Simultaneous Ligand and Receptor Tracking through NMR Spectroscopy Enabled by Distinct 19F Labels
Abstract
:1. Introduction
2. Results
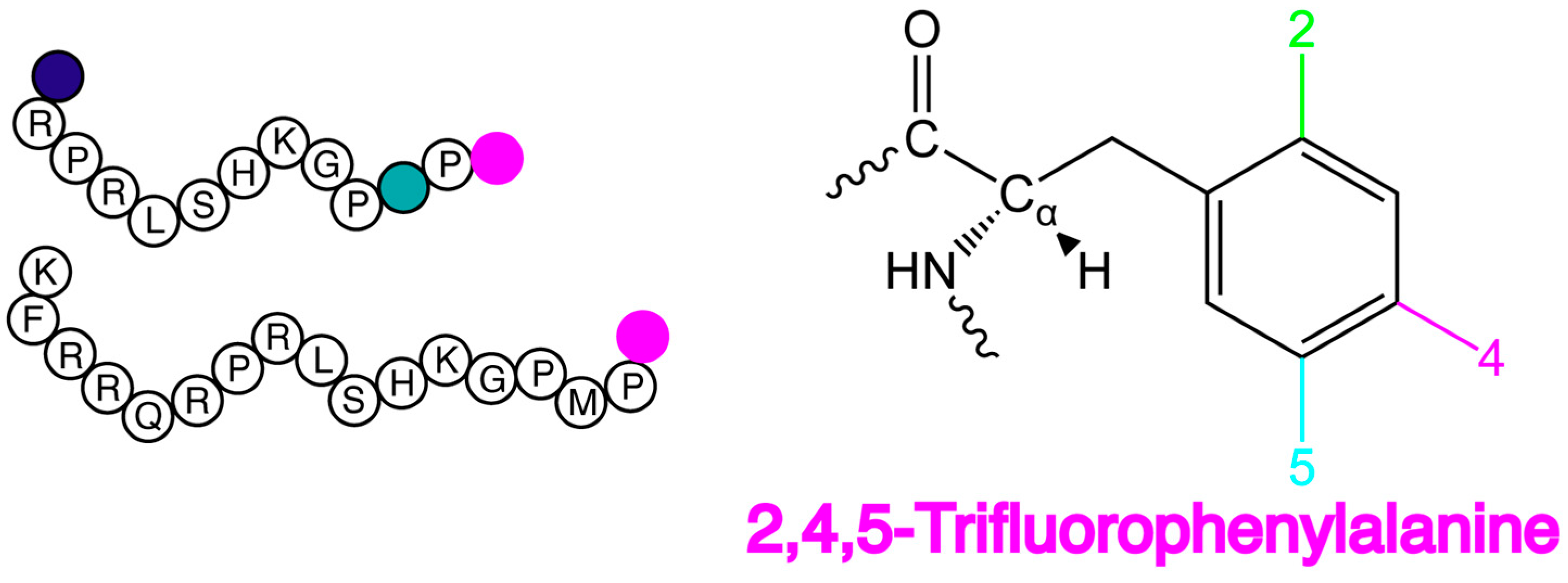
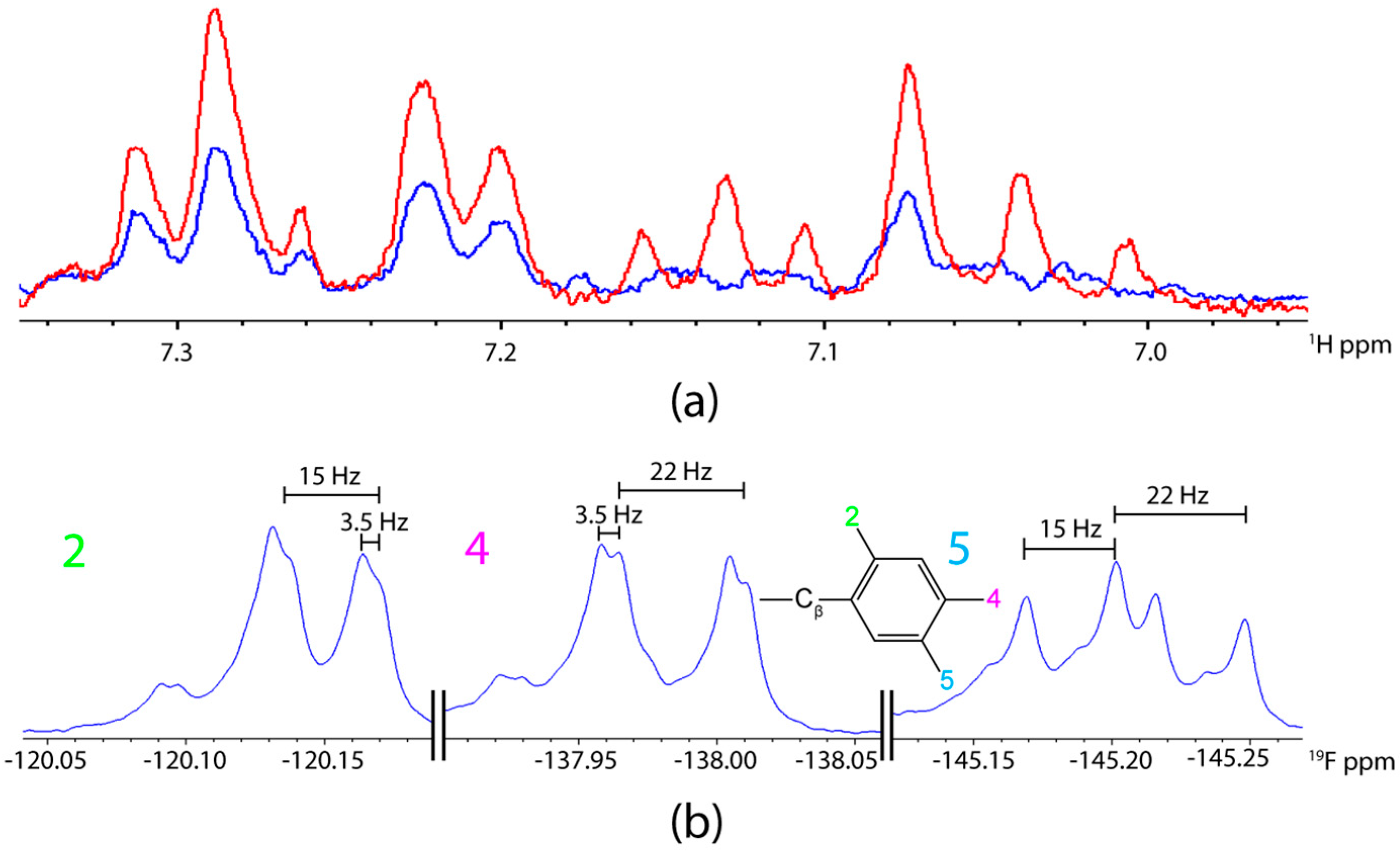
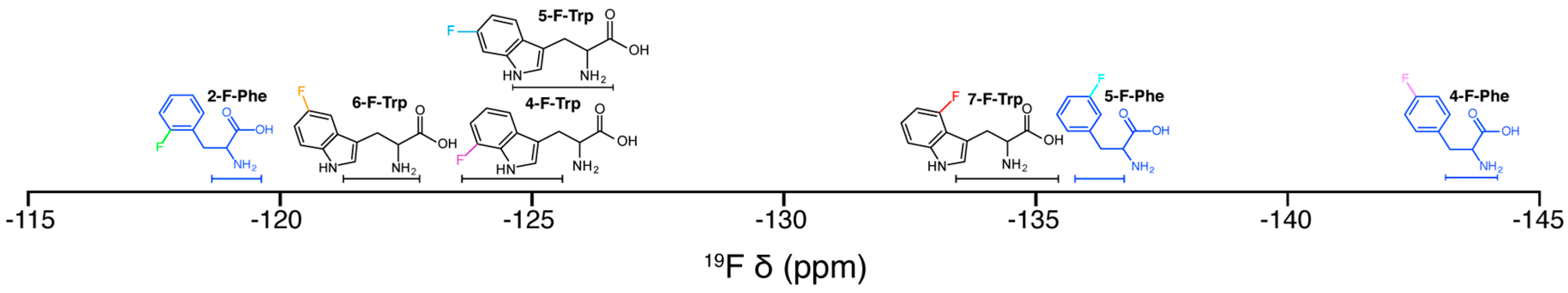
2.1. F NMR Spectroscopic Behaviour of 2,4,5F-Phe
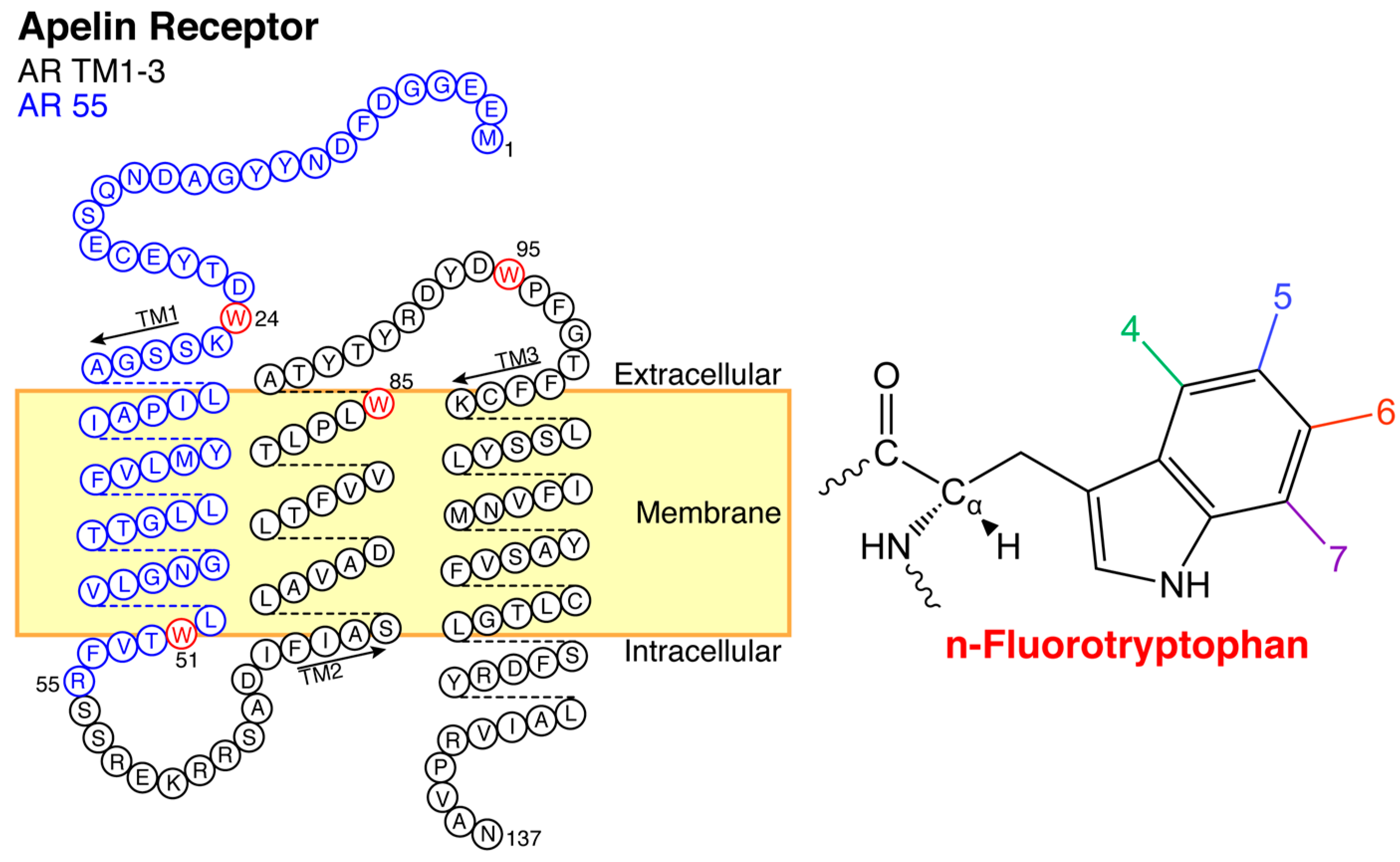
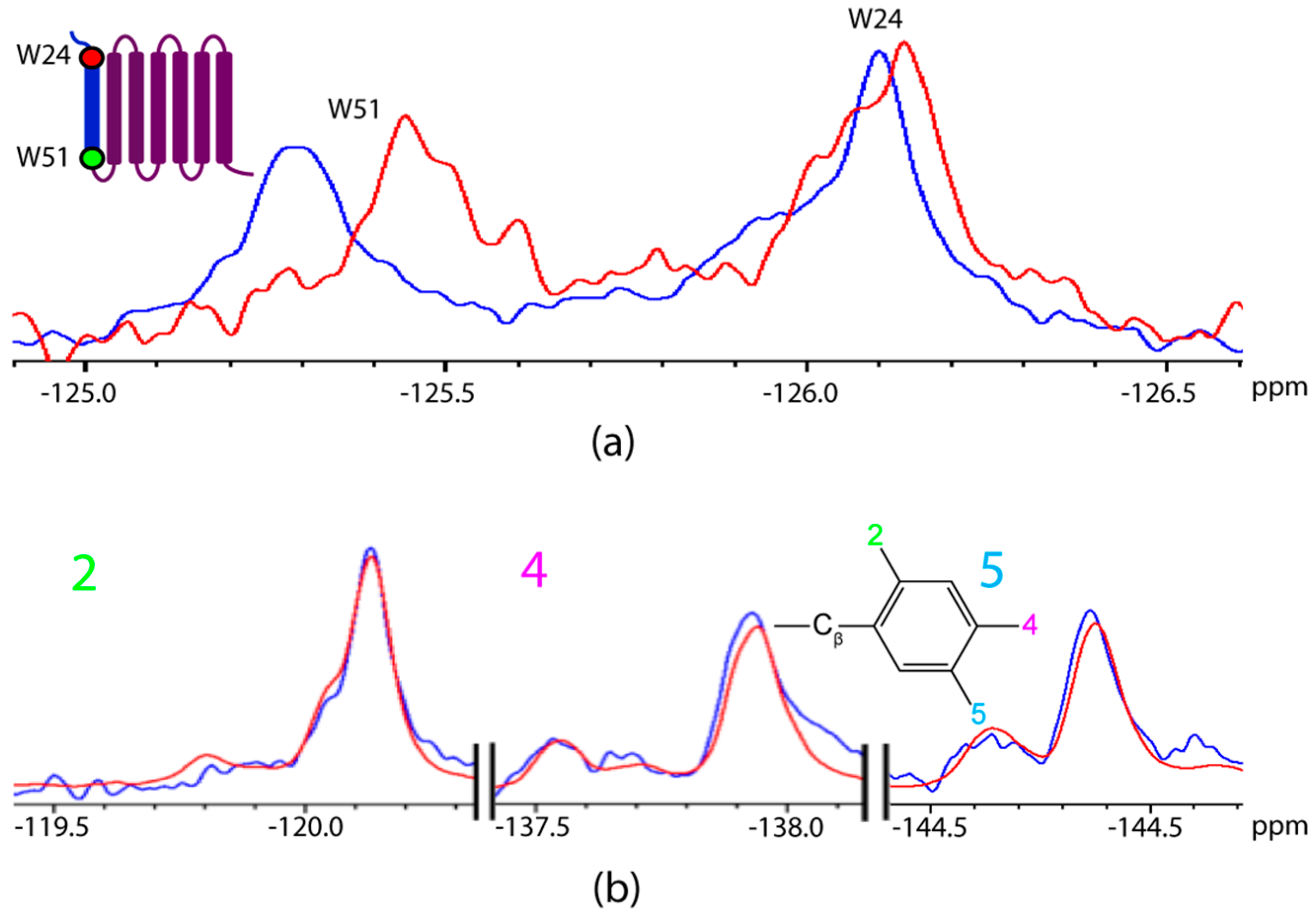
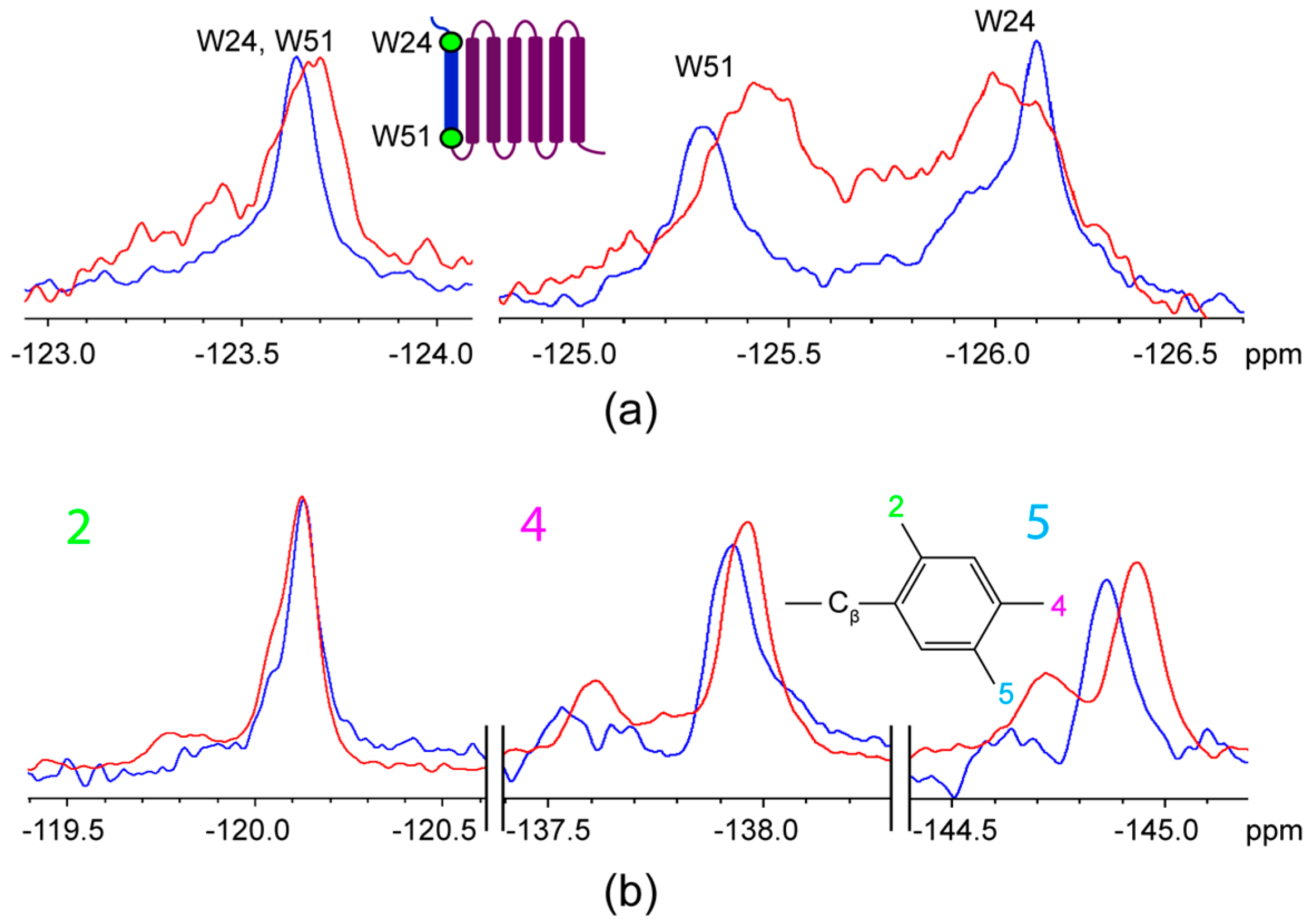
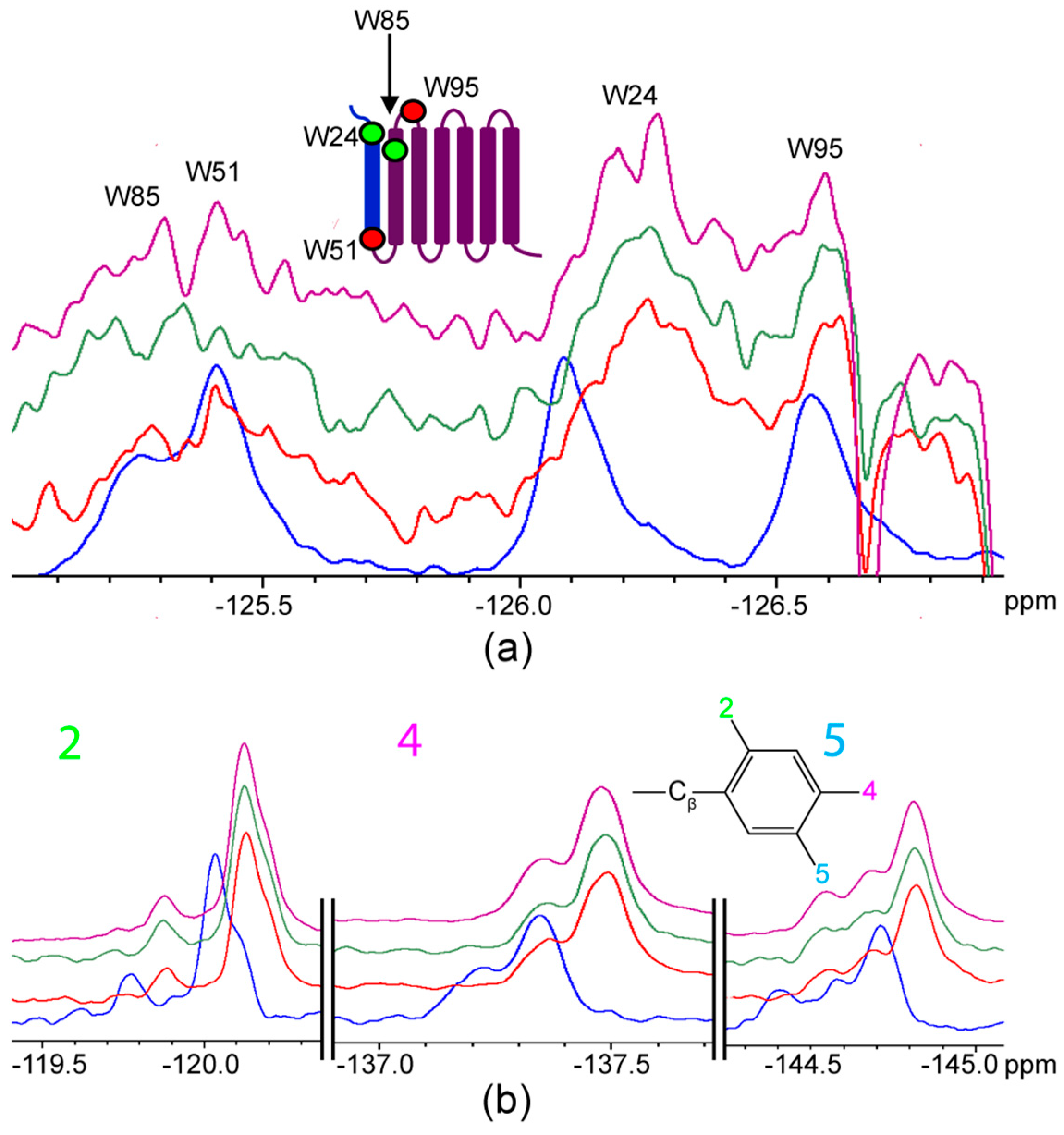
2.2. Titration of AR Fragments with F*-ap-13
3. Discussion
4. Materials and Methods
4.1. Apelin Receptor Fragments
4.2. F*-ap-13 and F*-ap-17 Synthesis and Purification
4.3. 19F NMR Spectroscopy
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AR | Apelin receptor (APJ) |
| AR55 | apelin receptor fragment comprising N-terminal tail and first transmembrane segment |
| DPC | Dodecylphosphocholine |
| GPCR | G-protein-coupled receptor |
| NMR | Nuclear magnetic resonance |
| RP-HPLC | Reverse phase high performance liquid chromatography |
| SDS | Sodium dodecyl sulphate |
| TFA | Trifluoroacetic acid |
| TM | Transmembrane |
| TM1-3 | apelin receptor fragment comprising N-terminal tail and first three transmembrane segments |
References
- Chapman, N.A.; Dupre, D.J.; Rainey, J.K. The apelin receptor: Physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem. Cell Biol. 2014, 92, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Kenward, C.; Rainey, J.K. Apelinergic system structure and function. Compr. Physiol. 2018, 8, 407–450. [Google Scholar] [CrossRef]
- O’Dowd, B.F.; Heiber, M.; Chan, A.; Heng, H.H.; Tsui, L.C.; Kennedy, J.L.; Shi, X.; Petronis, A.; George, S.R.; Nguyen, T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993, 136, 355–360. [Google Scholar] [CrossRef]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Chng, S.C.; Ho, L.; Tian, J.; Reversade, B. ELABELA: A hormone essential for heart development signals via the apelin receptor. Dev. Cell 2013, 27, 672–680. [Google Scholar] [CrossRef]
- Pauli, A.; Norris, M.L.; Valen, E.; Chew, G.L.; Gagnon, J.A.; Zimmerman, S.; Mitchell, A.; Ma, J.; Dubrulle, J.; Reyon, D.; et al. Toddler: An embryonic signal that promotes cell movement via apelin receptors. Science 2014, 343, 746. [Google Scholar] [CrossRef] [PubMed]
- Szokodi, I.; Tavi, P.; Foldes, G.; Voutilainen-Myllyla, S.; Ilves, M.; Tokola, H.; Pikkarainen, S.; Piuhola, J.; Rysa, J.; Toth, M.; et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 2002, 91, 434–440. [Google Scholar] [CrossRef]
- Mesmin, C.; Fenaille, F.; Becher, F.; Tabet, J.C.; Ezan, E. Identification and characterization of apelin peptides in bovine colostrum and milk by liquid chromatography-mass spectrometry. J. Proteome Res. 2011, 10, 5222–5231. [Google Scholar] [CrossRef]
- Shin, K.; Chapman, N.A.; Sarker, M.; Kenward, C.; Huang, S.K.; Weatherbee-Martin, N.; Pandey, A.; Dupre, D.J.; Rainey, J.K. Bioactivity of the putative apelin proprotein expands the repertoire of apelin receptor ligands. Biochim. Biophys. Acta 2017, 1861, 1901–1912. [Google Scholar] [CrossRef]
- Huang, S.K.; Shin, K.; Sarker, M.; Rainey, J.K. Apela exhibits isoform- and headgroup-dependent modulation of micelle binding, peptide conformation and dynamics. Biochim. Biophys. Acta 2017, 1859, 767–778. [Google Scholar] [CrossRef]
- Langelaan, D.N.; Bebbington, E.M.; Reddy, T.; Rainey, J.K. Structural insight into G-protein coupled receptor binding by apelin. Biochemistry 2009, 48, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Langelaan, D.N.; Rainey, J.K. Headgroup-dependent membrane catalysis of apelin-receptor interactions is likely. J. Phys. Chem. B 2009, 113, 10465–10471. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Sarker, M.; Huang, S.K.; Rainey, J.K. Apelin conformational and binding equilibria upon micelle interaction primarily depend on membrane-mimetic headgroup. Sci. Rep. 2017, 7, 15433. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Speckert, M.; Rainey, J.K. Bicelle composition-dependent modulation of phospholipid dynamics by apelin peptides. Biochem. Cell Biol. 2019, 97, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Medhurst, A.D.; Jennings, C.A.; Robbins, M.J.; Davis, R.P.; Ellis, C.; Winborn, K.Y.; Lawrie, K.W.; Hervieu, G.; Riley, G.; Bolaky, J.E.; et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 2003, 84, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Ceraudo, E.; Galanth, C.; Carpentier, E.; Banegas-Font, I.; Schonegge, A.M.; Alvear-Perez, R.; Iturrioz, X.; Bouvier, M.; Llorens-Cortes, C. Biased signaling favoring gi over beta-arrestin promoted by an apelin fragment lacking the C-terminal phenylalanine. J. Biol. Chem. 2014, 289, 24599–24610. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Kuc, R.E.; Brame, A.L.; Dyson, A.; Singer, M.; Glen, R.C.; Cheriyan, J.; Wilkinson, I.B.; Davenport, A.P.; Maguire, J.J. [Pyr(1)]Apelin-13(1-12) is a biologically active ACE2 metabolite of the endogenous cardiovascular peptide [Pyr(1)]Apelin-13. Front. Neurosci. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Saldivia, V.R.; Nguyen, T.; Cheng, R.; George, S.R.; O’Dowd, B.F. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology 2005, 146, 231–236. [Google Scholar] [CrossRef]
- Murza, A.; Besserer-Offroy, E.; Cote, J.; Berube, P.; Longpre, J.M.; Dumaine, R.; Lesur, O.; Auger-Messier, M.; Leduc, R.; Sarret, P.; et al. C-Terminal modifications of apelin-13 significantly change ligand binding, receptor signaling, and hypotensive action. J. Med. Chem. 2015, 58, 2431–2440. [Google Scholar] [CrossRef]
- Murza, A.; Parent, A.; Besserer-Offroy, E.; Tremblay, H.; Karadereye, F.; Beaudet, N.; Leduc, R.; Sarret, P.; Marsault, E. Elucidation of the structure-activity relationships of apelin: Influence of unnatural amino acids on binding, signaling, and plasma stability. ChemMedChem 2012, 7, 318–325. [Google Scholar] [CrossRef]
- Langelaan, D.N.; Reddy, T.; Banks, A.W.; Dellaire, G.; Dupre, D.J.; Rainey, J.K. Structural features of the apelin receptor N-terminal tail and first transmembrane segment implicated in ligand binding and receptor trafficking. Biochim. Biophys. Acta 2013, 1828, 1471–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langelaan, D.N.; Pandey, A.; Sarker, M.; Rainey, J.K. Preserved transmembrane segment topology, structure, and dynamics in disparate micellar environments. J. Phys. Chem. Lett. 2017, 8, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yue, Y.; Ma, Y.; Zhang, Q.; Zhou, Q.; Song, Y.; Shen, Y.; Li, X.; Ma, X.; Li, C.; et al. Structural basis for apelin control of the human apelin receptor. Structure 2017, 25, 858–866.E4. [Google Scholar] [CrossRef] [PubMed]
- Kitevski-LeBlanc, J.L.; Prosser, R.S. Current applications of 19F NMR to studies of protein structure and dynamics. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 62, 1–33. [Google Scholar] [CrossRef]
- Costa, J.L.; Dobson, C.M.; Kirk, K.L.; Poulsen, F.M.; Valeri, C.R.; Vecchione, J.J. Studies of human platelets by 19F and 31P NMR. FEBS Lett. 1979, 99, 141–146. [Google Scholar] [CrossRef]
- Klein-Seetharaman, J.; Getmanova, E.V.; Loewen, M.C.; Reeves, P.J.; Khorana, H.G. NMR spectroscopy in studies of light-induced structural changes in mammalian rhodopsin: Applicability of solution 19F NMR. Proc. Natl. Acad. Sci. USA 1999, 96, 13744–13749. [Google Scholar] [CrossRef]
- Liu, J.J.; Horst, R.; Katritch, V.; Stevens, R.C.; Wuthrich, K. Biased signaling pathways in b2-adrenergic receptor characterized by 19F-NMR. Science 2012, 335, 1106–1110. [Google Scholar] [CrossRef]
- Manglik, A.; Kim, T.H.; Masureel, M.; Altenbach, C.; Yang, Z.; Hilger, D.; Lerch, M.T.; Kobilka, T.S.; Thian, F.S.; Hubbell, W.L.; et al. Structural insights into the dynamic process of b2-adrenergic receptor signaling. Cell 2015, 161, 1101–1111. [Google Scholar] [CrossRef]
- Nygaard, R.; Zou, Y.; Dror, R.O.; Mildorf, T.J.; Arlow, D.H.; Manglik, A.; Pan, A.C.; Liu, C.W.; Fung, J.J.; Bokoch, M.P.; et al. The dynamic process of b2-adrenergic receptor activation. Cell 2013, 152, 532–542. [Google Scholar] [CrossRef]
- Susac, L.; Eddy, M.T.; Didenko, T.; Stevens, R.C.; Wuthrich, K. A2A adenosine receptor functional states characterized by 19F-NMR. Proc. Natl. Acad. Sci. USA 2018, 115, 12733–12738. [Google Scholar] [CrossRef]
- Ye, L.; Neale, C.; Sljoka, A.; Lyda, B.; Pichugin, D.; Tsuchimura, N.; Larda, S.T.; Pomes, R.; Garcia, A.E.; Ernst, O.P.; et al. Mechanistic insights into allosteric regulation of the A2A adenosine G protein-coupled receptor by physiological cations. Nat. Commun. 2018, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Van Eps, N.; Zimmer, M.; Ernst, O.P.; Prosser, R.S. Activation of the A2A adenosine G-protein-coupled receptor by conformational selection. Nature 2016, 533, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Kenward, K.; Shin, K.; Rainey, J.K. Mixed fluorotryptophan substitutions at the same residue expand the versatility of 19F protein NMR spectroscopy. Chem. Eur. J. 2018, 24, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Crowley, P.B.; Kyne, C.; Monteith, W.B. Simple and inexpensive incorporation of 19F-tryptophan for protein NMR spectroscopy. Chem. Commun. 2012, 48, 10681–10683. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Sarker, M.; Liu, X.Q.; Rainey, J.K. Small expression tags enhance bacterial expression of the first three transmembrane segments of the apelin receptor. Biochem. Cell Biol. 2014, 92, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, W.E.; Sykes, B.D. Fluorine-19 nuclear magnetic resonance study of fluorotyrosine alkaline phosphatase: The influence of zinc on protein structure and a conformational change induced by phosphate binding. Biochemistry 1976, 15, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J.W.; Phillips, L.; Wray, V. Fluorine coupling constants. Prog. NMR Spectrosc. 1976, 10, 83–756. [Google Scholar] [CrossRef]
- Matei, E.; Gronenborn, A.M. 19F paramagnetic relaxation rnhancement: A valuable tool for distance measurements in proteins. Angew. Chem. Int. Ed. Engl. 2016, 55, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Rule, G.S.; Pratt, E.A.; Simplaceanu, V.; Ho, C. Nuclear magnetic resonance and molecular genetic studies of the membrane-bound D-lactate dehydrogenase of Escherichia coli. Biochemistry 1987, 26, 549–556. [Google Scholar] [CrossRef]
- Sarker, M.; Orrell, K.E.; Xu, L.; Tremblay, M.L.; Bak, J.J.; Liu, X.Q.; Rainey, J.K. Tracking transitions in spider wrapping silk conformation and dynamics by 19F nuclear magnetic resonance spectroscopy. Biochemistry 2016, 55, 3048–3059. [Google Scholar] [CrossRef]
- Iturrioz, X.; Gerbier, R.; Leroux, V.; Alvear-Perez, R.; Maigret, B.; Llorens-Cortes, C. By interacting with the C-terminal Phe of apelin, Phe255 and Trp259 in helix VI of the apelin receptor are critical for internalization. J. Biol. Chem. 2010, 285, 32627–32637. [Google Scholar] [CrossRef] [PubMed]
- Bordag, N.; Keller, S. Alpha-Helical transmembrane peptides: A “Divide and Conquer” approach to membrane proteins. Chem. Phys. Lipids 2010, 163, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhang, X.; Fan, X.; Argyris, E.; Fang, J.; Acheampong, E.; DuBois, G.C.; Pomerantz, R.J. The N-terminal domain of APJ, a CNS-based coreceptor for HIV-1, is essential for its receptor function and coreceptor activity. Virology 2003, 317, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.L.; Rashid, S.; Wajda, B.; Wolmarans, A.; LaPointe, P.; Spyracopoulos, L. The Hsp90 chaperone: 1H and 19F dynamic nuclear magnetic resonance spectroscopy reveals a perfect enzyme. Biochemistry 2019, 58, 1869–1877. [Google Scholar] [CrossRef]
- Boeszoermenyi, A.; Chhabra, S.; Dubey, A.; Radeva, D.L.; Burdzhiev, N.T.; Chanev, C.D.; Petrov, O.I.; Gelev, V.M.; Zhang, M.; Anklin, C.; et al. Aromatic 19F-13C TROSY: A background-free approach to probe biomolecular structure, function, and dynamics. Nat. Methods 2019, 16, 333–340. [Google Scholar] [CrossRef]
- Marley, J.; Lu, M.; Bracken, C. A method for efficient isotopic labeling of recombinant proteins. J. Biomol. NMR 2001, 20, 71–75. [Google Scholar] [CrossRef]

| Δδ (ppm) | |||
|---|---|---|---|
| Species | Location | 1:1 ligand:receptor | 1:4 ligand:receptor |
| 4F-Trp AR55 | W24 | −0.03 a | 0.11 |
| 4F-Trp AR55 | W51 | −0.15–0.3 b | −0.13 |
| 6F-Trp AR55 | W24/W51 | - | 0.05 c |
| F*-ap-13 | 2F on Phe | 0 | 0.01 |
| F*-ap-13 | 4F on Phe | −0.02 | −0.04 |
| F*-ap-13 | 5F on Phe | −0.01 | −0.07 |
| Δδ (ppm) | ||||
|---|---|---|---|---|
| Species | Location | 1:2 ligand:receptor | 1:1 ligand:receptor | 2:1 ligand:receptor |
| 4F-Trp TM1-3 | W24 | −0.17 | −0.16 | −0.16 |
| 4F-Trp TM1-3 | W51 | 0 | 0.07 | 0 |
| 4F-Trp TM1-3 | W85 | −0.02 | 0.06 | −0.04 |
| 4F-Trp TM1-3 | W95 | −0.04 | −0.04 | −0.03 |
| F*-ap-13 | 2F on Phe | −0.10 | −0.09 | −0.9 |
| F*-ap-13 | 4F on Phe | 0.14 | 0.14 | 0.13 |
| F*-ap-13 | 5F on Phe | −0.11 | −0.10 | −0.10 |
| Condition | Concentration(s) | Experiment | No. of Scans |
|---|---|---|---|
| 19F NMR experiments | |||
| F*-ap-13 no surfactant | F*-ap-13: 0.5 mM | zgflqn | 384 |
| F*-ap-13 with SDS | F*-ap-13: 0.5 mM | zgflqn | 384 |
| F*-ap-13 with DPC | F*-ap-13: 0.5 mM | zgflqn | 384 |
| F*-ap-17 no surfactant | F*-ap-17: 0.25 mM | Zgfhigqn a | 1024 |
| F*-ap-17 with SDS | F*-ap-17: 0.25 mM | Zgfhigqn a | 1024 |
| F*-ap-17 with DPC | F*-ap-17: 0.25 mM | Zgfhigqn a | 1024 |
| F*-ap-17 in 100% D2O no surfactant | F*-ap-17: 0.25 mM | Zgfhigqn a | 1024 |
| 1:1 AR55:F*-ap-13 | AR55 (4′): 0.5 mM F*-ap-13: 0.5 mM | Zgfhigqn a | 22,000 |
| 1:2 TM1-3: F*-ap-13 | TM1-3 (4′): 0.05 mM F*-ap-13: 0.1 mM | Zgfhigqn a | 19,456 |
| 1:1 TM1-3: F*-ap-13 | TM1-3 (4′): 0.1 mM F*-ap-13: 0.1 mM | Zgfhigqn a | 3072 |
| 2:1 TM1-3:F*-ap-13 | TM1-3 (4′): 0.15 mM F*-ap-13: 0.1 mM | Zgfhigqn a | 3072 |
| 4:1 AR55:F*-ap-13 | AR55 (4′): 0.25 mM AR55 (6′): 0.25 mM F*-ap-13: 0.125 mM | Zgfhigqn a | 24,576 |
| 1H NMR experiments | |||
| F*-ap-17 in 100% D2O no surfactant | F*-ap-17: 0.25 mM | zg | 16 |
| F*-ap-17 Soft Decoupling (100% D2O, no surfactant, −135 ppm, pl24 of 25 dB) | F*-ap-17: 0.25 mM | In-house experiment b | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simmons, J.R.; Murza, A.; Lumsden, M.D.; Kenward, C.; Marsault, É.; Rainey, J.K. Simultaneous Ligand and Receptor Tracking through NMR Spectroscopy Enabled by Distinct 19F Labels. Int. J. Mol. Sci. 2019, 20, 3658. https://doi.org/10.3390/ijms20153658
Simmons JR, Murza A, Lumsden MD, Kenward C, Marsault É, Rainey JK. Simultaneous Ligand and Receptor Tracking through NMR Spectroscopy Enabled by Distinct 19F Labels. International Journal of Molecular Sciences. 2019; 20(15):3658. https://doi.org/10.3390/ijms20153658
Chicago/Turabian StyleSimmons, Jeffrey R., Alexandre Murza, Michael D. Lumsden, Calem Kenward, Éric Marsault, and Jan K. Rainey. 2019. "Simultaneous Ligand and Receptor Tracking through NMR Spectroscopy Enabled by Distinct 19F Labels" International Journal of Molecular Sciences 20, no. 15: 3658. https://doi.org/10.3390/ijms20153658
APA StyleSimmons, J. R., Murza, A., Lumsden, M. D., Kenward, C., Marsault, É., & Rainey, J. K. (2019). Simultaneous Ligand and Receptor Tracking through NMR Spectroscopy Enabled by Distinct 19F Labels. International Journal of Molecular Sciences, 20(15), 3658. https://doi.org/10.3390/ijms20153658





