechanisms and Pathophysiological Implications. Int. J. Mol. Sci. 2019, 20, 3022. [Google Scholar] [CrossRef] [PubMed]
- Buntinx, M.; Vanderlocht, J.; Hellings, N.; Vandenabeele, F.; Lambrichts, I.; Raus, J.; Ameloot, M.; Stinissen, P.; Steels, P. Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury:morphologyand oligodendrocyte-specific gene expression. J. Neurocytol. 2003, 32, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Accetta, R.; Damiano, S.; Morano, A.; Mondola, P.; Paternò, R.; Avvedimento, E.V.; Santillo, M. Reactive Oxygen Species Derived from NOX3 and NOX5 Drive Differentiation of Human Oligodendrocytes. Front. Cell. Neurosci. 2016, 10, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, Y.T.; Kwok-Wai Lau, W.K.; Yu, M.S.; Lai, C.S.W.; Yeung, S.C.; So, K.F.; Chang, R.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. NeuroToxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Chen, F.; Yu, Y.; Haigh, S.; Johnson, J.; Lucas, R.; Stepp, D.W.; Fulton, D.J.R. Regulation of NADPH Oxidase 5 by Protein Kinase C Isoforms. PLoS ONE 2014, 9, e88405. [Google Scholar] [CrossRef]
- McLaurin, J.; Trudel, G.C.; Shaw, I.T.; Antel, J.P.; Cashman, N.R. A human glial hybrid cell line differentially expressing genes subserving oligodendrocyte and astrocyte phenotype. J. Neurobiol. 1995, 26, 283–293. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J. Oxidative stress and its impact on neurons and glia in multiplesclerosis lesions. Biochim. Biophys. Acta 2016, 1862, 506–510. [Google Scholar] [CrossRef]
- Lapato, A.S.; Tiwari-Woodruff, S.K. Connexins and pannexins: At the junction of neuro-glial homeostasis & disease. J. Neurosci. Res. 2018, 96, 31–44. [Google Scholar] [PubMed]
- Rai, N.K.; Ashok, A.; Rai, A.; Tripathi, S.; Nagar, G.K.; Mitra, K.; Bandyopadhyay, S. Exposure to As, Cd and Pb-mixture impairs myelin and axondevelopment in rat brain, optic nerve and retina. Toxicol. Appl. Pharmacol. 2013, 273, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Lee, P.R.; Fields, R.D. Control of local protein synthesis and initial events in myelination by action potentials. Science 2011, 333, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Arellano, R.O.; Sánchez-Gómez, M.V.; Alberdi, E.; Canedo-Antelo, M.; Chara, J.C.; Palomino, A.; Pérez-Samartín, A.; Matute, C. Axon-to-Glia Interaction Regulates GABAA Receptor Expression in Oligodendrocytes. Mol. Pharmacol. 2016, 89, 63–74. [Google Scholar] [CrossRef]
- Peferoen, L.; Kipp, M.; van der Valk, P.; van Noort, J.M.; Amor, S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 2014, 141, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, S.; Sun, Q.; Li, Z.; Xu, F.; Hou, C.; Harada, T.; Chu, M.; Xu, K.; Feng, X.; et al. Inhibition of TROY Promotes OPC Differentiation and Increases Therapeutic Efficacy of OPC Graft for Spinal Cord Injury. Stem Cells Dev. 2014, 23, 2104–2118. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Stys, P.K. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009, 8, 280–291. [Google Scholar] [CrossRef]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef]
- Larocca, J.N.; Rodriguez-Gabin, A.G. Myelin Biogenesis: Vesicle Transport in Oligodendrocytes. Neurochem. Res. 2002, 27, 1313–1329. [Google Scholar] [CrossRef]
- Yaffe, Y.; Hugger, I.; Yassaf, I.N.; Shepshelovitch, J.; Sklan, E.H.; Elkabetz, Y.; Yeheskel, A.; Pasmanik-Chor, M.; Benzing5, C.; Macmillan, A.; et al. The myelin proteolipid plasmolipin forms oligomers and induces liquid-ordered membranes in the Golgi complex. J. Cell Sci. 2015, 128, 2293–2302. [Google Scholar] [CrossRef] [Green Version]
- Accardi, A. Mechanisms of Ion and lipid transport by TMEM16 scramblases. Biophys. J. 2016, 110, 174. [Google Scholar] [CrossRef]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef]
- Yu-Feng, S.; Chao, H.; Xi, S.; Ya-Xiong, P.; Xu, L.; Zhi, L. Endoplasmic reticulum stress and dysregulation of calcium homeostasis mediate Cu-induced alteration in hepatic lipid metabolism of javelin goby Synechogobius hasta. Aquat. Toxicol. 2016, 175, 20–29. [Google Scholar]
- McNulty, T.J.; Taylor, C.W. Extracellular heavy-metal ions stimulate Ca2+ mobilization in hepatocytes. Biochem. J. 1999, 339, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids as a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Issa, Y.; Brunton, P.; Waters, C.M.; Watts, D.C. Citotoxicity of metal ions to human oligodendroglial cells and human fibroblast assessed by mitochondrial dehydrogenase activity. Dent. Mater. 2008, 24, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bulotta, S.; Verderio, C.; Benfante, R.; Borgese, N. Selective activation of the transcription factor ATF6 mediates endoplasmic reticulum proliferation triggered by a membrane protein. Proc. Natl. Acad. Sci. USA 2011, 108, 7832–7837. [Google Scholar] [CrossRef] [Green Version]

| METAL | USED DOSE |
|---|---|
| Nickel (Ni2+) | 200 µM |
| Aluminum (Al3+) | 25 µM |
| Lead (Pb2+) | 25 µM |
| Cobalt (Co2+) | 25 µM |
| Copper (Cu2+) | 10 µM |
| Cadmium (Cd2+) | 25 µM |
| Chrome (Cr 3+) | 1000 µM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Scicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. https://doi.org/10.3390/ijms20184554
Maiuolo J, Macrì R, Bava I, Gliozzi M, Musolino V, Nucera S, Carresi C, Scicchitano M, Bosco F, Scarano F, et al. Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction. International Journal of Molecular Sciences. 2019; 20(18):4554. https://doi.org/10.3390/ijms20184554
Chicago/Turabian StyleMaiuolo, Jessica, Roberta Macrì, Irene Bava, Micaela Gliozzi, Vincenzo Musolino, Saverio Nucera, Cristina Carresi, Miriam Scicchitano, Francesca Bosco, Federica Scarano, and et al. 2019. "Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction" International Journal of Molecular Sciences 20, no. 18: 4554. https://doi.org/10.3390/ijms20184554
APA StyleMaiuolo, J., Macrì, R., Bava, I., Gliozzi, M., Musolino, V., Nucera, S., Carresi, C., Scicchitano, M., Bosco, F., Scarano, F., Palma, E., Gratteri, S., & Mollace, V. (2019). Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction. International Journal of Molecular Sciences, 20(18), 4554. https://doi.org/10.3390/ijms20184554