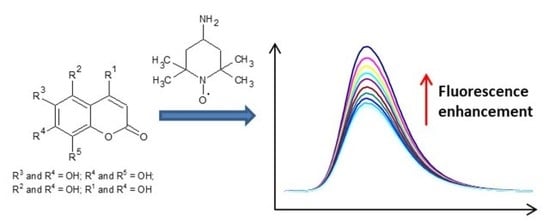
Dihydroxy-Substituted Coumarins as Fluorescent Probes for Nanomolar-Level Detection of the 4-Amino-TEMPO Spin Label
Abstract
1. Introduction
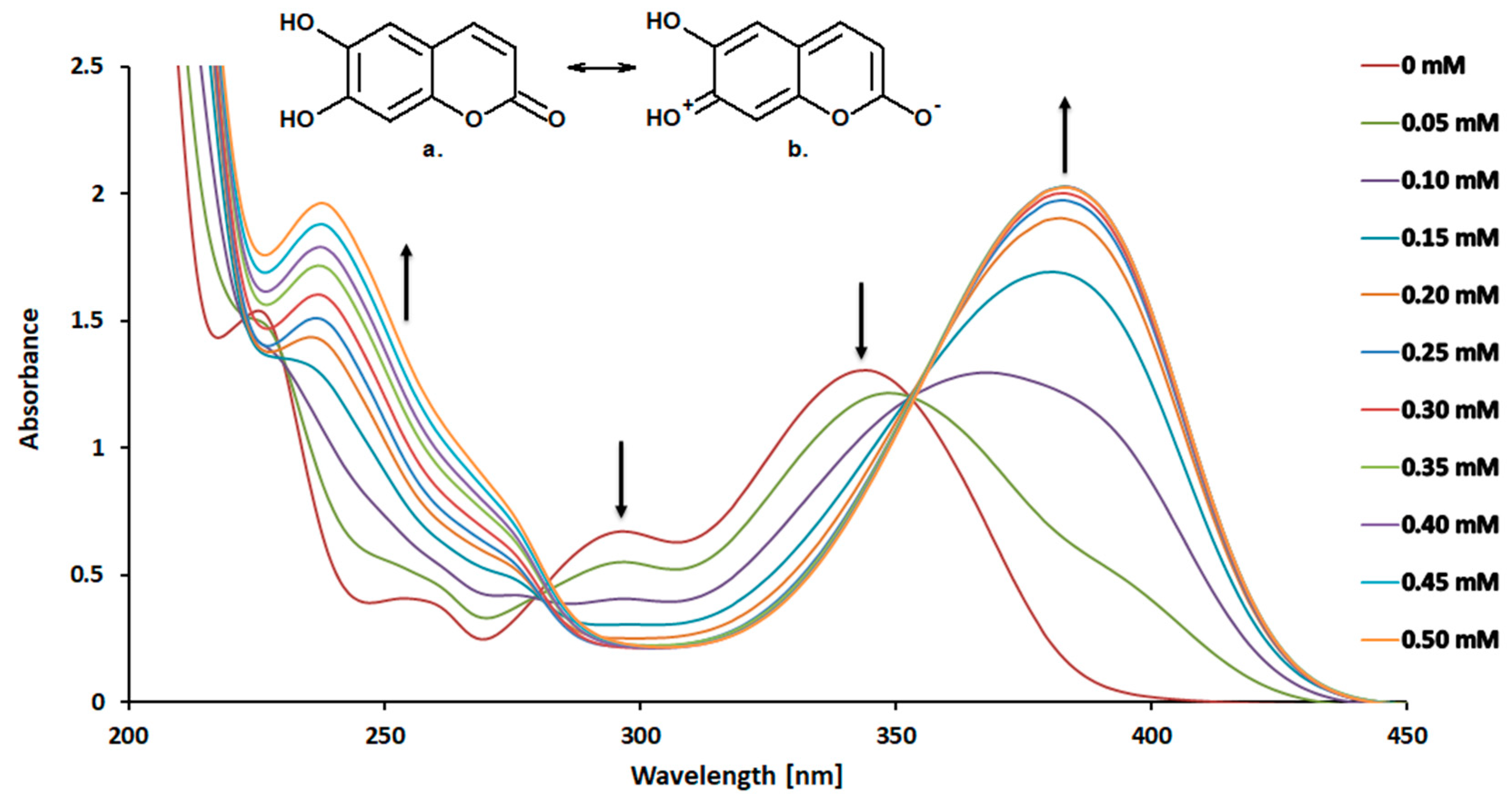
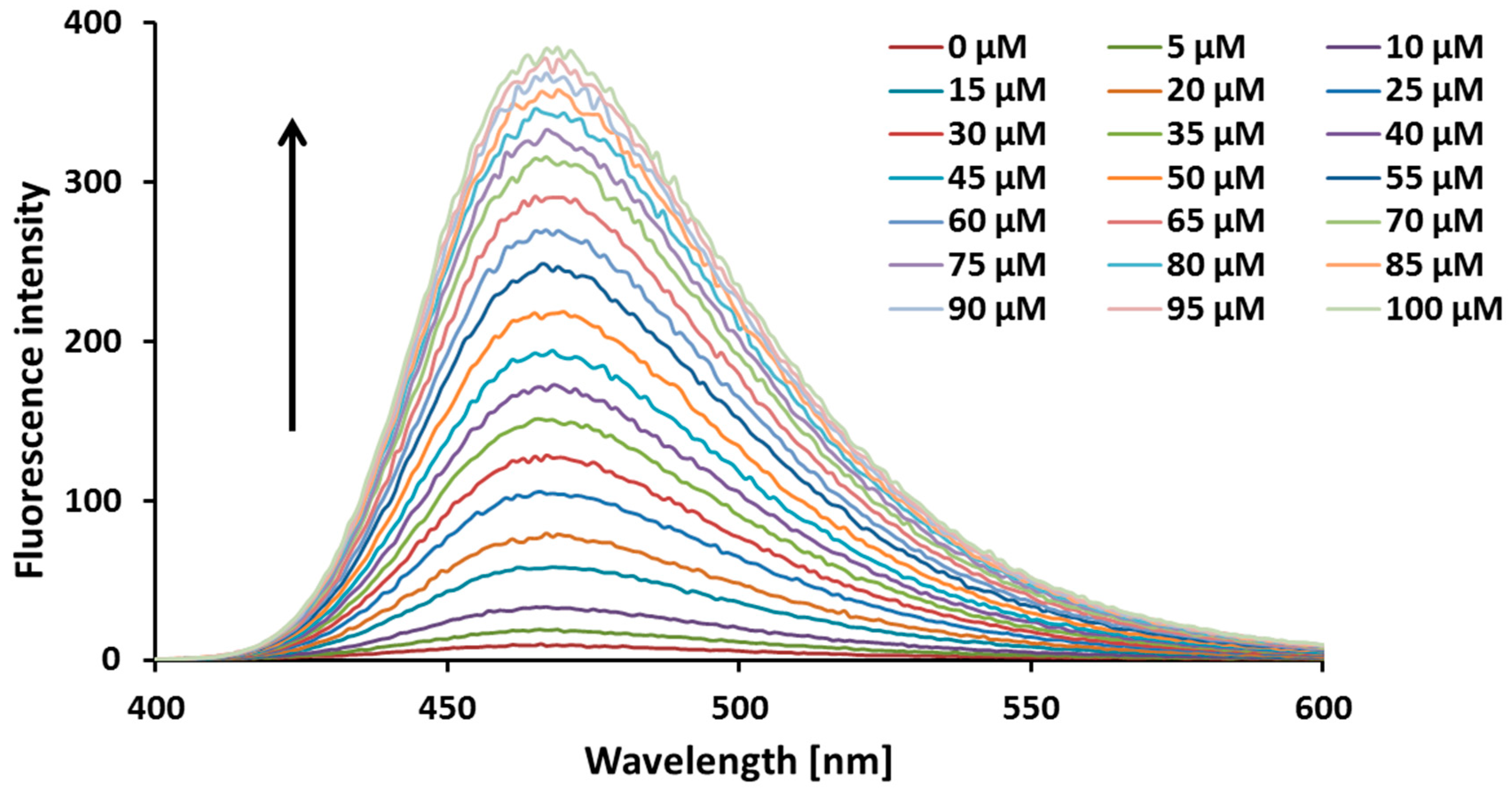
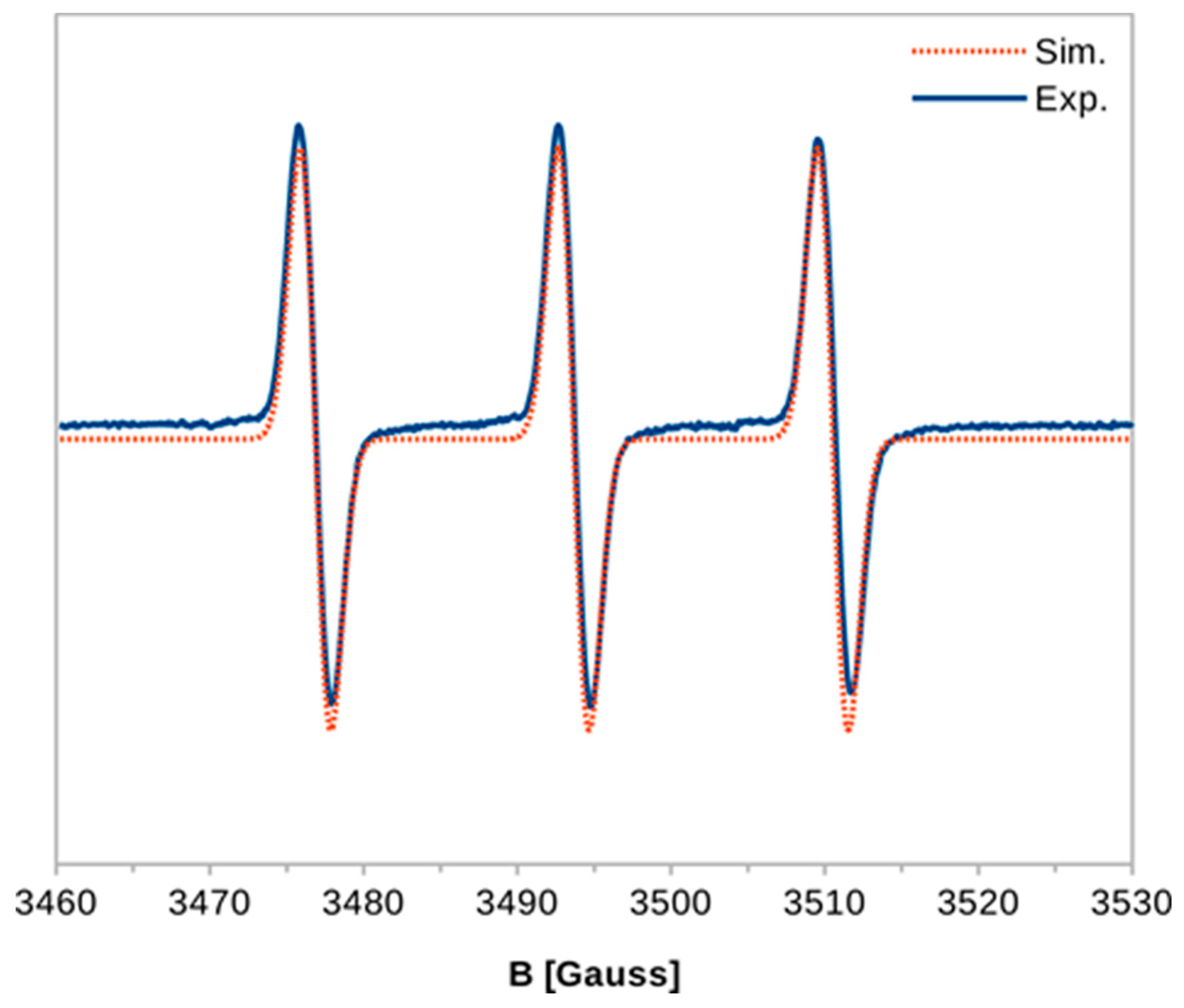
2. Results and Discussion
2.1. Chemical Studies
2.2. Biological Studies
3. Materials and Methods
3.1. Materials
3.2. UV-Vis Spectroscopy
3.3. Steady-State Fluorescence Spectroscopy
3.4. Time-Resolved Fluorescence Spectroscopy
3.5. Electron Paramagnetic Resonance
3.6. Potentiometric Titration
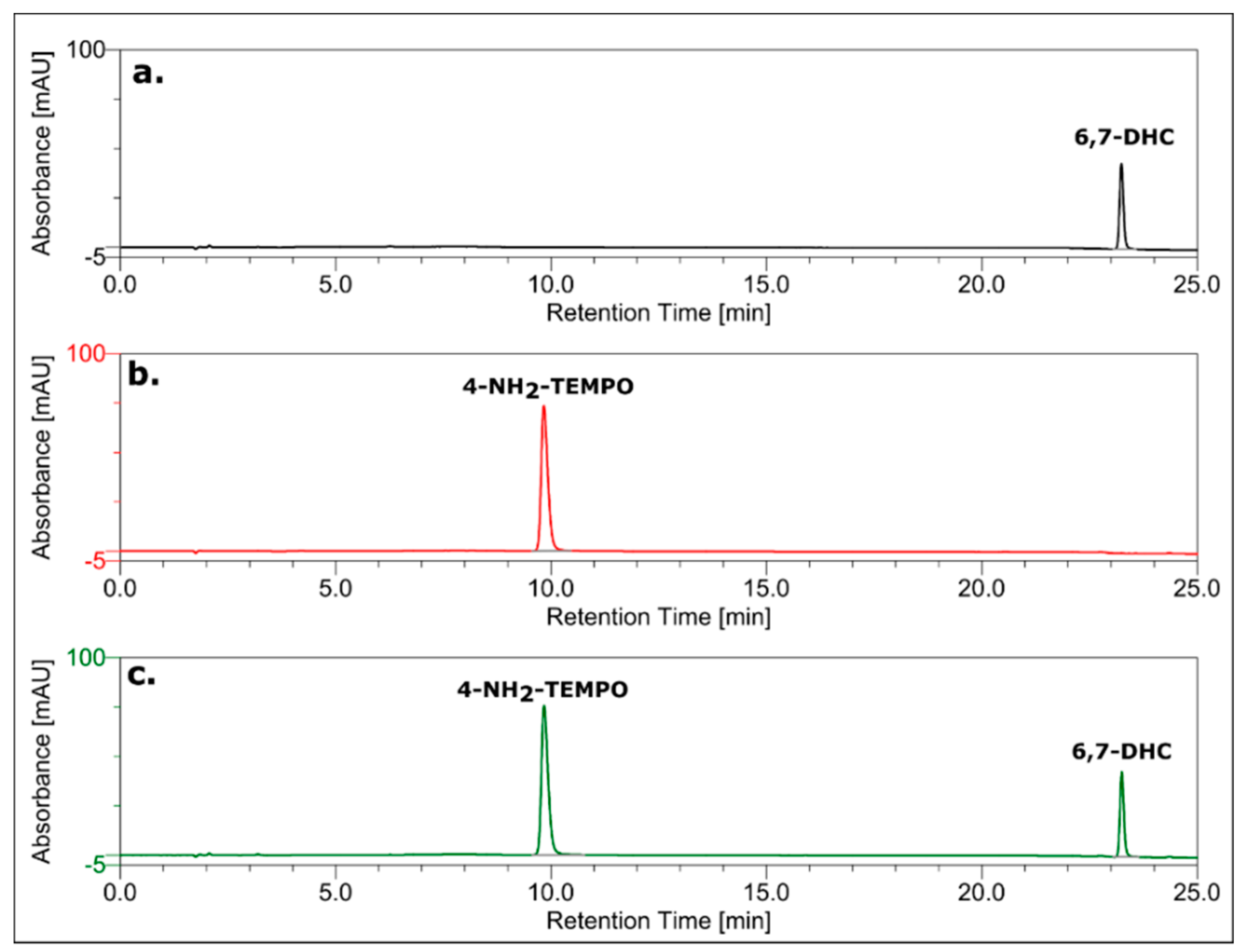
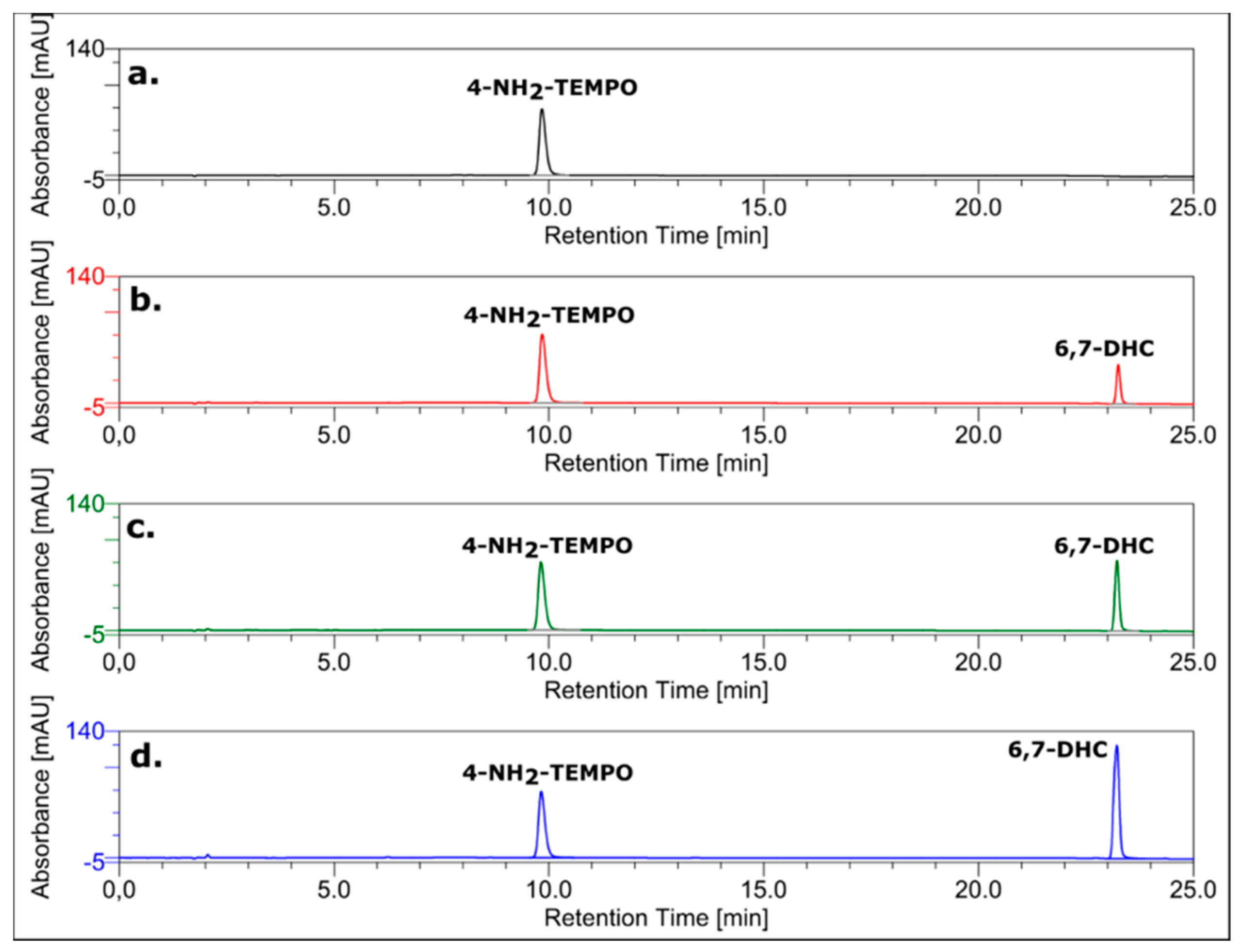
3.7. HPLC Analysis
3.8. Cell Culture
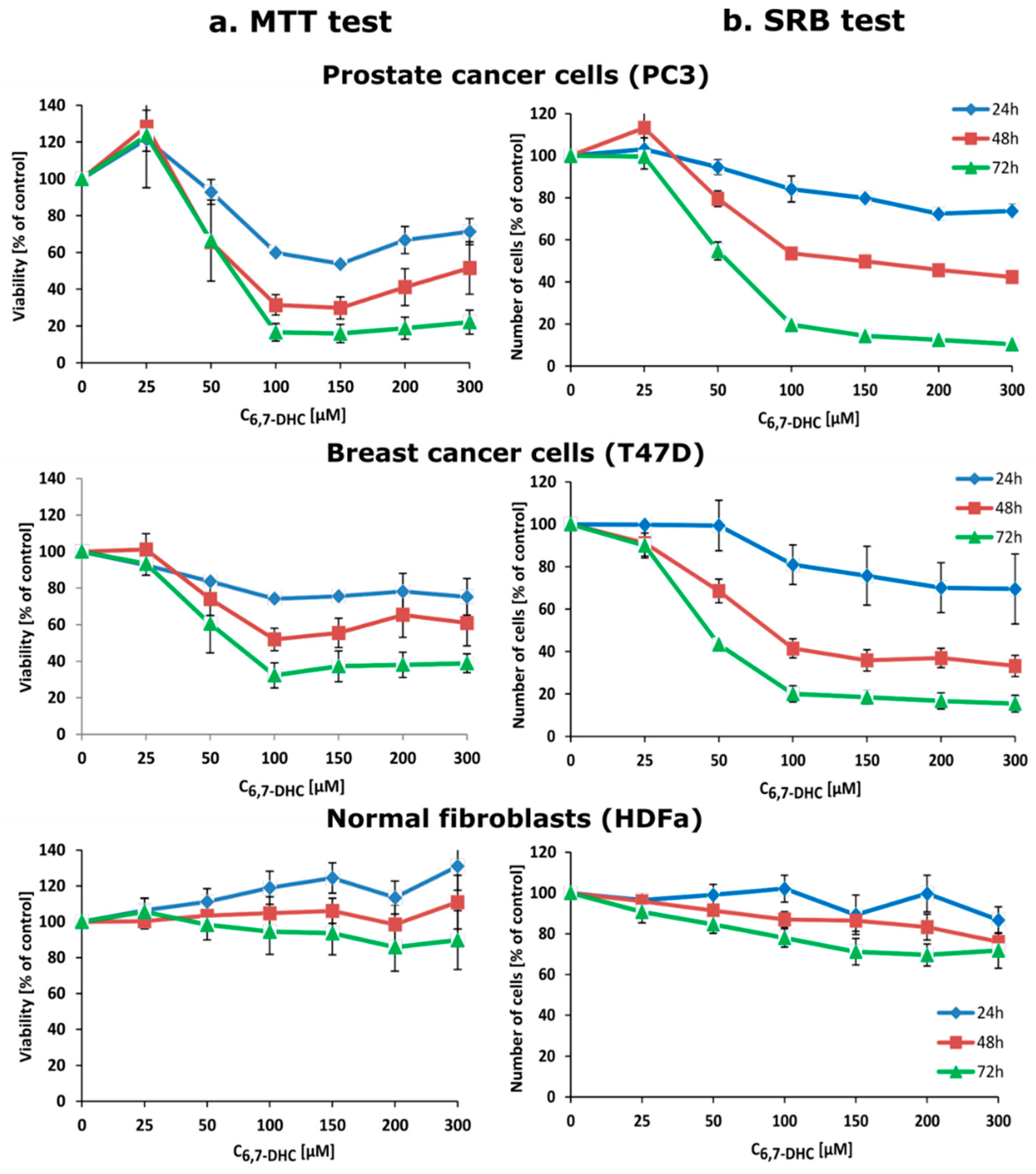
3.9. Cell Viability Assay
3.10. Indirect Analysis of Cell Number
3.11. Analysis of Cell Morphology in Phase Contrast Microscopy
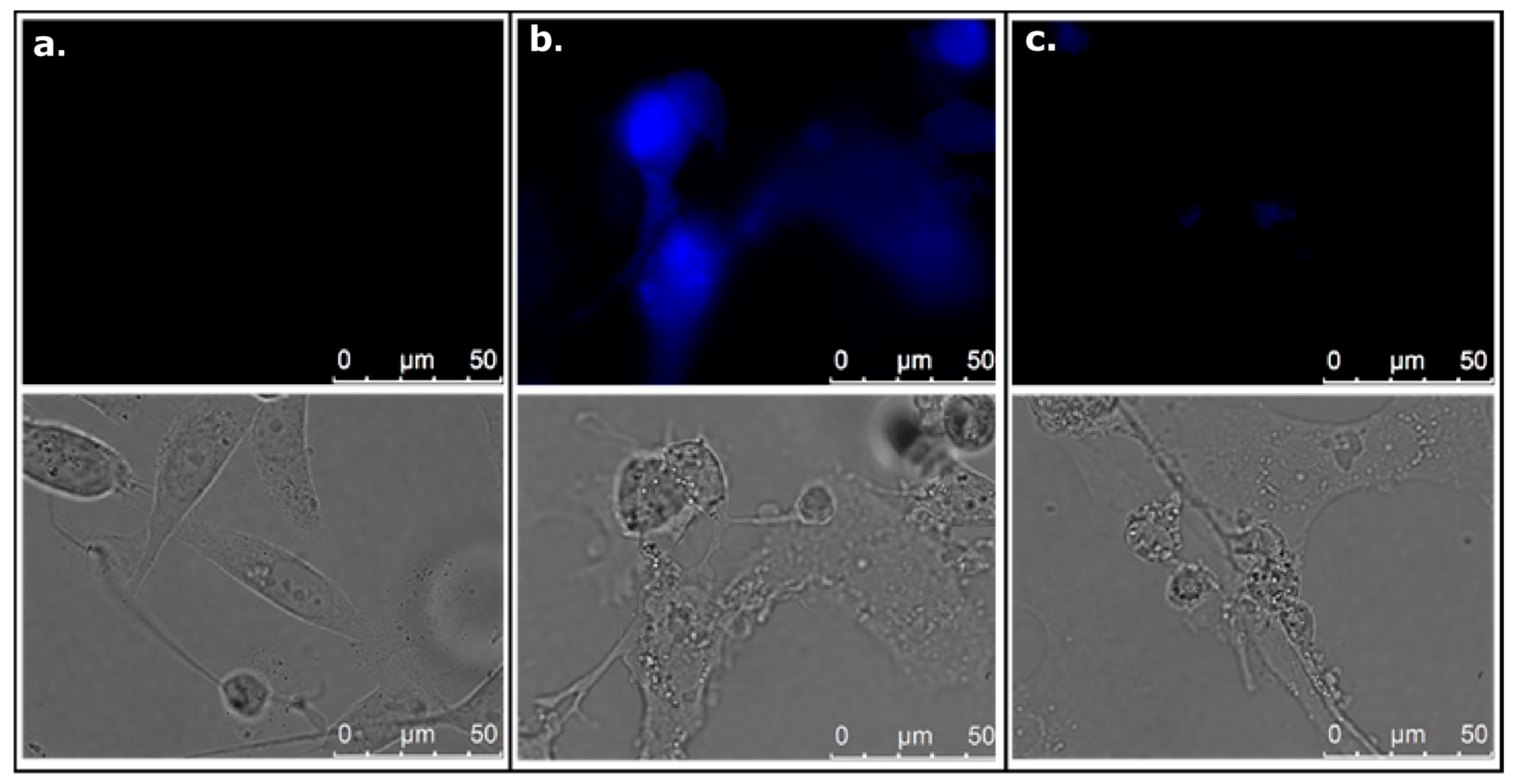
3.12. Analysis of Intracellular 6,7-Dihydroxycoumarin Localization Using Fluorescence Microscope
3.13. Impact of Cellular pH on 6,7-Dihydroxycoumarin Fluorescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Żamojć, K.; Zdrowowicz, M.; Rudnicki-Velasquez, P.B.; Krzymiński, K.; Zaborowski, B.; Niedziałkowski, P.; Jacewicz, D.; Chmurzyński, L. The development of 1,3-diphenylisobenzofuran as a highly selective probe for the detection and quantitative determination of hydrogen peroxide. Free Radic. Res. 2017, 51, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Giacosa, A.; Filiberti, R. Free radicals, oxidative damage and degenerative diseases. Eur. J. Cancer Prev. 1996, 5, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Żamojć, K.; Jacewicz, D.; Zdrowowicz, M.; Chmurzyński, L. Kinetics of the reaction between 1,3-diphenylisobenzofuran and nitrogen dioxide studied by steady-state fluorescence. Res. Chem. Intermed. 2013, 39, 3023–3031. [Google Scholar] [CrossRef]
- Marshall, D.L.; Christian, M.L.; Gryn’ova, G.; Coote, M.L.; Barker, P.J.; Blanksby, S.J. Oxidation of 4-substituted TEMPO derivatives reveals modifications at the 1- and 4-positions. Org. Biomol. Chem. 2011, 9, 4936–4947. [Google Scholar] [CrossRef] [PubMed]
- Chechik, V.; Ionita, G. Supramolecular complexes of spin-labelled cyclodextrins. Org. Biomol. Chem. 2006, 4, 3505–3510. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Ma, Y.; Loyns, C.; Price, P.; Rippon, D.; Chechik, V. Mechanistic insight into TEMPO-inhibited polymerisation: Simultaneous determination of oxygen and inhibitor concentrations by EPR. Org. Biomol. Chem. 2009, 7, 2685–2687. [Google Scholar] [CrossRef] [PubMed]
- Blinco, J.P.; Keddie, D.J.; Wade, T.; Barker, P.J.; George, G.A.; Bottle, S.E. Profluorescent nitroxides: Sensors and stabilizers of radical-mediated oxidative damage. Polym. Degrad. Stab. 2008, 93, 1613–1618. [Google Scholar] [CrossRef][Green Version]
- Brownlie, I.T.; Ingold, K.U. The inhibited autoxidation of styrene. Part VII. Inhibition by nitroxides and hydroxylamines. Can. J. Chem. 1967, 45, 2427–2432. [Google Scholar] [CrossRef]
- Rigo, A.; Argese, E.; Stevanato, R.; Orsega, E.F.; Viglino, P. A new method of detecting O2-production. Inorg. Chim. Acta 1977, 24, L71–L73. [Google Scholar] [CrossRef]
- Belkin, S.; Mehlhorn, R.J.; Packer, L. Determination of dissolved oxygen in photosynthetic systems by nitroxide spin-probe broadening. Arch. Biochem. Biophys. 1987, 252, 487–495. [Google Scholar] [CrossRef]
- Mehlhorn, R.J.; Packer, L. Electron spin resonance spin destruction methods for radical detection. Meth. Enzym. 1984, 105, 215–220. [Google Scholar] [PubMed]
- Offer, T.; Samuni, A. Nitroxides inhibit peroxyl radical-mediated DNA scission and enzyme inactivation. Free Radic. Biol. Med. 2002, 32, 872–881. [Google Scholar] [CrossRef]
- Aspee, A.; Maretti, L.; Scaiano, J.C. Monitoring photodecomposition of dibenzyl ketone within NaY zeolite with a pre-fluorescent nitroxide compound. Photochem. Photobiol. Sci. 2003, 2, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Aspée, A.; García, O.; Maretti, L.; Sastre, R.; Scaiano, J.C. Free radical reactions in poly(methyl methacrylate) films monitored using a prefluorescent quinoline-TEMPO sensor. Macromolecules 2003, 36, 3550–3556. [Google Scholar] [CrossRef]
- Samuni, A.; Krishna, C.M.; Mitchell, J.B.; Collins, C.R.; Russo, A. Superoxide reaction with nitroxides. Free Radic. Res. Commun. 1990, 9, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Pearlman, A. Chemistry and Antihypertensive Effects of Tempol and Other Nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef]
- Samuni, A.; Godinger, D.; Aronovitch, J.; Russo, A.; Mitchell, J.B. Nitroxides block DNA scission and protect cells from oxidative damage. Biochemistry 1991, 30, 555–561. [Google Scholar] [CrossRef]
- Hahn, S.M.; Wilson, L.; Krishna, C.M.; Liebmann, J.; DeGraff, W.; Gamson, J.; Samuni, A.; Venzon, D.; Mitchell, J.B. Identification of nitroxide radioprotectors. Radiat. Res. 1992, 132, 87–93. [Google Scholar] [CrossRef]
- Maurel, V.; Laferrière, M.; Billone, P.; Godin, R.; Scaiano, J.C. Free Radical Sensor Based on CdSe Quantum Dots with Added 4-Amino-2,2,6,6-Tetramethylpiperidine Oxide Functionality. J. Phys. Chem. B 2006, 110, 16353–16358. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Laferrière, M.; Galian, R.E.; Maurel, V.; Billone, P. Non-linear effects in the quenching of fluorescent semiconductor nanoparticles by paramagnetic species. Phys. Status Solidi 2006, 203, 1337–1343. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Industrial oxidations with organocatalyst TEMPO and its derivatives. Org. Proc. Res. Dev. 2009, 14, 245–251. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Lazzari, D.; Vitali, M.; Andrews, S.M. EPR Imaging Determination of High Molecular Weight Nitroxide Radicals in the UV Degradation of Polycarbonate-Poly(acrylonitrile-butadiene-styrene) Polymers. Macromol. Chem. Phys. 2002, 203, 2239–2244. [Google Scholar] [CrossRef]
- Blinco, J.P.; Fairfull-Smith, K.E.; Morrow, B.J.; Bottle, S.E. Profluorescent nitroxides as sensitive probes of oxidative change and free radical reactions. Aust. J. Chem. 2001, 64, 373–389. [Google Scholar] [CrossRef]
- Makino, Y.; Uchiyama, S.; Ohno, K.; Arakawa, H. Low-cost fluorimetric determination of radicals based on fluorogenic dimerization of the natural phenol sesamol. Anal. Chem. 2010, 82, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Jacewicz, D.; Zamojc, K.; Chmurzyński, L. Analytical Methods for Determination of ·NO and ·NO2 and their Applicability in Biological Studies. Curr. Pharm. Anal. 2012, 8, 115–134. [Google Scholar] [CrossRef]
- Żamojć, K.; Zdrowowicz, M.; Jacewicz, D.; Wyrzykowski, D.; Chmurzyński, L. Fluorescent and luminescent probes for monitoring hydroxyl radical under biological conditions. Crit. Rev. Anal. Chem. 2016, 46, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Żamojć, K.; Zdrowowicz, M.; Jacewicz, D.; Wyrzykowski, D.; Chmurzyński, L. Fluorescent probes used for detection of hydrogen peroxide under biological conditions. Crit. Rev. Anal. Chem. 2016, 46, 171–200. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Use of Fluorescence Probes for Detection of Reactive Nitrogen Species: A Review. J. Fluoresc. 2006, 16, 119–139. [Google Scholar] [CrossRef]
- Żamojć, K.; Zdrowowicz, M.; Wiczk, W.; Jacewicz, D.; Chmurzyński, L. Dihydroxycoumarins as highly selective fluorescent probes for the fast detection of 4-hydroxy-TEMPO in aqueous solution. RSC Adv. 2015, 5, 63807–63812. [Google Scholar] [CrossRef]
- Żamojć, K.; Jacewicz, D.; Chmurzyński, L. Quenching of fluorescence of polycyclic aromatic hydrocarbons by 4-OH-TEMPO. Anal. Lett. 2013, 46, 349–355. [Google Scholar]
- Żamojć, K.; Wiczk, W.; Chmurzyński, L. The influence of the type of substituents and the solvent on the interactions between different coumarins and selected TEMPO analogues—Fluorescence quenching studies. Chem. Phys. 2018, 513, 188–194. [Google Scholar] [CrossRef]
- Żamojć, K.; Wiczk, W.; Zaborowski, B.; Jacewicz, D.; Chmurzyński, L. Fluorescence quenching of 7-amino-4-methylcoumarin by different TEMPO derivatives. Spectrochim. Acta A 2015, 136, 1875–1880. [Google Scholar] [CrossRef]
- Żamojć, K.; Wiczk, W.; Zaborowski, B.; Makowski, M.; Pranczk, J.; Jacewicz, D.; Chmurzyński, L. Fluorescence quenching of fluoroquinolone antibiotics by 4-hydroxy-TEMPO in aqueous solution. Spectrochim. Acta A 2014, 133, 887–891. [Google Scholar] [CrossRef]
- Izumi, H.; Torigoe, T.; Ishiguchi, H.; Uramoto, H.; Yoshida, Y.; Tanabe, M.; Ise, T.; Murakami, T.; Yoshida, T.; Nomoto, M.; et al. Cellular pH regulators: Potentially promising molecular targets for cancer chemotherapy. Cancer Treat. Rev. 2003, 29, 541–549. [Google Scholar] [CrossRef]
- Boscoboinik, D.; Gupta, R.; Epand, R. Investigation of the relationship between altered intracellular pH and multidrug resistance in mammalian cells. Br. J. Cancer 1990, 61, 568–572. [Google Scholar] [CrossRef]
- Wojtkowiak, J.W.; Verduzco, D.; Schramm, K.J.; Gillies, R.J. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol. Pharm. 2011, 8, 2032–2038. [Google Scholar] [CrossRef]
- Wong, P.; Lee, C.; Tannock, I.F. Reduction of Intracellular pH as a Strategy to Enhance the pH-Dependent Cytotoxic Effects of Melphalan for Human Breast Cancer Cells. Clin. Cancer Res. 2005, 11, 3553–3557. [Google Scholar] [CrossRef]
- Abu-Eittah, R.H.; El-Tawil, B.A.H. The electronic absorption spectra of some coumarins. A molecular orbital treatment. Can. J. Chem. 1985, 63, 1173–1179. [Google Scholar] [CrossRef]
- Goodwin, R.H.; Kavanagh, F. The Isolation of Scopoletin, a Blue-Fluorescing Compound from Oat Roots. Bull. Torrey Bot. Club 1949, 76, 255. [Google Scholar] [CrossRef]
- Traven, V.F.; Vorobjeva, L.I.; Chibisova, T.A.; Carberry, E.A.; Beyer, N.J. Electronic absorption spectra and structure of hydroxycoumarin derivatives and their ionized forms. Can. J. Chem. 1997, 75, 365–376. [Google Scholar] [CrossRef]
- Ito, T.; Yokoyama, H.; Ogata, T. Determination of absolute concentration of nitroxide radical by radio-frequency EPR imaging. Appl. Magn. Reson. 2001, 20, 257–263. [Google Scholar] [CrossRef]
- Yokoyama, H.; Sato, T.; Oteki, T.; Ohya, H.; Akatsuka, T. Estimation of the in vivo decay rate of EPR signals for a nitroxide radical in rat brains by a region-selected intensity determination method. Appl. Magn. Reson. 2005, 29, 363–373. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free Radical Scavenging by Natural Polyphenols: Atom versus Electron Transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Owenius, R.; Engström, M.; Lindgren, M.; Huber, M. Influence of Solvent Polarity and Hydrogen Bonding on the EPR Parameters of a Nitroxide Spin Label Studied by 9-GHz and 95-GHz EPR Spectroscopy and DFT Calculations. J. Phys. Chem. A 2001, 105, 10967–10977. [Google Scholar] [CrossRef]
- Knauer, B.R.; Napier, J.J. The nitrogen hyperfine splitting constant of the nitroxide functional group as a solvent polarity parameter. The relative importance for a solvent polarity parameter of its being a cybotactic probe vs. its being a model process. J. Am. Chem. Soc. 1976, 98, 4395–4400. [Google Scholar] [CrossRef]
- Jerzykiewicz, M.; Ćwieląg-Piasecka, I.; Witwicki, M.; Jezierski, A. EPR spin trapping and DFT studies on structure of active antioxidants in bioglycerol. Chem. Phys. Lett. 2010, 497, 135–141. [Google Scholar] [CrossRef]
- MacMillan, F.; Lendzian, F.; Lubitz, W. EPR and ENDOR characterization of semiquinone anion radicals related to photosynthesis. Magn. Reson. Chem. 1995, 33, S81–S93. [Google Scholar] [CrossRef]
- Burghaus, O.; Plato, M.; Rohrer, M.; Mobius, K. 3-mm High-field EPR on semiquinone radical anions Q·– related to photosynthesis and on the primary donor P·+ and acceptor QA·– in reaction centers of Rhodobacter sphaeroides R-26. J. Phys. Chem. 1993, 97, 7639–7647. [Google Scholar] [CrossRef]
- Witwicki, M.; Jezierska, J.; Ozarowski, A. Solvent effect on EPR, molecular and electronic properties of semiquinone radical derived from 3,4-dihydroxybenzoic acid as model for humic acid transient radicals: High-field EPR and DFT studies. Chem. Phys. Lett. 2009, 473, 160–166. [Google Scholar] [CrossRef]
- Taguchi, A.T.; O’Malley, P.J.; Wraight, C.A.; Dikanov, S.A. Hydrogen bond network around the semiquinone of the secondary quinone acceptor QB in bacterial photosynthetic reaction centers. J. Phys. Chem. B 2015, 119, 5805–5814. [Google Scholar] [CrossRef]
- Likhtenshtein, G.I.; Yamauchi, J.; Nakatsuji, S.I.; Smirnov, A.I.; Tamura, R. Nitroxides: Applications in Chemistry, Biomedicine, and Materials Science; John Wiley & Sons: Weinheim, Germany, 2008. [Google Scholar]
- Lewińska, A.; Frąckowiak, R.; Witwicki, M.; Jezierski, A.; Wilk, K.A. Experimental and Theoretical Approach to Aggregation Behavior of New Di- N -Oxide Surfactants in an Aquatic Environment. J. Phys. Chem. B 2012, 116, 14324–14332. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: Baltimore, MD, USA, 2013. [Google Scholar]
- Brandariz, I.; Barriada, J.; Vilarino, T.; de Vicente, M.E.S. Comparison of several calibration procedures for glass electrodes in proton concentration. Mon. Chem. 2004, 135, 1475–1488. [Google Scholar] [CrossRef]
- Tesmar, A.; Wyrzykowski, D.; Jacewicz, D.; Żamojć, K.; Pranczk, J.; Chmurzynski, L. Buffer contribution to formation enthalpy of copper(II)–bicine complex determined by isothermal titration calorimetry method. J. Therm. Anal. Calorim. 2016, 126, 97–102. [Google Scholar] [CrossRef]
- Kostrowicki, J.; Liwo, A. A general method for the determination of the stoichiometry of unknown species in multicomponent systems from physicochemical measurements. Comput. Chem. 1987, 11, 195–210. [Google Scholar] [CrossRef]
- Kostrowicki, J. Determination of equilibrium parameters by minimization of an extended sum of squares. Talanta 1990, 37, 645–650. [Google Scholar] [CrossRef]
- Wiczk, A.; Hofman, D.; Konopa, G.; Herman-Antosiewicz, A. Sulforaphane, a cruciferous vegetable-derived isothiocyanate, inhibits protein synthesis in human prostate cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1295–1305. [Google Scholar] [CrossRef]
- Pawlik, A.; Słomińska-Wojewódzka, M.; Herman-Antosiewicz, A. Sensitization of estrogen receptor-positive breast cancer cell lines to 4-hydroxytamoxifen by isothiocyanates present in cruciferous plants. Eur. J. Nutr. 2016, 55, 1165–1180. [Google Scholar] [CrossRef]
- Hać, A.; Domachowska, A.; Narajczyk, M.; Cyske, K.; Pawlik, A.; Herman-Antosiewicz, A. S6K1 controls autophagosome maturation in autophagy induced by sulforaphane or serum deprivation. Eur. J. Cell Boil. 2015, 94, 470–481. [Google Scholar] [CrossRef]
- Pawlik, A.; Wała, M.; Hać, A.; Felczykowska, A.; Herman-Antosiewicz, A. Sulforaphene, an isothiocyanate present in radish plants, inhibits proliferation of human breast cancer cells. Phytomedicine 2017, 29, 1–10. [Google Scholar] [CrossRef]
- Berezhnov, A.V.; Soutar, M.P.M.; Fedotova, E.I.; Frolova, M.S.; Plun-Favreau, H.; Zinchenko, V.P.; Abramov, A.Y. Intracellular pH Modulates Autophagy and Mitophagy. J. Boil. Chem. 2016, 291, 8701–8708. [Google Scholar] [CrossRef]


| Method | Fluorescence Spectroscopy (for 6,7-Dihydroxycoumarin) | UV-Vis Spectroscopy | |
|---|---|---|---|
| Validation Parameter | |||
| Range of quantitation (M) | 5 × 10−8–5 × 10−5 | 2.5 × 10−3–5 × 10−2 | |
| Coefficient of determination (R2) | 0.9912 | 0.9986 | |
| Coefficient of variation CV (%) | 2.56 | 2.87 | |
| Accuracy (%) | 92–106 | 92–105 | |
| Limit of detection (LOD) (M) | 1.67 × 10−8 | 0.833 × 10−3 | |
| Limit of quantitation (LOQ) (M) | 5 × 10−8 | 2.5 × 10−3 | |
| Coumarin Derivative | λex (nm) | λem (nm) |
|---|---|---|
| 6,7-dihydroxycoumarin | 350 | 467 |
| 7,8-dihydroxy-6-methoxycoumarin | 380 | 517 |
| 5,7-dihydroxy-4-methylcoumarin | 330 | 457 |
| 7,8-dihydroxy-4-phenylcoumarin | 340 | 510 |
| 5,7-dihydroxy-4-phenylcoumarin | 360 | 460 |
| 7,8-dihydroxy-4-methylcoumarin | 330 | 528 |
| 3-[4-(bromomethyl)phenyl]-7-(diethylamino)coumarin | 410 | 492 |
| 3-acetylcoumarin | 370 | 458 |
| 3-chlorocoumarin | 330 | 442 |
| 7-hydroxycoumarin | 360 | 456 |
| 7-hydroxy-4-methylcoumarin | 340 | 451 |
| 7-diethylamino-4-methylcoumarin | 400 | 470 |
| 7-ethoxy-4-methylcoumarin | 330 | 387 |
| 7-methoxycoumarin | 340 | 394 |
| 5-methoxypsoralene | 320 | 455 |
| 7-hydroxy-4-methyl-3-coumarinylacetic acid | 350 | 456 |
| 7,8-dihydroxycoumarin | 330 | 450 |
| 4,7-dihydroxycoumarin | 310 | 418 |
| 6-methoxy-7-hydroxycoumarin | 350 | 462 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żamojć, K.; Zdrowowicz, M.; Hać, A.; Witwicki, M.; Rudnicki-Velasquez, P.B.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L. Dihydroxy-Substituted Coumarins as Fluorescent Probes for Nanomolar-Level Detection of the 4-Amino-TEMPO Spin Label. Int. J. Mol. Sci. 2019, 20, 3802. https://doi.org/10.3390/ijms20153802
Żamojć K, Zdrowowicz M, Hać A, Witwicki M, Rudnicki-Velasquez PB, Wyrzykowski D, Wiczk W, Chmurzyński L. Dihydroxy-Substituted Coumarins as Fluorescent Probes for Nanomolar-Level Detection of the 4-Amino-TEMPO Spin Label. International Journal of Molecular Sciences. 2019; 20(15):3802. https://doi.org/10.3390/ijms20153802
Chicago/Turabian StyleŻamojć, Krzysztof, Magdalena Zdrowowicz, Aleksandra Hać, Maciej Witwicki, Paweł Błażej Rudnicki-Velasquez, Dariusz Wyrzykowski, Wiesław Wiczk, and Lech Chmurzyński. 2019. "Dihydroxy-Substituted Coumarins as Fluorescent Probes for Nanomolar-Level Detection of the 4-Amino-TEMPO Spin Label" International Journal of Molecular Sciences 20, no. 15: 3802. https://doi.org/10.3390/ijms20153802
APA StyleŻamojć, K., Zdrowowicz, M., Hać, A., Witwicki, M., Rudnicki-Velasquez, P. B., Wyrzykowski, D., Wiczk, W., & Chmurzyński, L. (2019). Dihydroxy-Substituted Coumarins as Fluorescent Probes for Nanomolar-Level Detection of the 4-Amino-TEMPO Spin Label. International Journal of Molecular Sciences, 20(15), 3802. https://doi.org/10.3390/ijms20153802