Building a Bridge between Chemotherapy and Immunotherapy in Malignant Pleural Mesothelioma: Investigating the Effect of Chemotherapy on Immune Checkpoint Expression
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of Chemotherapeutics Is Cell Line Dependent
2.2. Chemotherapeutics Have A Variable Influence On ICP Expression
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Peripheral Blood Mononuclear Cells (PBMC)
4.3. Sulforhodamine B (SRB) Assay
4.4. Allogeneic Co-cultures
4.5. Flow Cytometry
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ab | antibody |
| APC | allophycocyanin |
| TLA | three letter acronym |
| LD | linear dichroism |
| DC | dendritic cell |
| DMSO | dimethyl sulfoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| EPP | extrapleural pneumonectomy |
| FCM | flow cytometry |
| IC | inhibitory concentration |
| ICD | immunogenic cell death |
| ICP | immune checkpoint |
| ICPB | immune checkpoint blockade |
| LAG-3 | lymphocyte activation gene-3 |
| MFI | mean fluorescence intensity |
| MPM | malignant pleural mesothelioma |
| NSCLC | non-small cell lung cancer |
| OD | optic density |
| PBMC | peripheral blood mononuclear cells |
| PBS | phosphate-buffered saline |
| PD-1 | programmed death-1 |
| PD-L1 | programmed death ligand-1 |
| PD-L2 | programmed death ligand-2 |
| PE | phycoerythrin |
| SRB | sulphorodamine B |
| TIM-3 | T-cell immunoglobuline mucine-3 |
References
- Neumann, V.; Loseke, S.; Tannapfel, A. Mesothelioma and analysis of tissue fiber content. Recent Results Cancer Res. 2011, 189, 79–95. [Google Scholar] [PubMed]
- Bianchi, C.; Bianchi, T. Malignant mesothelioma: Global incidence and relationship with asbestos. Ind. Health 2007, 45, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Delgermaa, V.; Takahashi, K.; Park, E.K.; Le, G.V.; Hara, T.; Sorahan, T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull. World Health Organ. 2011, 89, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.W.; Lake, R.A. Advances in malignant mesothelioma. N. Engl. J. Med. 2005, 353, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Bagia, M.; Nowak, A.K. Novel targeted therapies and vaccination strategies for mesothelioma. Curr. Treat. Options Oncol. 2011, 12, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Astoul, P.; Baas, P.; Berghmans, T.; Clayson, H.; de Vuyst, P.; Dienemann, H.; Galateau-Salle, F.; Hennequin, C.; Hillerdal, G.; et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur. Respir. J. 2010, 35, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Schlesser, M.; Frenay, C.; Astoul, P. Malignant pleural mesothelioma. Eur. Respir. J. 1998, 12, 972–981. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef]
- Ismail-Khan, R.; Robinson, A.; Williams, C.C., Jr.; Garrett, C.R.; Bepler, G.; Simon, G.R. Malignant pleural mesothelioma: A comprehensive review. Cancer Control 2006, 13, 255–263. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet. Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, R.J.; Sharon, E.; Hassan, R. Chemotherapy and targeted therapies for unresectable malignant mesothelioma. Lung Cancer 2011, 73, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Ellis, P.; Davies, A.M.; Evans, W.K.; Haynes, A.E.; Lloyd, N.S.; the Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. The use of chemotherapy in patients with advanced malignant pleural mesothelioma: A systematic review and practice guideline. J. Thorac. Oncol. 2006, 1, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010, 29, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, F.; Turrisi, G.; Marinozzi, C.; Aliberti, C.; Fiorentini, G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2011, 14, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Wouters, A.; Pauwels, B.; Lardon, F.; Pattyn, G.G.; Lambrechts, H.A.J.; Baay, M.; Baay, M.; Meijnders, P.; Vermorken, J.B. In vitro study on the schedule-dependency of the interaction between pemetrexed, gemcitabine and irradiation in non-small cell lung cancer and head and neck cancer cells. BMC Cancer 2010, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; Schellens, J.H.M. OA13.03 Long term overall survival for patients with malignant pleural mesothelioma on Pembrolizumab enrolled in KEYNOTE-028. J. Thorac. Oncol. 2017, 12, S294. [Google Scholar] [CrossRef]
- Quispel-Janssen, J.; Zago, G.; Schouten, R.; Buikhuisen, W.; Monkhorst, K.; Thunissen, E.; Baas, P. A phase II study of Nivolumab in malignant pleural mesothelioma (NIVOMES): With translational research (TR) biopsies. J. Thorac. Oncol. 2017, 12, S292. [Google Scholar] [CrossRef]
- Calabro, L.; Morra, A.; Fonsatti, E.; Cutaia, O.; Amato, G.; Giannarelli, D.; Di Giacomo, A.M.; Danielli, R.; Altomonte, M.; Mutti, L.; et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: An open-label, single-arm, phase 2 trial. Lancet. Oncol. 2013, 14, 1104–1111. [Google Scholar] [CrossRef]
- Satoh, T.; Tatsuta, T.; Sugawara, S.; Hara, A.; Hosono, M. Synergistic anti-tumor effect of bullfrog sialic acid-binding lectin and pemetrexed in malignant mesothelioma. Oncotarget 2017, 8, 42466–42477. [Google Scholar] [CrossRef] [Green Version]
- Hudson, A.L.; Weir, C.; Moon, E.; Harvie, R.; Klebe, S.; Clarke, S.J.; Pavlakis, N.; Howell, V.M. Establishing a panel of chemo-resistant mesothelioma models for investigating chemo-resistance and identifying new treatments for mesothelioma. Sci. Rep. 2014, 4, 6152. [Google Scholar] [CrossRef] [Green Version]
- Cortes-Dericks, L.; Froment, L.; Boesch, R.; Schmid, R.A.; Karoubi, G. Cisplatin-resistant cells in malignant pleural mesothelioma cell lines show ALDH(high)CD44(+) phenotype and sphere-forming capacity. BMC Cancer 2014, 14, 304. [Google Scholar] [CrossRef]
- Nannizzi, S.; Veal, G.J.; Giovannetti, E.; Mey, V.; Ricciardi, S.; Ottley, C.J.; Del Tacca, M.; Danesi, R. Cellular and molecular mechanisms for the synergistic cytotoxicity elicited by oxaliplatin and pemetrexed in colon cancer cell lines. Cancer Chemother Pharm. 2010, 66, 547–558. [Google Scholar] [CrossRef]
- Musk, A.W.; Olsen, N.; Alfonso, H.; Reid, A.; Mina, R.; Franklin, P.; Sleith, J.; Hammond, N.; Threlfall, T.; Shilkin, K.B.; et al. Predicting survival in malignant mesothelioma. Eur. Respir. J. 2011, 38, 1420–1424. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Symanowski, J.T.; Peikert, T. Systematic review of response rates of sarcomatoid malignant pleural mesotheliomas in clinical trials. Lung Cancer 2014, 86, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Rosen, L.E.; Karrison, T.; Ananthanarayanan, V.; Gallan, A.J.; Adusumilli, P.S.; Alchami, F.S.; Attanoos, R.; Brcic, L.; Butnor, K.J.; Galateau-Salle, F.; et al. Nuclear grade and necrosis predict prognosis in malignant epithelioid pleural mesothelioma: A multi-institutional study. Mod. Pathol. 2018, 31, 598–606. [Google Scholar] [CrossRef]
- Pelosi, G.; Papotti, M.; Righi, L.; Rossi, G.; Ferrero, S.; Bosari, S.; Calabrese, F.; Kern, I.; Maisonneuve, P.; Sonzogni, A.; et al. Pathologic Grading of Malignant Pleural Mesothelioma: An Evidence-Based Proposal. J. Thorac. Oncol. 2018, 13, 1750–1761. [Google Scholar] [CrossRef] [Green Version]
- Takezawa, K.; Okamoto, I.; Okamoto, W.; Takeda, M.; Sakai, K.; Tsukioka, S.; Kuwata, K.; Yamaguchi, H.; Nishio, K.; Nakagawa, K. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br. J. Cancer 2011, 104, 1594–1601. [Google Scholar] [CrossRef] [Green Version]
- Chamizo, C.; Zazo, S.; Domine, M.; Cristobal, I.; Garcia-Foncillas, J.; Rojo, F.; Madoz-Gurpide, J. Thymidylate synthase expression as a predictive biomarker of pemetrexed sensitivity in advanced non-small cell lung cancer. BMC Pulm. Med. 2015, 15, 132. [Google Scholar] [CrossRef]
- Ozasa, H.; Oguri, T.; Uemura, T.; Miyazaki, M.; Maeno, K.; Sato, S.; Ueda, R. Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci. 2010, 101, 161–166. [Google Scholar] [CrossRef]
- Sigmond, J.; Backus, H.H.; Wouters, D.; Temmink, O.H.; Jansen, G.; Peters, G.J. Induction of resistance to the multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpression. Biochem. Pharm. 2003, 66, 431–438. [Google Scholar] [CrossRef]
- Righi, L.; Papotti, M.G.; Ceppi, P.; Bille, A.; Bacillo, E.; Molinaro, L.; Ruffini, E.; Scagliotti, G.V.; Selvaggi, G. Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J. Clin. Oncol. 2010, 28, 1534–1539. [Google Scholar] [CrossRef]
- Kitazono-Saitoh, M.; Takiguchi, Y.; Kitazono, S.; Ashinuma, H.; Kitamura, A.; Tada, Y.; Kurosu, K.; Sakaida, E.; Sekine, I.; Tanabe, N.; et al. Interaction and cross-resistance of cisplatin and pemetrexed in malignant pleural mesothelioma cell lines. Oncol. Rep. 2012, 28, 33–40. [Google Scholar]
- Kim, J.H.; Lee, K.W.; Jung, Y.; Kim, T.Y.; Ham, H.S.; Jong, H.; Jung, K.H.; Im, S.; Kim, T.Y.; Kim, N.K.; et al. Cytotoxic effects of pemetrexed in gastric cancer cells. Cancer Sci. 2005, 96, 365–371. [Google Scholar] [CrossRef]
- Kano, Y.; Akutsu, M.; Tsunoda, S.; Izumi, T.; Kobayashi, H.; Inoue, K.; Mori, K.; Fujii, H.; Mano, H.; Odgerel, T.; et al. Schedule-dependent interactions between pemetrexed and cisplatin in human carcinoma cell lines in vitro. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2006, 16, 85–95. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Zitvogel, L.; Kroemer, G. The secret ally: Immunostimulation by anticancer drugs. Nat. Rev. Drug Discov. 2012, 11, 215–233. [Google Scholar] [CrossRef]
- Lake, R.A.; Robinson, B.W. Immunotherapy and chemotherapy--a practical partnership. Nat. Rev. Cancer 2005, 5, 397–405. [Google Scholar] [CrossRef]
- Shurin, G.V.; Tourkova, I.L.; Kaneno, R.; Shurin, M.R. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J. Immunol. 2009, 183, 137–144. [Google Scholar] [CrossRef]
- Zitvogel, L.; Kroemer, G. Anticancer immunochemotherapy using adjuvants with direct cytotoxic effects. J. Clin. Investig. 2009, 119, 2127–2130. [Google Scholar] [CrossRef]
- Chacon, J.A.; Schutsky, K.; Powell, D.J. The Impact of Chemotherapy, Radiation and Epigenetic Modifiers in Cancer Cell Expression of Immune Inhibitory and Stimulatory Molecules and Anti-Tumor Efficacy. Vaccines (Basel) 2016, 4. [Google Scholar] [CrossRef]
- Qin, X.; Liu, C.; Zhou, Y.; Wang, G. Cisplatin induces programmed death-1-ligand 1(PD-L1) over-expression in hepatoma H22 cells via Erk /MAPK signaling pathway. Cell Mol. Biol. (Noisy-le-grand) 2010, 56, OL1366–OL1372. [Google Scholar]
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C.; et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88. [Google Scholar] [CrossRef]
- Tel, J.; Hato, S.V.; Torensma, R.; Buschow, S.I.; Figdor, C.G.; Lesterhuis, W.J.; de Vries, I.J. The chemotherapeutic drug oxaliplatin differentially affects blood DC function dependent on environmental cues. Cancer Immunol. Immunother 2012, 61, 1101–1111. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Y.; Lv, C.; Huang, M.; Li, M.; Dong, B.; Liu, X.; An, G.; Zhang, W.; Zhang, J.; et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016, 107, 1563–1571. [Google Scholar] [CrossRef]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-kappaB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef]
- Zhang, P.; Su, D.M.; Liang, M.; Fu, J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol. Immunol 2008, 45, 1470–1476. [Google Scholar] [CrossRef]
- Yang, H.; Bueso-Ramos, C.; DiNardo, C.; Estecio, M.R.; Davanlou, M.; Geng, Q.R.; Fang, Z.; Nguyen, M.; Pierce, S.; Wei, Y.; et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014, 28, 1280–1288. [Google Scholar] [CrossRef]
- Ghebeh, H.; Lehe, C.; Barhoush, E.; Al-Romaih, K.; Tulbah, A.; Al-Alwan, M.; Hendrayani, S.F.; Manogaran, P.; Alaiya, A.; Al-Tweigeri, T.; et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010, 12, R48. [Google Scholar] [CrossRef]
- Oki, Y.; Buglio, D.; Zhang, J.; Ying, Y.; Zhou, S.; Sureda, A.; Ben-Yehuda, D.; Zinzani, P.L.; Prince, H.M.; Harrison, S.J.; et al. Immune regulatory effects of panobinostat in patients with Hodgkin lymphoma through modulation of serum cytokine levels and T-cell PD1 expression. Blood Cancer J. 2014, 4, e236. [Google Scholar] [CrossRef]
- Sheng, J.; Fang, W.; Yu, J.; Chen, N.; Zhan, J.; Ma, Y.; Yang, Y.; Huang, Y.; Zhao, H.; Zhang, L. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci. Rep. 2016, 6, 20090. [Google Scholar] [CrossRef]
- What is Supplementary Information and How Can I Find It? Available online: https://www.nature.com/articles/srep20090#supplementary-information (accessed on 29 January 2016).
- Marcq, E.; Siozopoulou, V.; De Waele, J.; van Audenaerde, J.; Zwaenepoel, K.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. Oncoimmunology 2017, 6, e1261241. [Google Scholar] [CrossRef]
- Zhang, L.; Du, H.; Xiao, T.W.; Liu, J.Z.; Liu, G.Z.; Wang, J.X.; Li, G.Y.; Wang, L.X. Prognostic value of PD-1 and TIM-3 on CD3+ T cells from diffuse large B-cell lymphoma. Biomed. Pharm. 2015, 75, 83–87. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112. [Google Scholar] [CrossRef]

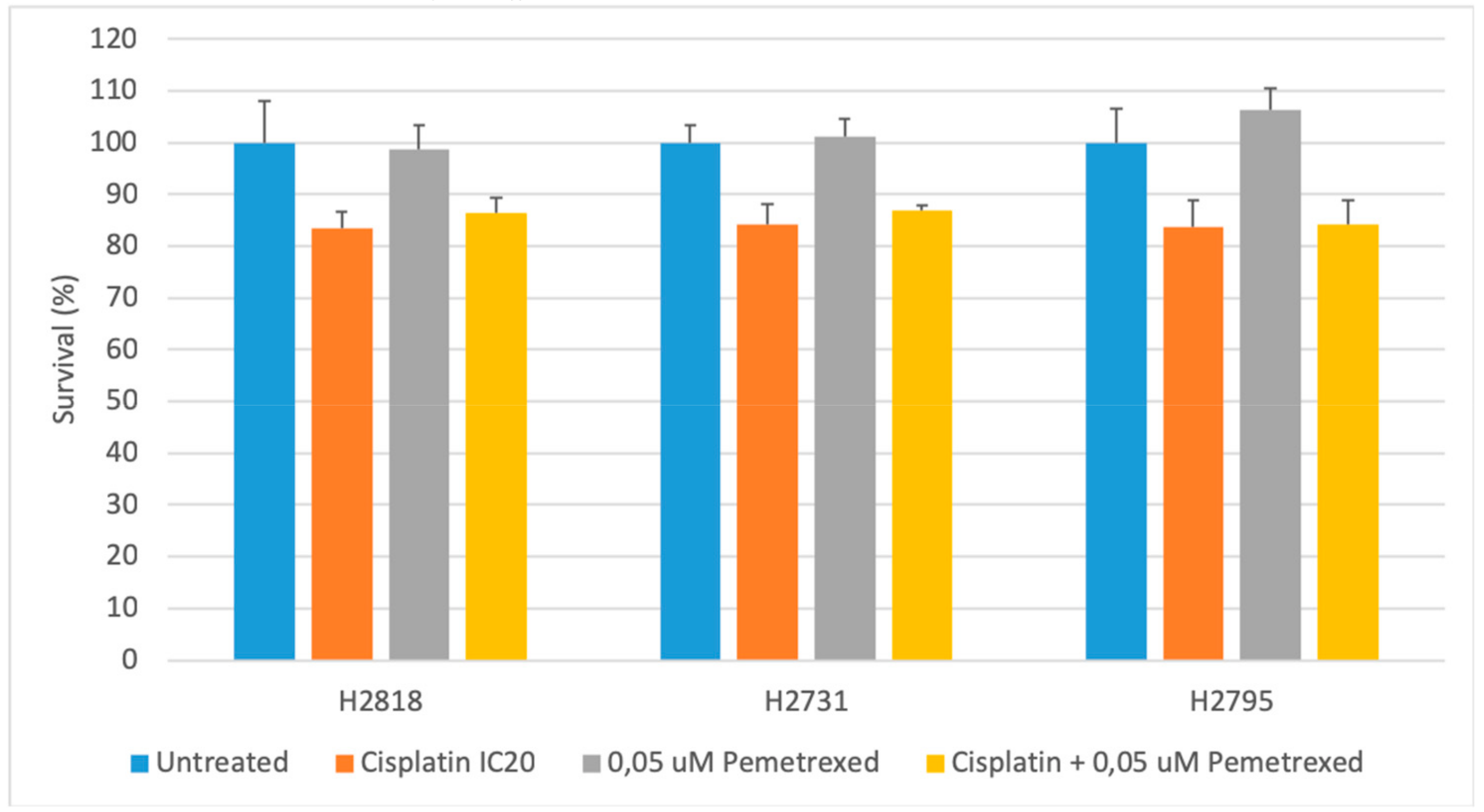
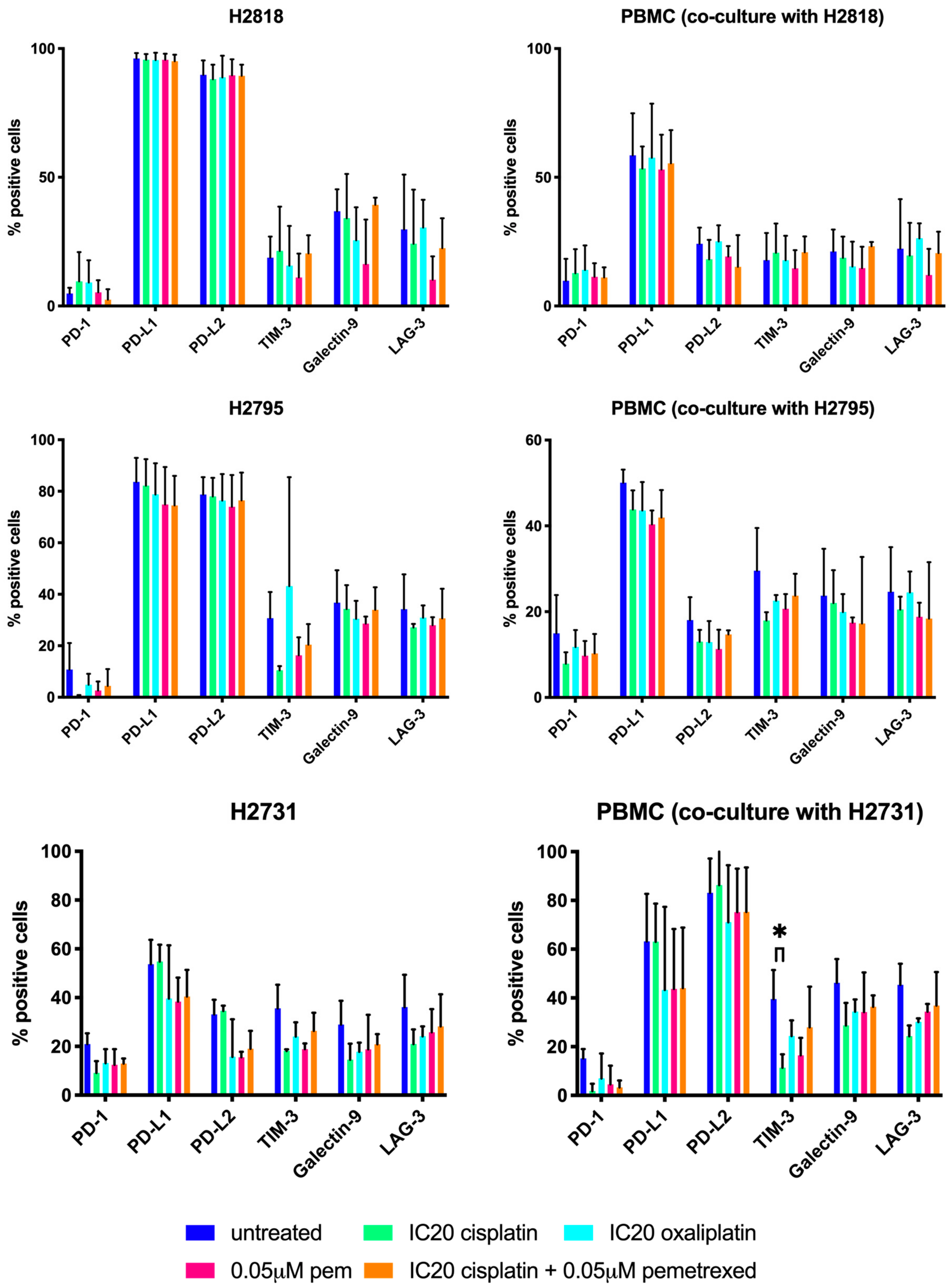



| Chemotherapy | Cell Line | IC50 | p-Values | ||
|---|---|---|---|---|---|
| NCI-H2818 | NCI-H2795 | NCI-H2731 | |||
| Cisplatin | NCI-H2818 | 2.31 ± 0.34 | 0.008 | 0.007 | |
| NCI-H2795 | 7.78 ± 0.44 | 0.008 | 1.000 | ||
| NCI-H2731 | 7.89 ± 0.44 | 0.007 | 1.000 | ||
| Oxaliplatin | NCI-H2818 | 3.47 ± 1.52 | 0.001 | 0.030 | |
| NCI-H2795 | 18.23 ± 3.98 | 0.001 | 0.012 | ||
| NCI-H2731 | 9.77 ± 2.33 | 0.030 | 0.012 | ||
| Cell Line | IC20 (µM) | ||
|---|---|---|---|
| Cisplatin | Oxaliplatin | Pemetrexed[18] | |
| NCI-H2818 | 0.50 | 0.17 | 0.05 |
| NCI-H2795 | 2.70 | 1.86 | 0.05 |
| NCI-H2731 | 2.34 | 1.01 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcq, E.; Van Audenaerde, J.R.; De Waele, J.; Jacobs, J.; Van Loenhout, J.; Cavents, G.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L. Building a Bridge between Chemotherapy and Immunotherapy in Malignant Pleural Mesothelioma: Investigating the Effect of Chemotherapy on Immune Checkpoint Expression. Int. J. Mol. Sci. 2019, 20, 4182. https://doi.org/10.3390/ijms20174182
Marcq E, Van Audenaerde JR, De Waele J, Jacobs J, Van Loenhout J, Cavents G, Pauwels P, van Meerbeeck JP, Smits EL. Building a Bridge between Chemotherapy and Immunotherapy in Malignant Pleural Mesothelioma: Investigating the Effect of Chemotherapy on Immune Checkpoint Expression. International Journal of Molecular Sciences. 2019; 20(17):4182. https://doi.org/10.3390/ijms20174182
Chicago/Turabian StyleMarcq, Elly, Jonas RM Van Audenaerde, Jorrit De Waele, Julie Jacobs, Jinthe Van Loenhout, Glenn Cavents, Patrick Pauwels, Jan P van Meerbeeck, and Evelien LJ Smits. 2019. "Building a Bridge between Chemotherapy and Immunotherapy in Malignant Pleural Mesothelioma: Investigating the Effect of Chemotherapy on Immune Checkpoint Expression" International Journal of Molecular Sciences 20, no. 17: 4182. https://doi.org/10.3390/ijms20174182






