Is TRPA1 Burning Down TRPV1 as Druggable Target for the Treatment of Chronic Pain?
Abstract
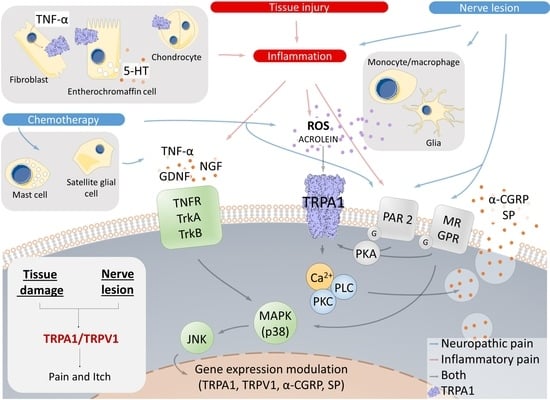
:1. Introduction
2. TRP Channels Family
2.1. TRPV1 Channel
2.2. TRPA1 Channel
3. TRPA1 in Inflammatory Pain
3.1. The Double Face of TRPA1 in Gastrointestinal Pain Models
3.2. TRPA1 Is Involved in Joint and Muscle Pain
3.3. TRPA1 and TRPV1 Cooperate in Skin Pathologies
3.4. TRPA1 Is a Sentinel for External Threats in the Airways and Urinary Tract
3.5. TRPA1 Senses Oxidation in the Cardiovascular System
3.6. TRPA1 Role in Eye Diseases
4. TRPA1 in Neuropathic Pain
4.1. TRPA1 Is Essential for Nerve Injury and Chemotherapy-Induced Neuropathic Pain
4.2. TRPA1 Is Upregulated in Neuropathic Pain
4.3. TRPA1 Modulation in Neuropathic Pain Shares Convergent Signalling Pathways in Different Models of Neuropathic Pain
4.4. Oxidative Stress Is a Major Integrator of TRPA1 Regulation in Neuropathic Pain
5. TRPA1 in Clinical Trials
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eccleston, C.; Wells, C.; Morlion, B. European Pain Management; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Gureje, O.; Simon, G.E.; Von Korff, M. A cross-national study of the course of persistent pain in primary care. Pain 2001, 92, 195–200. [Google Scholar] [CrossRef]
- Saastamoinen, P.; Laaksonen, M.; Kääriä, S.M.; Lallukka, T.; Leino-Arjas, P.; Rahkonen, O.; Lahelma, E. Pain and disability retirement: a prospective cohort study. Pain 2012, 153, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Viana, F. Molecular and cellular limits to somatosensory specificity. Mol. Pain 2008, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Ann. Intern. Med. 2004, 140, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V.V. Peripheral sensitization of sensory neurons. Ethn. Dis. 2011, 20, S1-3-6. [Google Scholar]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer-Montiel, A.; Fernández-Carvajal, A.; Planells-Cases, R.; Fernández-Ballester, G.; González-Ros, J.M.; Messeguer, A.; González-Muñiz, R. Advances in modulating thermosensory TRP channels. Expert Opin. Ther. Pat. 2012, 22, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Minke, B. Drosophila mutant with a transducer defect. Biophys. Struct. Mech. 1977, 3, 59–64. [Google Scholar] [CrossRef]
- Montell, C.; Jones, K.; Hafen, E.; Rubin, G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 1985, 230, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.D.; Alessandri-Haber, N. TRP channels: targets for the relief of pain. Biochim. Biophys. Acta 2007, 1772, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sato, Y.; Taniguchi, K. Distribution of TRPV1- and TRPV2- immunoreactive afferent nerve endings in rat trachea. J. Anat. 2007, 211, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, E.; Katsura, H.; Kobayashi, K.; Yamanaka, H.; Tsuzuki, K.; Noguchi, K.; Sakagami, M. Changes in transient receptor potential channels in the rat geniculate ganglion after chorda tympani nerve injury. Neuroreport 2015, 26, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Jeffry, J.A.; Yu, S.Q.; Sikand, P.; Parihar, A.; Evans, M.S.; Premkumar, L.S. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS ONE 2009, 4, e7021. [Google Scholar] [CrossRef] [PubMed]
- Sikand, P.; Premkumar, L.S. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J. Physiol. 2007, 581, 631–647. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef]
- Woodbury, C.J.; Zwick, M.; Wang, S.; Lawson, J.J.; Caterina, M.J.; Koltzenburg, M.; Albers, K.M.; Koerber, H.R.; Davis, B.M. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J. Neurosci. 2004, 24, 6410–6415. [Google Scholar] [CrossRef]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Bolcskei, K.; Helyes, Z.; Szabo, A.; Sandor, K.; Elekes, K.; Nemeth, J.; Almasi, R.; Pinter, E.; Petho, G.; Szolcsanyi, J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain 2005, 117, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Jaquemar, D.; Schenker, T.; Trueb, B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 1999, 274, 7325–7333. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Nagata, K.; Duggan, A.; Kumar, G.; Garcia-Anoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005, 25, 4052–4061. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P.; Beacham, D.; Ensor, E.; Koltzenburg, M. Cold-sensitive, menthol insensitive neurons in the murine sympathetic nervous system. Neuroreport 2004, 15, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Obata, K.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Sakagami, M.; Noguchi, K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp. Neurol. 2006, 200, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Chen, J.; Faltynek, C.R.; Moreland, R.B.; Neelands, T.R. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur. J. Neurosci. 2008, 27, 605–611. [Google Scholar] [CrossRef]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Babes, A.; Sauer, S.K.; Moparthi, L.; Kichko, T.I.; Neacsu, C.; Namer, B.; Filipovic, M.; Zygmunt, P.M.; Reeh, P.W.; Fischer, M.J. Photosensitization in porphyrias and photodynamic therapy involves TRPA1 and TRPV. J. Neurosci. 2016, 36, 5264–5278. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Nakamura, S.; Zhao, M.; So, K.; Inoue, K.; Numata, T.; Takahashi, N.; Shirakawa, H.; Mori, Y.; Nakagawa, T.; et al. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat. Commun. 2016, 7, 12840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessac, B.F.; Sivula, M.; von Hehn, C.A.; Escalera, J.; Cohn, L.; Jordt, S.E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Investig. 2008, 118, 1899–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Camino, D.; Murphy, S.; Heiry, M.; Barrett, L.B.; Earley, T.J.; Cook, C.A.; Petrus, M.J.; Zhao, M.; D’Amours, M.; Deering, N.; et al. TRPA1 contributes to cold hypersensitivity. J. Neurosci. 2010, 30, 15165–15174. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chang, R.B.; Waters, H.N.; McKemy, D.D.; Liman, E.R. The nociceptor ion cannel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 2008, 283, 32691–32703. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.S.; Woolf, C.J.; Corey, D.P. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006, 50, 277–289. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Montiel, A.; García-Martínez, C.; Morenilla-Palao, C.; García-Sanz, N.; Fernández-Carvajal, A.; Fernández-Ballester, G.; Planells-Cases, R. Molecular architecture of the vanilloid receptor. Insights for drug design. Eur. J. Biochem. 2004, 271, 1820–1826. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef]
- Hinman, A.; Chuang, H.H.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Mendes, S.J.F.; Sousa, F.I.A.B.; Pereira, D.M.S.; Ferro, T.A.F.; Pereira, I.C.P.; Silva, B.L.R.; Pinheiro, A.J.M.C.R.; Mouchrek, A.Q.S.; Monteiro-Neto, V.; Costa, S.K.P.; et al. Cinnamaldehyde modulates LPS-induced systemic inflammatory response syndrome through TRPA1-dependent and independent mechanisms. Int. Immunopharmacol. 2016, 34, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, E.J.; Simkin, D.; Kim, D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience 2008, 154, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Zurborg, S.; Yurgionas, B.; Jira, J.A.; Caspani, O.; Heppenstall, P.A. Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 2007, 10, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Mizuno, Y.; Kozai, D.; Yamamoto, S.; Kiyonaka, S.; Shibata, T.; Uchida, K.; Mori, Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2008, 2, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Marsakova, L.; Barvik, I.; Zima, V.; Zimova, L.; Vlachova, V. The First Extracellular Linker Is Important for Several Aspects of the Gating Mechanism of Human TRPA1 Channel. Front. Mol. Neurosci. 2017, 10, 16. [Google Scholar] [CrossRef]
- Gui, J.; Liu, B.; Cao, G.; Lipchik, A.M.; Perez, M.; Dekan, Z.; Mobli, M.; Daly, N.L.; Alewood, P.F.; Parker, L.L.; et al. A tarantula-venom peptide antagonizes the TRPA1 nociceptor ion channel by binding to the S1–S4 gating domain. Curr. Biol. 2014, 24, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Kremeyer, B.; Lopera, F.; Cox, J.J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.G.; Jones, N.G.; Paterson, K.J.; Fricker, F.R.; et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Dubin, A.E.; Bursulaya, B.; Viswanath, V.; Jegla, T.J.; Patapoutian, A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J. Neurosci. 2008, 28, 9640–9651. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Fukuda, T.; Okada, H.; Kitao, S.; Ishida, Y.; Kato, K.; Takahashi, C.; Katayama, M.; Uchida, K.; Tominaga, M. Identification of significant amino acids in multiple transmembrane domains of human transient receptor potential ankyrin 1 (TRPA1) for activation by eudesmol, an oxygenized sesquiterpene in hop essential oil. J. Biol. Chem. 2015, 290, 3161–3171. [Google Scholar] [CrossRef] [PubMed]
- de la Roche, J.; Eberhardt, M.J.; Klinger, A.B.; Stanslowsky, N.; Wegner, F.; Koppert, W.; Reeh, P.W.; Lampert, A.; Fischer, M.J.; Leffler, A. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J. Biol. Chem. 2013, 288, 20280–20292. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, H.; Latorre, R.; Grandl, J. The pore-domain of TRPA1 mediates the inhibitory effect of the antagonist 6-methyl-5-(2-(trifluoromethyl)phenyl)-1H-indazole. PLoS ONE 2014, 9, e106776. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.F.; Kort, M.E.; Huth, J.R.; Sun, C.; Miesbauer, L.J.; Cassar, S.C.; Neelands, T.; Scott, V.E.; Moreland, R.B.; et al. Molecular determinants of species-specific activation or blockade of TRPA1 channels. J. Neurosci. 2008, 28, 5063–5071. [Google Scholar] [CrossRef]
- Vallin, K.S.; Sterky, K.J.; Nyman, E.; Bernström, J.; From, R.; Linde, C.; Minidis, A.B.; Nolting, A.; Närhi, K.; Santangelo, E.M.; et al. N-1-Alkyl-2-oxo-2-aryl amides as novel antagonists of the TRPA1 receptor. Bioorg. Med. Chem. Lett. 2012, 22, 5485–5492. [Google Scholar] [CrossRef]
- Eid, S.R.; Crown, E.D.; Moore, E.L.; Liang, H.A.; Choong, K.C.; Dima, S.; Henze, D.A.; Kane, S.A.; Urban, M. O HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol. Pain 2008, 4, 48. [Google Scholar] [CrossRef]
- Klement, G.; Eisele, L.; Malinowsky, D.; Nolting, A.; Svensson, M.; Terp, G.; Weigelt, D.; Dabrowski, M. Characterization of a ligand binding site in the human transient receptor potential ankyrin 1 pore. Biophys. J. 2013, 104, 798–806. [Google Scholar] [CrossRef]
- Chen, J.; Joshi, S.K.; DiDomenico, S.; Perner, R.J.; Mikusa, J.P.; Gauvin, D.M.; Segreti, J.A.; Han, P.; Zhang, X.F.; Niforatos, W.; et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011, 152, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, M.; Uchida, K.; Fujita, F.; Tominaga, M. Inhibitory effects of monoterpenes on human TRPA1 and the structural basis of their activity. J. Physiol. Sci. 2014, 64, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ton, H.T.; Phan, T.X.; Abramyan, A.M.; Shi, L.; Ahern, G.P. Identification of a putative binding site critical for general anesthetic activation of TRPA. Proc. Natl. Acad. Sci. USA 2017, 114, 3762–3767. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.P.; Akyuz, N.; Corey, D.P. The Outer Pore and Selectivity Filter of TRPA. PLoS ONE 2016, 11, e0166167. [Google Scholar] [CrossRef]
- Zimova, L.; Sinica, V.; Kadkova, A.; Vyklicka, L.; Zima, V.; Barvik, I.; Vlachova, V. Intracellular cavity of sensor domain controls allosteric gating of TRPA1 channel. Sci. Signal. 2018, 11, eaan8621. [Google Scholar] [CrossRef] [Green Version]
- Banke, T.G.; Chaplan, S.R.; Wickenden, A.D. Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am. J. Physiol. Cell Physiol. 2010, 298, C1457–C1468. [Google Scholar] [CrossRef]
- Chen, J.; Kim, D.; Bianchi, B.R.; Cavanaugh, E.J.; Faltynek, C.R.; Kym, P.R.; Reilly, R.M. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol. Pain 2009, 5, 3. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Faria, R.X. TRPing on the pore phenomenon: what do we know about transient receptor potential ion channel-related pore dilation up to now? J. Bioenerg. Biomembr. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Biggs, J.E.; Stemkowski, P.L.; Knaus, E.E.; Chowdhury, M.A.; Ballanyi, K.; Smith, P.A. Suppression of network activity in dorsal horn by gabapentin permeation of TRPV1 channels: implications for drug access to cytoplasmic targets. Neurosci. Lett. 2015, 584, 397–402. [Google Scholar] [CrossRef]
- Banke, T.G. The dilated TRPA1 channel pore state is blocked by amiloride and analogues. Brain Res. 2011, 1381, 21–30. [Google Scholar] [CrossRef]
- Sousa-Valente, J.; Andreou, A.P.; Urban, L.; Nagy, I. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br. J. Pharmacol. 2014, 171, 2508–2527. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug. Discov. 2009, 8, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Vay, L.; Gu, C.; McNaughton, P.A. The thermos-TRP ion channel family: properties and therapeutic implications. Br. J. Pharmacol. 2012, 165, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Holtzer, P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011, 131, 142–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Dubin, A.E.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 2009, 64, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Brierly, S.M.; Hughes, P.A.; Page, A.J.; Kwan, K.Y.; Martin, C.M.; O’Donnell, T.A.; Cooper, N.J.; Harrington, A.M.; Adam, B.; Liebregts, T.; et al. The Ion Channel TRPA1 Is Required for Normal Mechanosensation and Is Modulated by Algesic Stimuli. Gastroenterology 2009, 137, 2084–2095. [Google Scholar] [CrossRef]
- Nozawa, K.; Kawabata-shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, M.; Jarmuz, A.; Wasilewski, A.; Sałaga, M.; Fichna, J. Role of transient receptor potential channels in intestinal inflammation and visceral pain: Novel targets in inflammatory bowel diseases. Inflamm. Bowel. Dis. 2015, 21, 419–427. [Google Scholar] [CrossRef]
- Feng, C.; Yan, X.; Chen, X.; Wang, E.; Liu, Q.; Zhang, L.; Chen, J.; Fang, J.Y.; Chen, S. Vagal anandamide signaling via cannabinoid receptor 1 contributes to luminal 5-HT modulation of visceral nociception in rats. Pain 2014, 155, 1591–1604. [Google Scholar] [CrossRef]
- Malin, S.; Molliver, D.; Christianson, J.A.; Schwartz, E.S.; Cornuet, P.; Albers, K.M.; Davis, B.M. TRPV1 and TRPA1 Function and Modulation are Target Tissue- Dependent. J. Neurosci. 2011, 31, 10516–10528. [Google Scholar] [CrossRef]
- Shen, S.; Al-thumairy, H.W.; Hashmi, F.; Qiao, L. Regulation of transient receptor potential cation channel subfamily V1 protein synthesis by the phosphoinositide 3- kinase/Akt pathway in colonic hypersensitivity. Exp. Neurol. 2017, 295, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kogure, Y.; Wang, S.; Tanaka, K.I.; Hao, Y.; Yamamoto, S.; Nishiyama, N.; Noguchi, K.; Dai, Y. Elevated H2O2 levels in trinitrobenzene sulfate-induced colitis rats contributes to visceral hyperalgesia through interaction with the transient receptor potential ankyrin 1 cation channel. J. Gastroenterol. Hepatol. 2016, 31, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Zuo, X.; Zhen, Y.; Yu, Y.; Gao, L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci. Lett. 2008, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kistner, K.; Siklosi, N.; Babes, A.; Khalil, M.; Selescu, T.; Zimmermann, K.; Wirtz, S.; Becker, C.; Neurath, M.F.; Reeh, P.W.; et al. Systemic desensitization through TRPA1 channels by capsazepine and mustard oil—A novel strategy against inflammation and pain. Sci. Rep. 2016, 30, e28621. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.A.; Leffler, A.; Niedermirtl, F.; Babes, A.; Tribbensee, S.M.M.; Khalil, M.; Siklosi, N.; Nau, C.; Ivanović-Burmazović, I.; Neuhuber, W.L.; et al. TRPA1 and Substance P Mediate Colitis in Mice. Gastroenterology 2011, 141, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Borrelli, F.; Fasolino, I.; Capasso, R.; Piscitelli, F.; Ii, F.; Domenico, V. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br. J. Pharmacol. 2013, 169, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Nozawa, K.; Doihara, H.; Keto, Y.; Kaku, H. Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. Eur. J. Pharmacol. 2014, 723, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, M.; Shahbazian, A.; Bock, E.; Pabst, M.A.; Holzer, P. Chemo-nociceptive signalling from the colon is enhanced by mild colitis and blocked by inhibition of transient receptor potential ankyrin 1 channels. Br. J. Pharmacol. 2010, 1, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, E.M.; Souza, L.K.M.; Araújo, T.S.L.; Nogueira, K.M.; Sousa, F.B.M.; Araújo, A.R.; Martins, C.; Pacífico, D.M.; de C Brito, G.A.; Souza, E.P.; et al. Carvacrol reduces irinotecan-induced intestinal mucositis through inhibition of inflammation and oxidative damage via TRPA1 receptor activation. Chem. Biol. Interact. 2016, 260, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kurahara, L.H.; Hiraishi, K.; Hu, Y.; Koga, K.; Onitsuka, M.; Doi, M.; Aoyagi, K.; Takedatsu, H.; Kojima, D.; Fujihara, Y.; et al. Activation of Myofibroblast TRPA1 by Steroids and Pirfenidone Ameliorates Fibrosis in Experimental Crohn’s Disease. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 antagonists for pain relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Nummenmaa, E.; Hämäläinen, M.; Moilanen, L.J.; Paukkeri, E.L.; Nieminen, R.M.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Transient receptor potential ankyrin 1 (TRPA1) is functionally expressed in primary human osteoarthritic chondrocytes. Arthritis Res. Ther. 2016, 18, e185. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Á.; Tékus, V.; Boros, M.; Pozsgai, G.; Botz, B.; Borbély, É.; Szolcsányi, J.; Pintér, E.; Helyes, Z. Transient receptor potential ankyrin 1 (TRPA1) receptor is involved in chronic arthritis: In vivo study using TRPA1-deficient mice. Arthritis Res. Ther. 2016, 18, e6. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, L.J.; Hämäläinen, M.; Nummenmaa, E.; Ilmarinen, P.; Vuolteenaho, K.; Nieminen, R.M.; Lehtimäki, L.; Moilanen, E. Monosodium iodoacetate-induced inflammation and joint pain are reduced in TRPA1 deficient mice - potential role of TRPA1 in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Russell, F.A.; Spina, D.; McDougall, J.J.; Graepel, R.; Gentry, C.; Staniland, A.A.; Mountford, D.M.; Keeble, J.E.; Malcangio, M.; et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factorα-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum. 2011, 63, 819–829. [Google Scholar] [CrossRef]
- Pereira, L.M.S.; Lima-Júnior, R.C.P.; Bem, A.X.C.; Teixeira, C.G.; Grassi, L.S.; Medeiros, R.P.; Marques-Neto, R.D.; Callado, R.B.; Aragão, K.S.; Wong, D.V.; et al. Blockade of TRPA1 with HC-030031 attenuates visceral nociception by a mechanism independent of inflammatory resident cells, nitric oxide and the opioid system. Eur. J. Pain 2013, 17, 223–233. [Google Scholar] [CrossRef]
- Lowin, T.; Bleck, J.; Schneider, M.; Pongratz, G. Selective killing of proinflammatory synovial fibroblasts via activation of transient receptor potential ankyrin (TRPA1). Biochem. Pharmacol. 2018, 154, 293–302. [Google Scholar] [CrossRef]
- Lowin, T.; Apitz, M.; Anders, S.; Straub, R.H. Anti-inflammatory effects of N-acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2 dependent manner. Arthritis Res. Ther. 2015, 17, e321. [Google Scholar] [CrossRef]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Moilanen, L.J.; Hämäläinen, M.; Lehtimäki, L.; Nieminen, R.M.; Moilanen, E. Urate crystal induced inflammation and joint pain are reduced in transient receptor potential ankyrin 1 deficient mice - Potential role for transient receptor potential ankyrin1 in gout. PLoS ONE 2015, 10, e0117770. [Google Scholar] [CrossRef]
- Maruyama, K.; Takayama, Y.; Kondo, T.; Ishibashi, K.; Sahoo, B.R.; Kanemaru, H.; Kumagai, Y.; Martino, M.M.; Tanaka, H.; Ohno, N.; et al. Nociceptors Boost the Resolution of Fungal Osteoinflammation via the TRP Channel-CGRP-Jdp2 Axis. Cell Rep. 2017, 19, 2730–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgar, J.; Zhang, Y.; Saloman, J.L.; Wang, S.; Chung, M.K.; Ro, J.Y. The role of TRPA1 in muscle pain and mechanical hypersensitivity under inflammatory conditions in rats. Neuroscience 2015, 310, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.K.; Park, J.; Asgar, J.; Ro, J.Y. Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol. Pain 2016, 4, e1744806916668526. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, A.; Akopian, A.N.; Hargreaves, K.M. NGF Up-regulates TRPA1: Implications for orofacial pain. J. Dent. Res. 2007, 86, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Brigoli, B.; Lim, J.; Karley, A.; Chung, M.K. Roles of TRPV1 and TRPA1 in Spontaneous Pain from Inflamed Masseter Muscle. Neuroscience 2018, 384, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, D.; Kang, S.; Brennan, T.J. Muscle reactive oxygen species (ROS) contribute to post-incisional guarding via the TRPA1 receptor. PLoS ONE 2017, 12, e0170410. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Pagano, E.; Moriello, A.S.; Alvino, F.G.; Sorrentino, N.C.; D’Orsi, L.; Gazzerro, E.; Capasso, R.; De Leonibus, E.; De Petrocellis, L.; et al. Effects of non-euphoric plant cannabinoids on muscle quality and performance of dystrophic mdx mice. Br. J. Pharmacol. 2019, 176, 1568–1584. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Nelson, A.M.; Batia, L.; Morita, T.; Estandian, D.; Owens, D.M.; Lumpkin, E.A.; Bautista, D.M. The Ion Channel TRPA1 Is Required for Chronic Itch. J. Neurosci. 2013, 33, 9283–9294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodji, X.; Arkless, K.L.; Kee, Z.; Cleary, S.J.; Aubdool, A.A.; Evans, E.; Caton, P.; Pitchford, S.C.; Brain, S.D. Sensory nerves mediate spontaneous behaviors in addition to inflammation in a murine model of psoriasis. FASEB J. 2019, 33, 1578–1594. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Vong, C.T.; Quek, S.; Cheong, J.; Awal, S.; Gentry, C.; Aubdool, A.A.; Liang, L.; Bodkin, J.V.; Bevan, S.; et al. Superoxide generation and leukocyte accumulation: Key elements in the mediation of leukotriene B4-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J. 2013, 27, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Escalera, J.; Balakrishna, S.; Fan, L.; Caceres, A.I.; Robinson, E.; Sui, A.; McKay, M.C.; McAlexander, M.A.; Herrick, C.A.; et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013, 27, 3549–3563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Ru-Rong, J. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci. Bull. 2012, 28, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 2017, 8, e980. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-Dependent Pruritus in IL-13-Induced Chronic Atopic Dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Conklin, D.J. Acute cardiopulmonary toxicity of inhaled aldehydes: role of TRPA. Ann. N. Y. Acad. Sci. 2016, 1374, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Page, C.P.; Barnes, P.J. Pharmacology and Therapeutics of Asthma and COPD. In Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2017; Volume 237. [Google Scholar]
- Zholos, A.; McGarvey, L.; Ennis, M. TRP Channels as Therapeutic Targets: From Basic Science to Clinical Use; Elsevier Inc.: London, UK, 2015. [Google Scholar]
- Taylor-Clark, T.E.; Undem, B.J. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J. Physiol. 2010, 588, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.H.; Liu, M.H.; Ko, H.K.; Perng, D.W.; Lee, T.S.; Kou, Y.R. Lung Epithelial TRPA1 Transduces the Extracellular ROS into Transcriptional Regulation of Lung Inflammation Induced by Cigarette Smoke: The Role of Influxed Ca2+. Mediat. Inflamm. 2015, 2015, e148367. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas Krohn, I.; Callebaut, I.; Alpizar, Y.A.; Steelant, B.; Van Gerven, L.; Skov, P.S.; Kasran, A.; Talavera, K.; Wouters, M.M.; Ceuppens, J.L.; et al. MP29-02 reduces nasal hyperreactivity and nasal mediators in patients with house dust mite-allergic rhinitis. Allergy 2018, 73, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lee, L.Y. Role of calcium ions in the positive interaction between TRPA1 and TRPV1 channels in bronchopulmonary sensory neurons. J. Appl. Physiol. 2015, 118, 1533–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.J.; Lin, R.L.; Ruan, T.; Khosravi, M.; Lee, L.Y. A synergistic effect of simultaneous TRPA1 and TRPV1 activations on vagal pulmonary C-fiber afferents. J. Appl. Physiol. 2015, 118, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Alenmyr, L.; Herrmann, A.; Högestätt, E.D.; Greiff, L.; Zygmunt, P.M. TRPV1 and TRPA1 stimulation induces MUC5B secretion in the human nasal airway in vivo. Clin. Physiol. Funct. Imaging 2011, 31, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Prandini, P.; De Logu, F.; Fusi, C.; Provezza, L.; Nassini, R.; Montagner, G.; Materazzi, S.; Munari, S.; Gilioli, E.; Bezzerri, V.; et al. Transient receptor potential ankyrin 1 channels modulate inflammatory response in respiratory cells from patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2016, 55, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Streng, T.; Axelsson, H.E.; Hedlund, P.; Andersson, D.A.; Jordt, S.E.; Bevan, S.; Andersson, K.E.; Högestätt, E.D.; Zygmunt, P.M. Distribution and Function of the Hydrogen Sulfide-Sensitive TRPA1 Ion Channel in Rat Urinary Bladder. Eur. Urol. 2008, 53, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.B.M.; Sperotto, N.D.M.; Andrade, E.L.; Pereira, T.C.B.; Leite, C.E.; De Souza, A.H.; Bogo, M.R.; Morrone, F.B.; Gomez, M.V.; Campos, M.M. Spinal blockage of P/Q-or N-type voltage-gated calcium channels modulates functional and symptomatic changes related to haemorrhagic cystitis in mice. Br. J. Pharmacol. 2015, 172, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Du, S.; Kong, C.; Zhang, Z.; Mokhtar, A.D. Intrathecal administration of TRPA1 antagonists attenuate cyclophosphamide-induced cystitis in rats with hyper-reflexia micturition. BMC Urol. 2016, 16, e33. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C.; Forner, S.; Lima-Garcia, J.F.; Viana, A.F.; Calixto, J.B. Antagonism of the transient receptor potential ankyrin 1 (TRPA1) attenuates hyperalgesia and urinary bladder overactivity in cyclophosphamide-induced haemorrhagic cystitis. Chem. Biol. Interact. 2013, 203, 440–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, E.L.; Forner, S.; Bento, A.F.; Ferraz, D.; Leite, P.; Dias, M.A.; Leal, P.C.; Koepp, J.; Calixto, J.B. TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am. J. Physiol. Renal. Physiol. 2011, 300, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Kamei, J.; Aizawa, N.; Nakagawa, T.; Kaneko, S.; Kume, H.; Homma, Y.; Igawa, Y. Attenuated lipopolysaccharide-induced inflammatory bladder hypersensitivity in mice deficient of transient receptor potential ankilin. Sci. Rep. 2018, 8, e15622. [Google Scholar] [CrossRef] [PubMed]
- Oyama, S.; Dogishi, K.; Kodera, M.; Kakae, M.; Nagayasu, K.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Pathophysiological role of transient receptor potential ankyrin 1 in a mouse long-lasting cystitis model induced by an intravesical injection of hydrogen peroxide. Front. Physiol. 2017, 8, e877. [Google Scholar] [CrossRef] [PubMed]
- Chanpimol, S.; Seamon, B.; Hernandez, H.; Harris-love, M.; Blackman, M.R. Effects of Estrogen Receptor β Stimulation in a Rat Model of Non-Bacterial Prostatic Inflammation. Prostate 2017, 77, 803–811. [Google Scholar]
- Lim, J.Y.; Park, C.K.; Hwang, S.W. Biological Roles of Resolvins and Related Substances in the Resolution of Pain. Biomed. Res. Int. 2015, 2015, e830930. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Z.; Xie, J.; Yu, A.; Stock, J.; Du, J.; Yue, L. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2014, 308, H157–H182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Shyue, S.K.; Kou, Y.R.; Lu, T.M.; Lee, T.S. Transient receptor potential ankyrin 1 channel involved in atherosclerosis and macrophage-foam cell formation. Int. J. Biol. Sci. 2016, 12, 812–823. [Google Scholar] [CrossRef]
- Ogawa, N.; Kurokawa, T.; Mori, Y. Sensing of redox status by TRP channels. Cell Calcium 2016, 60, 115–122. [Google Scholar] [CrossRef]
- Oehler, B.; Kistner, K.; Martin, C.; Schiller, J.; Mayer, R.; Mohammadi, M.; Sauer, R.S.; Filipovic, M.R.; Nieto, F.R.; Kloka, J.; et al. Inflammatory pain control by blocking oxidized phospholipid-mediated TRP channel activation. Sci. Rep. 2017, 7, e5447. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Liu, J.; Ye, J.; Jiang, H.; Xu, Y.; Ye, D.; Wan, J. Inhibition of TRPA1 attenuates doxorubicin-induced acute cardiotoxicity by suppressing oxidative stress, the inflammatory response, and endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2018, 2018, e5179468. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Wang, M.; Ye, J.; Liu, J.; Jiang, H.; Ye, D.; Wan, J. TRPA1 inhibition ameliorates pressure overload-induced cardiac hypertrophy and fibrosis in mice. EBioMedicine 2018, 36, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mergler, S.; Valtink, M.; Takayoshi, S.; Okada, Y.; Miyajima, M.; Saika, S.; Reinach, P.S. Temperature-sensitive transient receptor potential channels in corneal tissue layers and cells. Ophthalmic. Res. 2014, 52, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.M.; Luna, C.; Quirce, S.; Belmonte, C.; Gallar, J. Corneal sensory nerve activity in an experimental model of UV keratitis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3403–3412. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Shirai, K.; Reinach, P.S.; Kitano-Izutani, A.; Miyajima, M.; Flanders, K.C.; Jester, J.V.; Tominaga, M.; Saika, S. TRPA1 is required for TGF-β signaling and its loss blocks inflammatory fibrosis in mouse corneal stroma. Lab. Investig. 2014, 94, 1030–1041. [Google Scholar] [CrossRef]
- Okada, Y.; Reinach, P.; Shirai, K.; Kitano-Izutani, A.; Miyajima, M.; Yamanaka, O.; Sumioka, T.; Saika, S. Transient Receptor Potential Channels and Corneal Stromal Inflammation. Cornea 2015, 34, S136–S141. [Google Scholar] [CrossRef]
- Katagiri, A.; Thompson, R.; Rahman, M.; Okamoto, K.; Bereiter, D.A. Evidence for TRPA1 involvement in central neural mechanisms in a rat model of dry eye. Neuroscience 2015, 290, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.S.; Madsen, C.S.; Finnerup, N.B. Pharmacology and treatment of neuropathic pains. Curr. Opin. Neurol. 2009, 22, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Brix Finnerup, N.; Hein Sindrup, S.; Staehelin Jensen, T. Management of painful neuropathies. Handb. Clin. Neurol. 2013, 115, 279–290. [Google Scholar]
- Andrade, E.L.; Meotti, F.C.; Calixto, J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2012, 133, 189–204. [Google Scholar] [CrossRef]
- Story, G.M.; Gereau, R.W. Numbing the senses: role of TRPA1 in mechanical and cold sensation. Neuron 2006, 50, 177–180. [Google Scholar] [CrossRef]
- Demartini, C.; Greco, R.; Zanaboni, A.M.; Francesconi, O.; Nativi, C.; Tassorelli, C.; Deseure, K. Antagonism of Transient Receptor Potential Ankyrin Type-1 Channels as a Potential Target for the Treatment of Trigeminal Neuropathic Pain: Study in an Animal Model. Int. J. Mol. Sci. 2018, 19, 3320. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, M.B.; de Melo Júnior, J.M.; Santos, S.A.; Melo, L.T.; Leite, L.H.; Vieira-Neto, A.E.; Moreira, R.A.; Monteiro-Moreira, A.C.; Campos, A.R. Frutalin reduces acute and neuropathic nociceptive behaviours in rodent models of orofacial pain. Chem. Biol. Interact. 2016, 256, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Fusi, C.; Materazzi, S.; Coppi, E.; Tuccinardi, T.; Marone, I.M.; De Logu, F.; Preti, D.; Tonello, R.; Chiarugi, A.; et al. The TRPA1 channel mediates the analgesic action of dipyrone and pyrazolone derivatives. Br. J. Pharmacol. 2015, 172, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, W.; Kim, J.H.; Woo, M.K.; Baek, J.Y.; Kim, S.Y.; Chung, S.H.; Kim, H.J. A Prospective Study of Chronic Oxaliplatin-Induced Neuropathy in Patients with Colon Cancer: Long-Term Outcomes and Predictors of Severe Oxaliplatin-Induced Neuropathy. J. Clin. Neurol. 2018, 14, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Authier, N.; Balayssac, D.; Marchand, F.; Ling, B.; Zangarelli, A.; Descoeur, J.; Coudore, F.; Bourinet, E.; Eschalier, A. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics 2009, 6, 620–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.H.; Zhang, L.; Zhou, Q.G.; Fang, Y.; Ge, W.H. (+)-Borneol attenuates oxaliplatin-induced neuropathic hyperalgesia in mice. NeuroReport 2016, 27, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Bian, D.; Jiang, B.; Zhai, Q.; Gao, N.; Wang, R. TRPA1 contributed to the neuropathic pain induced by docetaxel treatment. Cell. Biochem. Funct. 2017, 35, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Isami, K.; Nakamura, S.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol. Pain 2012, 28, 8–55. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Gees, M.; Harrison, S.; De Siena, G.; Materazzi, S.; Moretto, N.; Failli, P.; Preti, D.; Marchetti, N.; Cavazzini, A.; et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 2011, 152, 1621–1631. [Google Scholar] [CrossRef]
- Park, J.H.; Chae, J.; Roh, K.; Kil, E.J.; Lee, M.; Auh, C.K.; Lee, M.A.; Yeom, C.H.; Lee, S. Oxaliplatin-induced Peripheral Neuropathy via TRP1 Stimulation in Mice Dorsal Root Ganglion Is Correlated with Aluminium Accumulation. PLoS ONE 2015, 10, e0124875. [Google Scholar]
- Lee, M.; Cho, S.; Roh, K.; Chae, J.; Park, J.H.; Park, J.; Lee, M.A.; Kim, J.; Auh, C.K.; Yeom, C.H.; et al. Glutathione alleviated peripheral neuropathy in oxaliplatin-treated mice by removing aluminum from dorsal root ganglia. Am. J. Transl. Res. 2017, 9, 926–939. [Google Scholar] [PubMed]
- Obata, K.; Yamanaka, H.; Dai, Y.; Mizushima, T.; Fukuoka, T.; Tokunaga, A.; Noguchi, K. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur. J. Neurosci. 2004, 20, 2881–2895. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wu, H.Y.; Chen, Z.; Ma, A.N.; Mao, X.F.; Li, T.F.; Li, X.Y.; Wang, Y.X.; Pertovaara, A. Mechanical antihypersensitivity effect induced by repeated spinal administrations of a TRPA1 antagonist or a gap junction decoupler in peripheral neuropathy. Pharmacol. Biochem. Behav. 2016, 150–151, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Chukyo, A.; Chiba, T.; Kambe, T.; Yamamoto, K.; Kawakami, K.; Taguchi, K.; Abe, K. Oxaliplatin-induced changes in expression of transient receptor potential channels in the dorsal root ganglion as a neuropathic mechanism for cold hypersensitivity. Neuropeptides 2018, 67, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.T.; Xia, Z.Y.; Pan, X.; Zhao, B.; Liu, Z.G. Puerarin ameliorates allodynia and hyperalgesia in rats with peripheral nerve injury. Neural. Regen. Res. 2018, 13, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ma, S.; Wang, Y.; Huang, T.; Zhu, Z.; Zhao, G. Mus musculus-microRNA-449a ameliorates neuropathic pain by decreasing the level of KCNMA1 and TRPA1, and increasing the level of TPTE. Mol. Med. Rep. 2017, 16, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Zheng, L.; Acosta, G.; Vega-Alvarez, S.; Chen, Z.; Muratori, B.; Cao, P.; Shi, R. Acrolein contributes to TRPA1 up-regulation in peripheral and central sensory hypersensitivity following spinal cord injury. J. Neurochem. 2015, 135, 987–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, Z.Y.; Wen, Y.R.; Zhang, D.R.; Borsello, T.; Bonny, C.; Strichartz, G.R.; Decosterd, I.; Ji, R.R. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006, 26, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, Z.; Tang, J.; Cao, P.; Shi, R. Acrolein Contributes to the Neuropathic Pain and Neuron Damage after Ischemic-Reperfusion Spinal Cord Injury. Neuroscience 2017, 384, 120–130. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Wang, Z.J. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 2011, 193, 440–451. [Google Scholar] [CrossRef]
- Butler, B.; Acosta, G.; Shi, R. Exogenous Acrolein intensifies sensory hypersensitivity after spinal cord injury in rat. J. Neurol. Sci. 2017, 379, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Park, J.; Butler, B.; Acosta, G.; Vega-Alvarez, S.; Zheng, L.; Tang, J.; McCain, R.; Zhang, W.; Ouyang, Z.; et al. Mitigation of sensory and motor deficits by acrolein scavenger phenelzine in a rat model of spinal cord contusive injury. J. Neurochem. 2016, 138, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Schäfers, M.; Svensson, C.I.; Sommer, C.; Sorkin, L.S. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 2003, 23, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Mizushima, T.; Katsura, H.; Fukuoka, T.; Tokunaga, A.; Noguchi, K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J. Neurosci. 2004, 24, 10211–10222. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, L.; Ruan, Y.; Geng, X.; Xu, M.; Yang, N.; Yu, L.; Jiang, Y.; Zhu, C.; Yang, Y.; et al. Facilitation of MrgprD by TRP-A1 promotes neuropathic pain. FASEB J. 2019, 33, 1360–1373. [Google Scholar] [CrossRef]
- Yamamoto, K.; Chiba, N.; Chiba, T.; Kambe, T.; Abe, K.; Kawakami, K.; Utsunomiya, I.; Taguchi, K. Transient receptor potential ankyrin 1 that is induced in dorsal root ganglion neurons contributes to acute cold hypersensitivity after oxaliplatin administration. Mol. Pain 2015, 11, e69. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Deng, T.; Shang, Z.; Wang, D.; Xiao, Y. Blocking TRPA1 and TNF-α Signal Improves Bortezomib-Induced Neuropathic Pain. Cell Physiol. Biochem. 2018, 51, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Fan, T.; Zhou, N.; Guo, H.; Zhang, W. Role of PAR2 in regulating oxaliplatin-induced neuropathic pain via TRPA. Transl. Neurosci. 2015, 6, 111–116. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Z.F.; Liao, M.F.; Yao, W.L.; Wang, J.; Wang, X.R. Blocking PAR2 attenuates oxaliplatin-induced neuropathic pain via TRPV1 and releases of substance P and CGRP in superficial dorsal horn of spinal cord. J. Neurol. Sci. 2015, 352, 62–97. [Google Scholar] [CrossRef]
- Wang, Q.; Wang Gao, D.; Li, J. Inhibition of PAR2 and TRPA1 signals alleviates neuropathic pain evoked by chemotherapeutic bortezomib. J. Biol. Regul. Homeost. Agents 2017, 31, 977–983. [Google Scholar]
- Wu, Z.; Wang, S.; Wu, I.; Mata, M.; Fink, D.J. Activation of TLR-4 to produce tumour necrosis factor-α in neuropathic pain caused by paclitaxel. Eur. J. Pain 2015, 19, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Materazzi, S.; Benemei, S.; Geppetti, P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev. Physiol. Biochem. Pharmacol. 2014, 167, 1–43. [Google Scholar] [PubMed]
- Trevisan, G.; Benemei, S.; Materazzi, S.; De Logu, F.; De Siena, G.; Fusi, C.; Fortes Rossato, M.; Coppi, E.; Marone, I.M.; Ferreira, J.; et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 2016, 139, 1361–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materazzi, S.; Fusi, C.; Benemei, S.; Pedretti, P.; Patacchini, R.; Nilius, B.; Prenen, J.; Creminon, C.; Geppetti, P.; Nassini, R. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. 2012, 463, 561–569. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kaneko, S. Roles of Transient Receptor Potential Ankyrin 1 in Oxaliplatin-Induced Peripheral Neuropathy. Biol. Pharm. Bull. 2017, 40, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, T.; Nakamura, S.; Meng, Z.; Hamano, S.; Inoue, K.; Numata, T.; Takahashi, N.; Nagayasu, K.; Shirakawa, H.; Mori, Y.; et al. Distinct Mechanism of Cysteine Oxidation-Dependent Activation and Cold Sensitization of Human Transient Receptor Potential Ankyrin 1 Channel by High and Low Oxaliplatin. Front. Physiol. 2017, 8, e878. [Google Scholar] [CrossRef]
- Trevisan, G.; Materazzi, S.; Fusi, C.; Altomare, A.; Aldini, G.; Lodovici, M.; Patacchini, R.; Geppetti, P.; Nassini, R. Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 2013, 73, 3120–3131. [Google Scholar] [CrossRef]
- Miao, F.; Wang, R.; Cui, G.; Li, X.; Wang, T.; Li, X. Engagement of MicroRNA-155 in Exaggerated Oxidative Stress Signal and TRPA1 in the Dorsal Horn of the Spinal Cord and Neuropathic Pain During Chemotherapeutic Oxaliplatin. Neurotox. Res. 2019. [Google Scholar] [CrossRef]
- Sagalajev, B.; Wei, H.; Chen, Z.; Albayrak, I.; Koivisto, A.; Pertovaara, A. Oxidative Stress in the Amygdala Contributes to Neuropathic Pain. Neuroscience 2018, 387, 92–103. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Gonçalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017, 8, e1887. [Google Scholar] [CrossRef]
- Horváth, Á.; Tékus, V.; Bencze, N.; Szentes, N.; Scheich, B.; Bölcskei, K.; Szőke, É.; Mócsai, A.; Tóth-Sarudy, É.; Mátyus, P.; et al. Analgesic effects of the novel semicarbazide-sensitive amine oxidase inhibitor SZV 1287 in mouse pain models with neuropathic mechanisms: Involvement of transient receptor potential vanilloid 1 and ankyrin 1 receptors. Pharmacol. Res. 2018, 131, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Canning, B.; McGarvey, L. Eight International London Cough Symposium 2014: Cough hypersensitivity syndrome as the basis for chronic cough. Pulm. Pharmacol. Ther. 2015, 35, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2507. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02432664 (accessed on 6 May 2019).
- Eberhardt, M.J.; Schillers, F.; Eberhardt, E.M.; Risser, L.; La Roche, J.D.; Herzog, C.; Echtermeyer, F.; Leffler, A. Reactive metabolites of acetaminophen activate and sensitize the capsaicin receptor TRPV. Sci. Rep. 2017, 7, e12775. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.G.; Namer, B.; Reeh, P.W.; Fischer, M.J.M. TRPA1 and TRPV1 antagonists do not inhibit human acidosis-induced pain. J. Pain 2017, 18, 526–534. [Google Scholar] [CrossRef] [PubMed]

| Effect | Region | Residues | Example Substances | References | ||
|---|---|---|---|---|---|---|
| Electrophilic agonists | Pre-S1 region | C621, C641, C655, K710 | Mustard oil, tetrahydrocannabinol, allicin, acrolein, formaldehyde, N-methylmaleimide, cinnamaldehyde, eugenol, gingerol, thymol | [41,42,43,44,45,46,47] | ||
| Modulators | Ankyrin-repeat domain | C414 | Ca2+ ions | [48,49,50,51,53] | ||
| S4 | ||||||
| S1–S4 | Negatively charged residues | Tarantula toxin | [52] | |||
| Modulator | S5–S6 | V875 | Menthol | [54] | ||
| Nonelectrophilic agonists | S873 | Eudesmol | [55] | |||
| Protons | [56] | |||||
| Antagonists | S5–S6 | T874, V876, F877, M956 | 6-Methyl-5-(2-(trifluoromethyl)phenyl)-1H-indazole | [57] | ||
| Selectivity filter | M911, M912 | CMP1, AZ868, HC-030031 | [58,59,60,61] | |||
| S6 | I940–I950 | |||||
| S5 | S873, T874 | F909 | A-967079 | [39,62] | ||
| Monoterpens | [63] | |||||
| Anaesthetics | M912 M953 | Propofol, isofluorane | [64] | |||
| Modulator | S1–S4 | H719, N722, K787, K796, R852, K989 | Phosphoinositides | [66] | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorgi, S.; Nikolaeva-Koleva, M.; Alarcón-Alarcón, D.; Butrón, L.; González-Rodríguez, S. Is TRPA1 Burning Down TRPV1 as Druggable Target for the Treatment of Chronic Pain? Int. J. Mol. Sci. 2019, 20, 2906. https://doi.org/10.3390/ijms20122906
Giorgi S, Nikolaeva-Koleva M, Alarcón-Alarcón D, Butrón L, González-Rodríguez S. Is TRPA1 Burning Down TRPV1 as Druggable Target for the Treatment of Chronic Pain? International Journal of Molecular Sciences. 2019; 20(12):2906. https://doi.org/10.3390/ijms20122906
Chicago/Turabian StyleGiorgi, Simona, Magdalena Nikolaeva-Koleva, David Alarcón-Alarcón, Laura Butrón, and Sara González-Rodríguez. 2019. "Is TRPA1 Burning Down TRPV1 as Druggable Target for the Treatment of Chronic Pain?" International Journal of Molecular Sciences 20, no. 12: 2906. https://doi.org/10.3390/ijms20122906