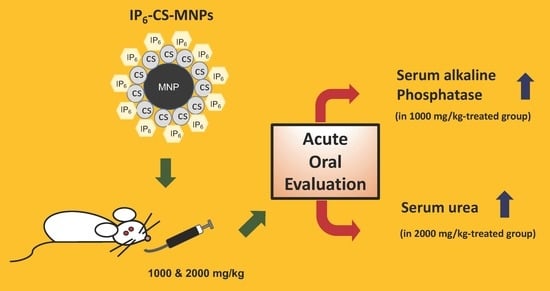
The Acute Effects of Oral Administration of Phytic Acid-Chitosan-Magnetic Iron Oxide Nanoparticles in Mice
Abstract
:1. Introduction
2. Results
2.1. Animal Observation, Mortality, and Body Weight Change
2.2. Relative Organ Weight
2.3. Biochemical Analysis
2.4. Histopathological Evaluation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Magnetic Iron Oxide Nanoparticle Synthesis
4.3. Coating of Magnetic Iron Oxide Nanoparticles with Chitosan
4.4. Phytic Acid-Chitosan-Iron Oxide Nanocomposite Synthesis
4.5. Experimental Animals and Grouping
4.6. Animal Observation
4.7. Serum Collection
4.8. Serum Biochemistry Analysis
4.9. Organ Collection
4.10. Histopathological Assessment
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| b.w | Body weight |
| creat | Creatinine |
| CS | Chitosan |
| H&E | Hematoxylin and eosin |
| IP6 | Phytic acid |
| IP6-CS-MNPs | Phytic acid-chitosan-magnetic iron oxide nanoparticles |
| IU/L | International units per liter |
| MNPs | Magnetic iron oxide nanoparticles |
References
- Markides, H.; Rotherham, M.A. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 13–15. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, F.; Sun, J.; Sheng, J.; Wang, F.; Sun, M. Bio and nanomaterials based on Fe3O4. Molecules 2014, 19, 21506–21528. [Google Scholar] [CrossRef] [PubMed]
- Thorat, N.D.; Bohara, R.A.; Malgras, V.; Tofail, S.A.M.; Ahamad, T.; Alshehri, S.M.; Wu, K.C.; Yamauchi, Y. Multimodal superparamagnetic nanoparticles with unusually enhanced specific absorption rate for synergetic cancer therapeutics and magnetic resonance imaging. Appl. Mater. Interfaces 2016, 8, 14656–14664. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; Ul-haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterisation, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Reza, R.T.; Pérez, C.A.M.; Martínez, A.M.; García-casillas, P.E.; De Ingeniería, I.; Autónoma, U.; Juárez, D.C.; Charro, A.; Chihuahua, C.J. Magnetite particle size dependence on the coprecipitation synthesis method for protein separation. NSTI-Nanotech 2010, 1, 534–538. [Google Scholar]
- Unsoy, G.; Gunduz, U.; Oprea, O.; Ficai, D.; Sonmez, M.; Radulescu, M.; Alexia, M.; Ficai, A. Magnetite: From synthesis to applications. Curr. Top. Med. Chem. 2015, 15, 1622–1640. [Google Scholar] [CrossRef]
- Predescu, A.M.; Matei, E.; Berbecaru, C.; Pantilimon, C.; Vidu, R.; Predescu, C.; Kuncser, V. Synthesis and characterization of dextran-coated iron oxide nanoparticles. R. Soc. Open Sci. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- William, T.; Menon, J. A review of malaria research in Malaysia. Med. J. Malays. 2014, 69, 82–87. [Google Scholar]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef]
- Jabir, N.R.; Tabrez, S.; Ashraf, G.M.; Shakil, S.; Damanhouri, G.A.; Kamal, M.A. Nanotechnology-based approaches in anticancer research. Int. J. Nanomed. 2012, 7, 4391–4408. [Google Scholar]
- Kim, S. Competitive biological activities of chitosan and its derivatives: Antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Int. J. Polym. Sci. 2018, 2018, 13. [Google Scholar] [CrossRef]
- Salomao Arias, L.; Pessan, J.P.; Viera, A.P.M.; Maria Toito de Lima, T.; Carlos Botazza Delbem, A.; Roberto Monteiro, D. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity and toxicity. Antibiotics 2018, 7, 1–32. [Google Scholar]
- Mitry, M.A.; Edwards, J.G. Doxorubicin induced heart failure: Phenotype and molecular mechanisms. IJC Hear. Vasc. 2016, 10, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Norhaizan, M.E.; Ng, S.K.; Norashareena, M.S.; Abdah, M.A. Antioxidant and cytotoxicity effect of rice bran phytic acid as an anticancer agent on ovarian, breast and liver cancer cell lines. Malays. J. Nutr. 2011, 17, 367–375. [Google Scholar] [PubMed]
- Shafie, N.H.; Esa, N.M.; Ithnin, H.; Saad, N.; Pandurangan, A.K. Pro-apoptotic effect of rice bran Inositol Hexaphosphate (IP6) on HT-29 colorectal cancer cells. Int. J. Mol. Sci. 2013, 14, 23545–23558. [Google Scholar] [CrossRef]
- Kapral, M.; Wawszczyk, J.; Jesse, K.; Paul-Samojedny, M.; Kúsmierz, D.; Wȩglarz, L. Inositol hexaphosphate inhibits proliferation and induces apoptosis of colon cancer cells by suppressing the AKT/mTOR signaling pathway. Molecules 2017, 22, 1657. [Google Scholar] [CrossRef]
- Norazalina, S.; Norhaizan, M.E.; Hairuszah, I.; Sabariah, A.R.; Husna, S.N.; Norsharina, I. Antiproliferation and apoptosis induction of phytic acid in hepatocellular carcinoma (HEPG2) cell lines. Afr. J. Biotechnol. 2011, 10, 16646–16653. [Google Scholar] [CrossRef]
- Shafie, N.H.; Mohd Esa, N.; Ithnin, H.; Md Akim, A.; Saad, N.; Pandurangan, A.K. Preventive inositol hexaphosphate extracted from rice bran inhibits colorectal cancer through involvement of Wnt/β-catenin and COX-2 pathways. BioMed Res. Int. 2013, 2013, 10. [Google Scholar] [CrossRef]
- Higuchi, M. Antioxidant properties of wheat bran against oxidative stress. In Wheat and Rice in Disease Prevention and Health: Benefits, Risks and Mechanisms of Whole Grains in Health Promotion; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 181–199. ISBN 9780124017160. [Google Scholar]
- Barahuie, F.; Dorniani, D.; Bullo, S.; Sivapragasam, G.; Hussein, M.Z.; Pandurangan, A.K.; Palanisamy, A.; Mohd Esa, N. Sustained release of anticancer agent phytic acid from its chitosan-coated magnetic nanoparticles for drug-delivery system. Int. J. Nanomed. 2017, 12, 2361–2372. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.; Chan, L.C. An intrinsic mitochondrial pathway is required for phytic acid-chitosan-iron oxide nanocomposite (Phy-CS-MNP) to induce G0/G1 cell cycle arrest and apoptosis in the human colorectal cancer (HT-29) cell line. Pharmaceutics 2018, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Erhirhie, E.O.; Ihekwereme, C.P.; Ilodigwe, E.E. Advances in acute toxicity testing: Strengths, weaknesses and regulatory acceptance. Interdiscip. Toxicol. 2018, 11, 5–12. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test, no. 423: Acute oral toxicity—Acute toxic class method. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Wada, S.; Yue, L.; Tazawa, K.; Furuta, I.; Nagae, H.; Takemori, S.; Minamimura, T. New local hyperthermia using dextran magnetite complex (DM) for oral cavity: Experimental study in normal hamster tongue. Oral Dis. 2001, 7, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Nurul, S.; Hazilawati, H.; Mohd, R.S.; Hanif, F.; Mohd, R.; Noordin, M.M.; Norhaizan, E. Subacute oral toxicity assesment of ethanol extract of Mariposa christia vespertilionis leaves in male Sprague dawley rats. Toxicol. Res. 2018, 34, 85–95. [Google Scholar]
- Kumari, M.; Rajak, S.; Singh, S.P.; Murty, U.S.N.; Mahboob, M.; Grover, P.; Rahman, M.F. Biochemical alterations induced by acute oral doses of iron oxide nanoparticles in Wistar rats. Drug Chem. Toxicol. 2012, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ewis, R.I.W.L.; Illington, R.I.B.; Ebryune, E.R.I.C.D.; Amer, A.R.G.; Ang, B.L. Recognition of adverse and nonadverse effects in toxicity studies. Toxicol. Pathol. 2002, 30, 66–74. [Google Scholar]
- Fields, D.; Higgins, P. Physiological mechanism impacting weight regulation. In Handbook of Childhood and Adolescent Obesity; Jelalian, E., Steele, R.G., Eds.; Springer: Boston, MA, USA, 2008; pp. 109–126. [Google Scholar]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.J.; Schafer, K. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Mahmudul, K.; Tamanna, N.; Haque, A. Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed. Food Sci. Hum. Wellness 2018, 7, 77–82. [Google Scholar]
- Webber, M.; Krishnan, A.; Thomas, N.G.; Cheung, B.M.Y. Association between serum alkaline phosphatase and C-reactive protein in the United States national health and nutrition examination survey 2005–2006. Clin. Chem. Lab. Med. 2010, 48, 167–173. [Google Scholar] [CrossRef]
- Higgins, C. Urea and the Clinical Value of Measuring Blood Urea Concentration. Available online: https://acutecaretesting.org/en/articles/urea-and-the-clinical-value-of-measuring-blood-urea-concentration (accessed on 23 April 2019).
- Qu, J.; Liu, G.; Wang, Y.; Hong, R. Preparation of Fe3O4—Chitosan nanoparticles used for hyperthermia. Adv. Powder Technol. 2010, 21, 461–467. [Google Scholar] [CrossRef]



| Day | Body Weight (g) | ||
|---|---|---|---|
| Control | 1000 mg/kg | 2000 mg/kg | |
| 0 | 18.94 ± 0.55 a | 18.07± 0.44 a | 18.69 ± 0.45 a |
| 7 | 18.61 ± 0.54 a | 17.88 ± 0.40 a | 18.59 ± 0.44 a |
| 14 | 19.2 ± 0.52 a | 18.48 ± 0.30 a | 19.15 ± 0.47 a |
| Organ | Relative Organ Weight | ||
|---|---|---|---|
| Control | 1000 mg/kg | 2000 mg/kg | |
| Liver | 4.32 ± 0.25 a | 4.51 ± 0.38 a | 4.60 ± 0.19 a |
| Kidney | 0.64 ± 0.02 a | 0.65 ± 0.04 a | 0.57 ± 0.03 a |
| Heart | 0.71 ± 0.11 a | 0.52 ± 0.03 a | 0.53 ± 0.04 a |
| Lung | 0.69 ± 0.05 a | 0.72 ± 0.10 a | 0.92 ± 0.11 a |
| Spleen | 0.42 ± 0.10 a | 0.31 ± 0.03 a | 0.38 ± 0.05 a |
| Brain | 2.18 ± 0.08 a | 2.26 ± 0.05 a | 2.05 ± 0.52 a |
| Colon | 1.80 ± 0.26 a | 2.94 ± 0.89 a | 2.13 ± 0.15 a |
| Parameter | Control | 1000 mg/kg b.w | 2000 mg/kg b.w |
|---|---|---|---|
| ALP (U/L) | 117.3 ± 5.68 a | 159.33 ± 6.73 b | 132.33 ± 6.51 a |
| AST (U/L) | 113.83 ± 20.46 a | 111.50 ± 13.60 a | 157.00 ± 26.10 a |
| ALT (U/L) | 23.67 ± 5.10 a | 18.67 ± 2.36 a | 20.17 ± 2.29 a |
| Creat (μmol/L) | 30.00 ± 1.21 a | 30.00 ± 0.82 a | 31.67 ± 1.33 a |
| Urea (mmol/L) | 8.02 ± 0.43 a | 7.67 ± 0.26 a | 9.75 ± 0.48 b |
| Groups | Dosage | Number of Mice |
|---|---|---|
| 1 | 1 mL deionized water | 6 |
| 2 | 1000 mg/kg b.w of IP6-CS-MNPs | 6 |
| 3 | 2000 mg/kg b.w of IP6-CS-MNPs | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Tamsir, N.; Mohd Esa, N.; Shafie, N.H.; Hussein, M.Z.; Hamzah, H.; Abdullah, M.A. The Acute Effects of Oral Administration of Phytic Acid-Chitosan-Magnetic Iron Oxide Nanoparticles in Mice. Int. J. Mol. Sci. 2019, 20, 4114. https://doi.org/10.3390/ijms20174114
Mohd Tamsir N, Mohd Esa N, Shafie NH, Hussein MZ, Hamzah H, Abdullah MA. The Acute Effects of Oral Administration of Phytic Acid-Chitosan-Magnetic Iron Oxide Nanoparticles in Mice. International Journal of Molecular Sciences. 2019; 20(17):4114. https://doi.org/10.3390/ijms20174114
Chicago/Turabian StyleMohd Tamsir, Norain, Norhaizan Mohd Esa, Nurul Husna Shafie, Mohd Zobir Hussein, Hazilawati Hamzah, and Maizaton Atmadini Abdullah. 2019. "The Acute Effects of Oral Administration of Phytic Acid-Chitosan-Magnetic Iron Oxide Nanoparticles in Mice" International Journal of Molecular Sciences 20, no. 17: 4114. https://doi.org/10.3390/ijms20174114