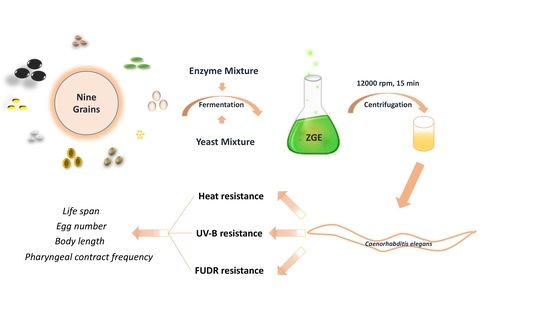
Zymolytic Grain Extract (ZGE) Significantly Extends the Lifespan and Enhances the Environmental Stress Resistance of Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
2.1. ZGE Does Not Inhibit the Propagation of E. coli OP50
2.2. ZGE Can Extend the Lifespan of C. Elegans N2 under Normal Culture Conditions
2.3. ZGE Does Not Influence the Reproductive Capacity of N2
2.4. ZGE Improves the Heat-Stress Resistance of C. elegans under Stress Conditions
2.5. ZGE Enhances the Radiation Resistance of Nematodes
2.6. ZGE Can Extend the Lifespan of C. elegans N2 Exposed to High Concentrations of FUDR
2.7. ZGE Promotes the Egg Hatching of C. elegans in the Presence of FUDR
2.8. ZGE Promotes the Larval Growth of C. elegans in the Presence of FUDR
2.9. Total Phenolic Content Analysis
3. Discussion
4. Materials and Methods
4.1. Nematode Strains and Maintenance
4.2. Test Drugs and Chemical Reagents
4.3. Effects of ZGE on the Growth of OP50 Strain
4.4. Life Span Assays under Normal Conditions
4.5. Self-Brood Size and Rate of Egg Production under Normal Conditions
4.6. Heat-Shock Assays
4.7. Anti-Ultraviolet Radiation Assays
4.8. Effects of ZGE on the Life Cycle of Nematode with High Concentration FUDR
4.9. Effects of ZGE on the FUDR Influence on Nematode Egg Hatching
4.10. Effects of ZGE on the Growth and Development of Nematodes Influenced by FUDR
4.11. Folin–Ciocalteu Method for the Determination of Total Polyphenols
4.12. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ZGE | Zymolytic grain extract |
| FUDR | 5-fluoro-2′-deoxyuridine |
| ROS | Reactive oxygen species |
| C. elegans | Caenorhabditis elegans |
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Kodera, Y.; Hirata, D.; Blackwell, T.K.; Mizunuma, M. Natural thioallyl compounds increase oxidative stress resistance and lifespan in Caenorhabditis elegans by modulating SKN-1/Nrf. Sci.Rep. 2016, 6, 21611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Leutner, S.; Eckert, A.; Muller, W.E. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J. Neural Transm. 2001, 108, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Sesti, F.; Liu, S.; Cai, S.Q. Oxidation of potassium channels by ROS: A general mechanism of aging and neurodegeneration? Trends Cell Biol. 2010, 20, 45–51. [Google Scholar] [CrossRef]
- Takano, H.; Zou, Y.; Hasegawa, H.; Akazawa, H.; Nagai, T.; Komuro, I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: Involvement of ROS in heart diseases. Antioxid. Redox Signal. 2003, 5, 789–794. [Google Scholar] [CrossRef]
- Chapple, I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef]
- Coutinho, L.G.; de Oliveira, A.H.S.; Witwer, M.; Leib, S.L.; Agnez-Lima, L.F. DNA repair protein APE1 is involved in host response during pneumococcal meningitis and its expression can be modulated by vitamin B6. J. Neuroinflamm. 2017, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, Y.; Guo, Y.; Xu, L.; Zhao, B.L. Significant longevity-extending effects of a tetrapeptide from maize on Caenorhabditis elegans under stress. Food Chem. 2012, 130, 254–260. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawata, A.; Katayama, T.; Fujisawa, S. Anti-inflammatory activity of the artificial antioxidants 2-tert-butyl-4-methoxyphenol (BHA), 2,6-di-tert-butyl-4-methylphenol (BHT) and 2,4,6-tri-tert-butylphenol (TBP), and their various combinations. In Vivo 2015, 29, 197–206. [Google Scholar] [PubMed]
- Xu, J.; Hou, H.; Hu, J.; Liu, B. Optimized microwave extraction, characterization and antioxidant capacity of biological polysaccharides from Eucommia ulmoides Oliver leaf. Sci. Rep. 2018, 8, 6561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, X.; Liu, L.; Li, Z.; Yang, Q.; Zhu, W.; Zhang, W.; Wang, J. Highly specific and sensitive determination of propyl gallate in food by a novel fluorescence sensor. Food Chem. 2018, 256, 45–52. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.Y.; Zhao, J.; Dong, Z.; Feng, D.Y.; Wu, R.; Shi, M.; Zhao, G. Ginsenoside Rd Protects SH-SY5Y Cells against 1-Methyl-4-phenylpyridinium Induced Injury. Int. J. Mol. Sci. 2015, 16, 14395–14408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zhou, S.; Wen, J.; Huang, M.; Xu, A. Mechanism of the antiulcerogenic effect of Ganoderma lucidum polysaccharides on indomethacin-induced lesions in the rat. Life Sci. 2002, 72, 731–745. [Google Scholar] [CrossRef]
- Ding, Q.; Yang, D.; Zhang, W.; Lu, Y.; Zhang, M.; Wang, L.; Li, X.; Zhou, L.; Wu, Q.; Pan, W.; et al. Antioxidant and anti-aging activities of the polysaccharide TLH-3 from Tricholoma lobayense. Int. J. Biol. Macromol. 2016, 85, 133–140. [Google Scholar] [CrossRef]
- Guo, H.; Ling, W.; Wang, Q.; Liu, C.; Hu, Y.; Xia, M.; Feng, X.; Xia, X. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods Hum. Nutr. 2007, 62, 1–6. [Google Scholar] [CrossRef]
- De Mello, V.D.; Schwab, U.; Kolehmainen, M.; Koenig, W.; Siloaho, M.; Poutanen, K.; Mykkanen, H.; Uusitupa, M. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: The Sysdimet study. Diabetologia 2011, 54, 2755–2767. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.S.; Fleenor, B.S. Whole grain consumption is negatively correlated with obesity-associated aortic stiffness: A hypothesis. Nutrition 2018, 45, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and In Vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.N.; Wu, H.L.; Yeh, H.I.; Chu, C.S.; Lin, H.C.; Lee, W.C. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids 2006, 41, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Zarei, I.; Brown, D.G.; Nealon, N.J.; Ryan, E.P. Rice Bran Metabolome Contains Amino Acids, Vitamins & Cofactors, and Phytochemicals with Medicinal and Nutritional Properties. Rice 2017, 10, 24. [Google Scholar] [PubMed] [Green Version]
- Jensen, M.K.; Koh-Banerjee, P.; Franz, M.; Sampson, L.; Gronbaek, M.; Rimm, E.B. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. Am. J. Clin. Nutr. 2006, 83, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, W.; Zhou, H.; Zhang, D.; Li, R.; Li, C.; Wang, S. The Relations between Minor Components and Antioxidant Capacity of Five Fruits and Vegetables Seed Oils in China. J. Oleo Sci. 2019, 68, 625–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leskovec, J.; Rezar, V.; Svete, A.N.; Salobir, J.; Levart, A. Antioxidative Effects of Olive Polyphenols Compared to Vitamin E in Piglets Fed a Diet Rich in N-3 PUFA. Animals 2019, 9, 161. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Guo, X.H.; Yu, B.; Wu, H.; Zhang, H.H.; Wu, J.X.; Jiang, C.L.; Kong, W.; Yu, X.H. Antiviral activity of a zymolytic grain based extract on human immunodeficiency virus type 1 In Vitro. Evid. Based Complement. Altern. Med. 2015, 2015, 642327. [Google Scholar] [CrossRef]
- Tuli, M.A.; Daul, A.; Schedl, T. Caenorhabditis nomenclature. WormBook 2018. [Google Scholar] [CrossRef] [PubMed]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wheeler, A.R. Maze exploration and learning in C. elegans. Lab Chip 2007, 7, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Sulston, J.E.; Horvitz, H.R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 1977, 56, 110–156. [Google Scholar] [CrossRef]
- Consortium, C.e.S. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [Google Scholar]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Coburn, C.; Allman, E.; Mahanti, P.; Benedetto, A.; Cabreiro, F.; Pincus, Z.; Matthijssens, F.; Araiz, C.; Mandel, A.; Vlachos, M.; et al. Anthranilate fluorescence marks a calcium-propagated necrotic wave that promotes organismal death in C. elegans. PLoS Biol. 2013, 11, e1001613. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Sung, J.; Joung, H. A fruit, milk and whole grain dietary pattern is positively associated with bone mineral density in Korean healthy adults. Eur. J. Clin. Nutr. 2015, 69, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Hirose, K.; Matsuo, K.; Ito, H.; Kuriki, K.; Suzuki, T.; Kato, T.; Hirai, T.; Kanemitsu, Y.; Tajima, K. Dietary risk factors for colon and rectal cancers: A comparative case-control study. J. Epidemiol. 2006, 16, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, H.; Roxo, M.; Silva, E.; Valente, K.; Braun, M.; Wang, X.; Wink, M. Bark Extract of the Amazonian Tree Endopleura uchi (Humiriaceae) Extends Lifespan and Enhances Stress Resistance in Caenorhabditis elegans. Molecules 2019, 24, 915. [Google Scholar] [CrossRef] [PubMed]
- Hole, A.S.; Grimmer, S.; Naterstad, K.; Jensen, M.R.; Paur, I.; Johansen, S.G.; Balstad, T.R.; Blomhoff, R.; Sahlstrom, S. Activation and inhibition of nuclear factor kappa B activity by cereal extracts: Role of dietary phenolic acids. J. Agric. Food Chem. 2009, 57, 9481–9488. [Google Scholar] [CrossRef] [PubMed]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef] [PubMed]
- Borneo, R.; Leon, A.E. Whole grain cereals: Functional components and health benefits. Food Funct. 2012, 3, 110–119. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Hung, Y.H.; Chou, C.C. Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int. J. Food Microbiol. 2008, 121, 150–156. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span--from yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Meng, F.; Li, J.; Rao, Y.; Wang, W.; Fu, Y. Gengnianchun Extends the Lifespan of Caenorhabditis elegans via the Insulin/IGF-1 Signalling Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 4740739. [Google Scholar] [CrossRef] [PubMed]
- Arantes-Oliveira, N.; Berman, J.R.; Kenyon, C. Healthy animals with extreme longevity. Science 2003, 302, 611. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Zhang, Z. Age-dependent effects of floxuridine (FUdR) on senescent pathology and mortality in the nematode Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2019, 509, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.I.; Venkatesh, D.; Murali, D.; Bhaskar, J.P.; Krishnan, V.; Balamurugan, K. Understanding the role of DAF-16 mediated pathway in Caenorhabditis elegans during UV-A mediated photoaging process. Arch. Gerontol. Geriatr. 2019, 82, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.L.; Wu, D.; Cypser, J.R.; Vaupel, J.W.; Johnson, T.E. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. 2005, 37, 894–898. [Google Scholar] [CrossRef]
- Tissenbaum, H.A. Using, C. elegans for aging research. Invertebr. Reprod. Dev. 2015, 59 (Suppl. 1), 59–63. [Google Scholar] [CrossRef]
- Johnson, T.E. Advantages and disadvantages of Caenorhabditis elegans for aging research. Exp. Gerontol. 2003, 38, 1329–1332. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Wang, X.; Lin, R.; Zhang, Y.; Ma, H.; Guo, Y.; Xu, L.; Zhao, B. The novel dipeptide Tyr-Ala (TA) significantly enhances the lifespan and healthspan of Caenorhabditis elegans. Food Funct. 2016, 7, 1975–1984. [Google Scholar] [CrossRef]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| ZGE Treatment | Thermotolerance, 35 °C (h) | ||
|---|---|---|---|
| (mg/mL) | Mean | Maximum | Mean Fold Increase/% |
| 0 | 45.32 ± 18.2 (46) | 77 | -- |
| 6.5 | 58.89 ± 14.24 (61) | 82 | 29.9 *** |
| Group (mg/mL) | 0 | 0.8125 | 1.625 | 3.25 | 6.5 |
|---|---|---|---|---|---|
| Body length (μM) | 630 ± 24.05 (n = 27) | 659 ± 86.27 (n = 30) | 831.1 ± 124.7 (n = 27) | 1146.11 ± 154.78 (n = 33) | 1284.74 ± 215.18 (n = 35) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Jiang, M.; Guo, Q.; Shi, W. Zymolytic Grain Extract (ZGE) Significantly Extends the Lifespan and Enhances the Environmental Stress Resistance of Caenorhabditis elegans. Int. J. Mol. Sci. 2019, 20, 3489. https://doi.org/10.3390/ijms20143489
Hou L, Jiang M, Guo Q, Shi W. Zymolytic Grain Extract (ZGE) Significantly Extends the Lifespan and Enhances the Environmental Stress Resistance of Caenorhabditis elegans. International Journal of Molecular Sciences. 2019; 20(14):3489. https://doi.org/10.3390/ijms20143489
Chicago/Turabian StyleHou, Lu, Mengying Jiang, Qiong Guo, and Wei Shi. 2019. "Zymolytic Grain Extract (ZGE) Significantly Extends the Lifespan and Enhances the Environmental Stress Resistance of Caenorhabditis elegans" International Journal of Molecular Sciences 20, no. 14: 3489. https://doi.org/10.3390/ijms20143489