Recent Progress in TRPM8 Modulation: An Update
Abstract
:1. Introduction
2. TRPM8 Agonists
3. TRPM8 Antagonists
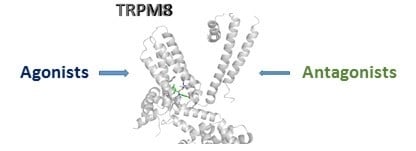
4. TRPM8 3D Structure
5. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Liu, Y.; Qin, N. TRPM8 in health and disease: Cold sensing and beyond. Adv. Exp. Med. Biol. 2011, 704, 185–208. [Google Scholar] [PubMed]
- Almaraz, L.; Manenschijn, J.-A.; de la Peña, E.; Viana, F. TRPM8. In Mammalian Transient Receptor Potential (TRP) cation Channels; Nilius, B., Flockerzi, V., Eds.; Springer: Berlin, Germany, 2014; ISBN 978-3-642-54215-2. [Google Scholar]
- Zakharian, E.; Cao, C.; Rohacs, T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 2010, 30, 12526–12534. [Google Scholar] [CrossRef]
- Daniels, R.L.; Takashima, Y.; McKemy, D.D. Activity of the Neuronal Cold Sensor TRPM8 Is Regulated by Phospholipase C via the Phospholipid Phosphoinositol 4,5-Bisphosphate. J. Biol. Chem. 2009, 284, 1570–1582. [Google Scholar] [CrossRef] [Green Version]
- Asuthkar, S.; Demirkhanyan, L.; Sun, X.; Velpula, K.K.; Zakharian, E.; Elustondo, P.A.; Krishnan, V.; Baskaran, P.; Thyagarajan, B.; Pavlov, E.V. The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8. J. Biol. Chem. 2015, 290, 2670–2688. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Valente, J.; Andreou, A.P.; Urban, L.; Nagy, I. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br. J. Pharmacol. 2014, 171, 2508–2527. [Google Scholar] [CrossRef]
- Lippoldt, E.K.; Elmes, R.R.; McCoy, D.D.; Knowlton, W.M.; McKemy, D.D. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J. Neurosci. 2013, 33, 12543–12552. [Google Scholar] [CrossRef]
- Tang, Z.; Kim, A.; Masuch, T.; Park, K.; Weng, H.; Wetzel, C.; Dong, X. Pirt functions as an endogenous regulator of TRPM8. Nat. Commun. 2013, 4, 3179/1–3179/9. [Google Scholar] [CrossRef]
- Weyer, A.; Lehto, S. Development of TRPM8 Antagonists to Treat Chronic Pain and Migraine. Pharmaceuticals 2017, 10, 37. [Google Scholar] [CrossRef]
- Dussor, G.; Cao, Y.-Q. TRPM8 and Migraine. Headache 2016, 56, 1406–1417. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, C.; Acosta, M.C.; Gallar, J.; Merayo-Lloves, J. What Causes Eye Pain? Curr. Ophthalmol. Rep. 2015, 3, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.M.; Wei, E.T.; Kim, S.J.; Yoon, K.C. TRPM8 Channels and Dry Eye. Pharmaceuticals 2018, 11, 125. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.; Wei, Z.; Wang, X.; Shen, P.; Wang, S.; Wang, A.; Chen, W.; Lu, Y. TRPM8: A potential target for cancer treatment. J. Cancer Res. Clin. Oncol. 2016, 142, 1871–1881. [Google Scholar] [CrossRef]
- Hantute-Ghesquier, A.; Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. TRPM family channels in cancer. Pharmaceuticals 2018, 11, 58. [Google Scholar] [CrossRef]
- Yee, N.S. TRPM8 Ion Channels as Potential Cancer Biomarker and Target in Pancreatic Cancer. Adv. Protein Chem. Struct. Biol. 2016, 104, 127–155. [Google Scholar]
- Henstroem, M.; Hadizadeh, F.; Beyder, A.; Bonfiglio, F.; Zheng, T.; Assadi, G.; Rafter, J.; Bujanda, L.; Agreus, L.; Andreasson, A.; et al. TRPM8 polymorphisms associated with increased risk of IBS-C and IBS-M. Gut 2017, 66, 1725–1728. [Google Scholar] [CrossRef]
- Alvarez-Berdugo, D.; Rofes, L.; Casamitjana, J.F.; Enrique, A.; Chamizo, J.; Vina, C.; Pollan, C.M.; Clave, P. TRPM8, ASIC1, and ASIC3 localization and expression in the human oropharynx. Neurogastroenterol. Motil. 2018, 30, e13398. [Google Scholar] [CrossRef]
- Bonvini, S.J.; Belvisi, M.G. Cough and airway disease: The role of ion channels. Pulm. Pharmacol. Ther. 2017, 47, 21–28. [Google Scholar] [CrossRef]
- Huang, F.; Ni, M.; Zhang, J.-M.; Li, D.-J.; Shen, F.-M. TRPM8 downregulation by angiotensin II in vascular smooth muscle cells is involved in hypertension. Mol. Med. Rep. 2017, 15, 1900–1908. [Google Scholar] [CrossRef]
- McKemy, D.D. Therapeutic potential of TRPM8 modulators. Open Drug Discov. J. 2010, 2, 81–88. [Google Scholar] [CrossRef]
- DeFalco, J.; Duncton, M.A.J.; Emerling, D. TRPM8 biology and medicinal chemistry. Curr. Top. Med. Chem. 2011, 11, 2237–2252. [Google Scholar] [CrossRef]
- Shailendra Kapoor, M.D. TRPM8 antagonists and their emerging role in the modulation of pain and allodynia. Biochem. Biophys. Res. Commun. 2012, 420, 937. [Google Scholar] [CrossRef] [PubMed]
- Journigan, V.B.; Zaveri, N.T. TRPM8 ion channel ligands for new therapeutic applications and as probes to study menthol pharmacology. Life Sci. 2013, 92, 425–437. [Google Scholar] [CrossRef]
- Pérez de Vega, M.J.; Gómez-Monterrey, I.; Ferrer-Montiel, A.; González-Muñiz, R. Transient Receptor Potential Melastatin 8 Channel (TRPM8) Modulation: Cool Entryway for Treating Pain and Cancer. J. Med. Chem. 2016, 59, 10006–10029. [Google Scholar] [CrossRef] [Green Version]
- Calixto, J.B.; Kassuya, C.A.L.; Andre, E.; Ferreira, J. Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacol. Ther. 2005, 106, 179–208. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Junior, E.V.M.; Oliveira, F.S.; de Almeida, R.N.; Nunes, X.P.; Barbosa-Filho, J.M. Antinociceptive activity of structural analogues of rotundifolone: Structure-activity relationship. Z. Fur Nat. C 2007, 62, 39–42. [Google Scholar] [CrossRef]
- Silva, D.F.; de, A.M.M.; Chaves, C.G.; Braz, A.L.; de, A.J.G.F.; Araujo, I.G.A.; Barbosa-Filho, J.M.; Correia, N.d.A.; de, M.I.A.; Gomes, M.A.; et al. TRPM8 Channel Activation Induced by Monoterpenoid Rotundifolone Underlies Mesenteric Artery Relaxation. PLoS ONE 2015, 10, e0143171. [Google Scholar] [CrossRef]
- Urata, T.; Mori, N.; Fukuwatari, T. Vagus nerve is involved in the changes in body temperature induced by intragastric administration of 1,8-cineole via TRPM8 in mice. Neurosci. Lett. 2017, 650, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Caceres, A.I.; Liu, B.; Jabba, S.V.; Achanta, S.; Morris, J.B.; Jordt, S.-E. Transient Receptor Potential Cation Channel Subfamily M Member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 2017, 174, 867–879. [Google Scholar] [CrossRef]
- Hoag, G.E. Topical analgesic pain relief and anti-inflammation formulations comprising plant exts., manufacture and methods of use thereof. Patent Number WO2017059088, 6 April 2017. [Google Scholar]
- Chen, G.-L.; Zou, F.; Chen, G.-L.; Lei, M.; Zhou, L.-P.; Zeng, B. Borneol Is a TRPM8 Agonist that Increases Ocular Surface Wetness. PLoS ONE 2016, 11, e0158868. [Google Scholar] [CrossRef]
- LeGay, C.M.; Gorobets, E.; Iftinca, M.; Ramachandran, R.; Altier, C.; Derksen, D.J. Natural-Product-Derived Transient Receptor Potential Melastatin 8 (TRPM8) Channel Modulators. Org. Lett. 2016, 18, 2746–2749. [Google Scholar] [CrossRef] [PubMed]
- Velazco, M.I.; Wuensche, L.; Deladoey, P. Use of cubebol as a flavoring ingredient. Patent Number EP 1040765, 4 October 2000. [Google Scholar]
- Ferrer Montiel, A.V.; Fernandez Carvajal, A.; Belmonte Martinez, C.; Gallar Martinez, J.; De la Torre, R.; Genazzani, A.; Tron, G.C.; Mercalli, V. Preparation and therapeutical uses of triazole derivatives as TRPM8 receptor agonists. Patent Number WO2017125634, 27 July 2017. [Google Scholar]
- Yelm, K.E.; Wos, J.A.; Bunke, G.M.; Frederick, H.; Haught, J.C.; Hoke, S.H.; Sreekrishna, K.T.; Lin, Y. Synthesis of aryl cyclohexane carboxamide derivatives useful as sensates in consumer products. Patent Number US2017/0057911, 2 March 2017. [Google Scholar]
- Wos, J.A.; Yelm, K.E.; Bunke, G.M.; Frederick, H.A.; Reilly, M.; Haught, J.C.; Sreekrishna, K.T.; Lin, Y. Synthesis of cyclohexane ester derivatives useful as cooling sensates in consumer products. Patent Number WO2017106279, 22 June 2017. [Google Scholar]
- Bharate, S.S.; Bharate, S.B. Modulation of Thermoreceptor TRPM8 by cooling compounds. ACS Chem. Neurosci. 2012, 3, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Staender, S.; Augustin, M.; Roggenkamp, D.; Blome, C.; Heitkemper, T.; Worthmann, A.C.; Neufang, G. Novel TRPM8 agonist cooling compound against chronic itch: Results from a randomized, double-blind, controlled, pilot study in dry skin. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1064–1068. [Google Scholar] [CrossRef]
- Misery, L.; Huet, F.; Misery, L.; Huet, F.; Santerre, A.; Neufang, G.; Batardiere, A.; Hornez, N.; Nedelec, A.S.; Le, C.F.; et al. Real-life study of anti-itching effects of a cream containing menthoxypropanediol, a TRPM8 agonist, in atopic dermatitis patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e67–e69. [Google Scholar] [CrossRef]
- Roggenkamp, D.; Worthmann, A.-C.; Sulzberger, M.; Wenck, H.; Staeb, F.; Neufang, G. Menthoxypropanediol inhibits nerve growth factor-induced nerve fibre sprouting in coculture models of sensory neurons and skin cells. Exp. Dermatol. 2016, 25, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.J.; Ericson, M.E.; Hosoi, J.; Seiffert, K.; Hordinsky, M.K.; Ansel, J.C.; Paus, R.; Scholzen, T.E. Neuropeptide Control Mechanisms in Cutaneous Biology: Physiological and Clinical Significance. J. Invest. Dermatol. 2006, 126, 1937–1947. [Google Scholar] [CrossRef]
- Takashima, Y.; Daniels, R.L.; Knowlton, W.; Teng, J.; Liman, E.R.; McKemy, D.D. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J. Neurosci. 2007, 27, 14147–14157. [Google Scholar] [CrossRef] [PubMed]
- Reaume, A.G.; Cong, W.; Greenway, F.; Coulter, A. Treatment of adipocytes. Patent Number US20180289707, 11 October 2018. [Google Scholar]
- Shirai, T.; Kumihashi, K.; Sakasai, M.; Kusuoku, H.; Shibuya, Y.; Ohuchi, A. Identification of a Novel TRPM8 Agonist from Nutmeg: A Promising Cooling Compound. ACS Med. Chem. Lett. 2017, 8, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Subkowski, T.; Bollschweiler, C.; Wittenberg, J.; Krohn, M.; Zinke, H. Screening for low molecular-weight modulators of the cold-menthol receptor TRPM8 for therapeutic and cosmetic use. Patent Number WO2010026094, 11 March 2010. [Google Scholar]
- Surburg, H.; Backes, M.; Oertling, H.; Machinek, A.; Loges, H.; Simchen, U.; Subkowski, T.; Bollscheiler, C.; Wittenberg, J.; Siegel, W. Use of physiological cooling active ingredients such as transient receptor potential cation channel 8 modulator for achieving cooling effect on skin or mucous membrane. Patent Number WO2011061330, 26 May 2011. [Google Scholar]
- Subkowski, T.; Bollschweiler, C.; Wittenberg, J.; Siegel, W.; Pelzer, R. Preparation of spiro compounds as low molecular weight modulators of the cold-menthol receptor TRPM8 and use thereof. Patent Number WO2013041621, 28 March 2013. [Google Scholar]
- Wei, E.T. Di-isopropyl-phosphinoyl-alkane compounds as topical agents for the treatment of sensory discomfort. Patent Number US20150164924, 18 June 2015. [Google Scholar]
- Wei, E.T. Dialkyl-phosphinoyl-alkane (DAPA) compounds and compositions for treatment of lower gastrointestinal tract disorders. Patent Number US20170189428, 6 July 2017. [Google Scholar]
- Yang, J.M.; Fengxian Li, F.; Liu, Q.; Rüedi, M.; Wei, E.T.; Lentsman, M.; Lee, H.S.; Choi, W.; Kim, S.J.; Yoon, K.C. TRPM8 agonist relieves dry eye discomfort. BMC Ophthalmol. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Priest, C.; Noncovich, A.; Patron, A.; Ung, J. Preparation of heteroaryl amide compounds useful as modulators of TRPM8. Patent Number WO2012061698, 10 May 2012. [Google Scholar]
- Chumakova, L.; Patron, A.; Priest, C.; Karanewsky, D.S.; Kimmich, R.; Boren, B.C.; Hammaker, J.R.; Chumakov, V.; Zhao, W.; Noncovich, A.; et al. Heterocycles and related compounds useful as modulators of TRPM8 and their preparation. Patent Number WO2014130582, 28 August 2014. [Google Scholar]
- Noncovich, A.; Priest, C.; Ung, J.; Patron, A.P.; Servant, G.; Brust, P.; Servant, N.; Faber, N.; Liu, H.; Gonsalves, N.S.; et al. Discovery and development of a novel class of phenoxyacetyl amides as highly potent TRPM8 agonists for use as cooling agents. Bioorg. Med. Chem. Lett. 2017, 27, 3931–3938. [Google Scholar] [CrossRef] [PubMed]
- Join, B.; Ongouta, J.; Backes, M.; Brodhage, R.; Machinek, A.; Loges, H.; Mundt, S.; Somers, T.; Subkowski, T.; Wittenberg, J.; et al. Use of physiological cooling active ingredients for modulation of menthol receptor TRPM8, and compositions comprising such active ingredients. Patent Number WO2019043164, 7 March 2019. [Google Scholar]
- Babes, R.-M.; Selescu, T.; Domocos, D.; Babes, A. The anthelminthic drug praziquantel is a selective agonist of the sensory transient receptor potential melastatin type 8 channel. Toxicol. Appl. Pharmacol. 2017, 336, 55–65. [Google Scholar] [CrossRef]
- Gunaratne, G.S.; Yahya, N.A.; Marchant, J.S.; Dosa, P.I.; Marchant, J.S. Activation of host transient receptor potential (TRP) channels by praziquantel stereoisomers. PLoS Negl. Trop. Dis. 2018, 12, e0006420. [Google Scholar] [CrossRef]
- Johnson, C.D.; Melanaphy, D.; Purse, A.; Stokesberry, S.A.; Dickson, P.; Zholos, A. V Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am. J. Physiol. 2009, 296, H1868–H1877. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, N.; Biebermann, H.; Mergler, S. 3-Iodothyronamine, a Novel Endogenous Modulator of Transient Receptor Potential Melastatin 8? Front. Endocrinol. 2017, 8, 198. [Google Scholar] [CrossRef]
- Schanze, N.; Rijntjes, E.; Del, O.M.; Hoefig, C.S.; Lehmphul, I.; Kohrle, J.; Schanze, N.; Jacobi, S.F.; Hoefig, C.S.; Mittag, J.; et al. 3-Iodothyronamine Decreases Expression of Genes Involved in Iodide Metabolism in Mouse Thyroids and Inhibits Iodide Uptake in PCCL3 Thyrocytes. Thyroid 2017, 27, 11–22. [Google Scholar] [CrossRef]
- Khajavi, N.; Reinach, P.S.; Slavi, N.; Skrzypski, M.; Lucius, A.; Strauss, O.; Koehrle, J.; Mergler, S. Thyronamine induces TRPM8 channel activation in human conjunctival epithelial cells. Cell. Signal. 2015, 27, 315–325. [Google Scholar] [CrossRef]
- Braunig, J.; Jyrch, S.; Biebermann, H.; Khajavi, N.; Braunig, J.; Jyrch, S.; Biebermann, H.; Khajavi, N.; Mergler, S.; Hoefig, C.S.; et al. 3-Iodothyronamine Activates a Set of Membrane Proteins in Murine Hypothalamic Cell Lines. Front. Endocrinol. 2018, 9, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucius, A.; Khajavi, N.; Huimann, P.; Ljubojevic, N.; Reinach, P.S.; Kohrle, J.; Dhandapani, P.; Grotzinger, C.; Mergler, S. 3-Iodothyronamine increases transient receptor potential melastatin channel 8 (TRPM8) activity in immortalized human corneal epithelial cells. Cell. Signal. 2016, 28, 136–147. [Google Scholar] [CrossRef]
- Mergler, S.; Cheng, Y.; Skosyrski, S.; Garreis, F.; Pietrzak, P.; Kociok, N.; Dwarakanath, A.; Reinach, P.S.; Kakkassery, V. Altered calcium regulation by thermosensitive transient receptor potential channels in etoposide-resistant WERI-Rb1 retinoblastoma cells. Exp. Eye Res. 2012, 94, 157–173. [Google Scholar] [CrossRef]
- Walcher, L.; Budde, C.; Bohm, A.; Ljubojevic, N.; Schweiger, M.W.; Waydbrink, H.v.d.; Reimers, I.; Mergler, S.; Reinach, P.S.; Dhandapani, P.; et al. TRPM8 Activation via 3-Iodothyronamine Blunts VEGF-Induced Transactivation of TRPV1 in Human Uveal Melanoma Cells. Front. Pharmacol. 2018, 9, 1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoefig, C.S.; Zucchi, R.; Koehrle, J. Thyronamines and Derivatives: Physiological Relevance, Pharmacological Actions, and Future Research Directions. Thyroid 2016, 26, 1656–1673. [Google Scholar] [CrossRef] [PubMed]
- Arcas, J.M.; Gonzalez, A.; Gers-Barlag, K.; Gonzalez-Gonzalez, O.; Bech, F.; Belmonte, C.; Gomis, A.; Viana, F.; Gonzalez-Gonzalez, O.; Bech, F.; et al. The Immunosuppressant Macrolide Tacrolimus Activates Cold-Sensing TRPM8 Channels. J. Neurosci. 2019, 39, 949–969. [Google Scholar] [CrossRef]
- Bas, E.; Naziroglu, M.; Naziroglu, M.; Pecze, L. ADP-Ribose and oxidative stress activate TRPM8 channel in prostate cancer and kidney cells. Sci. Rep. 2019, 9, 4100. [Google Scholar] [CrossRef]
- Janssens, A.; Gees, M.; Toth, B.I.; Ghosh, D.; Mulier, M.; Vennekens, R.; Vriens, J.; Talavera, K.; Voets, T. Definition of two agonist types at the mammalian cold-activated channel TRPM8. Elife 2016, 5, e17240/1–e17240/21. [Google Scholar] [CrossRef]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Arroyo, F.J.; Orlando, P.; Schiano Moriello, A.; Vitale, R.M.; Amodeo, P.; Sánchez, A.; Roncero, C.; Bianchini, G.; Martín, M.A.; et al. Tetrahydroisoquinoline-Derived Urea and 2,5-Diketopiperazine Derivatives as Selective Antagonists of the Transient Receptor Potential Melastatin 8 (TRPM8) Channel Receptor and Antiprostate Cancer Agents. J. Med. Chem. 2016, 59, 5661–5683. [Google Scholar] [CrossRef]
- Horne, D.B.; Tamayo, N.A.; Bartberger, M.D.; Bo, Y.; Clarine, J.; Davis, C.D.; Gore, V.K.; Kaller, M.R.; Lehto, S.G.; Ma, V.V.; et al. Optimization of Potency and Pharmacokinetic Properties of Tetrahydroisoquinoline Transient Receptor Potential Melastatin 8 (TRPM8) Antagonists. J. Med. Chem. 2014, 57, 2989–3004. [Google Scholar] [CrossRef] [PubMed]
- Lehto, S.G.; Weyer, A.D.; Zhang, M.; Youngblood, B.D.; Wang, J.; Wang, W.; Kerstein, P.C.; Davis, C.; Wild, K.D.; Stucky, C.L.; et al. AMG2850, a potent and selective TRPM8 antagonist, is not effective in rat models of inflammatory mechanical hypersensitivity and neuropathic tactile allodynia. Naunyn. Schmiedebergs. Arch. Pharmacol. 2015, 388, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.B.; Biswas, K.; Brown, J.; Bartberger, M.D.; Clarine, J.; Davis, C.D.; Gore, V.K.; Harried, S.; Horner, M.; Kaller, M.R.; et al. Discovery of TRPM8 Antagonist ( S )-6-(((3-Fluoro-4-(trifluoromethoxy)phenyl)(3-fluoropyridin-2-yl)methyl)carbamoyl)nicotinic Acid (AMG 333), a Clinical Candidate for the Treatment of Migraine. J. Med. Chem. 2018, 61, 8186–8201. [Google Scholar] [CrossRef]
- Bertamino, A.; Ostacolo, C.; Ambrosino, P.; Musella, S.; Di Sarno, V.; Ciaglia, T.; Soldovieri, M.V.; Iraci, N.; Fernandez Carvajal, A.; de la Torre-Martinez, R.; et al. Tryptamine-Based Derivatives as Transient Receptor Potential Melastatin Type 8 (TRPM8) Channel Modulators. J. Med. Chem. 2016, 59, 2179–2191. [Google Scholar] [CrossRef] [Green Version]
- Bertamino, A.; Iraci, N.; Ostacolo, C.; Ambrosino, P.; Musella, S.; Di Sarno, V.; Ciaglia, T.; Pepe, G.; Sala, M.; Soldovieri, M.V.; et al. Identification of a Potent Tryptophan-Based TRPM8 Antagonist With in Vivo Analgesic Activity. J. Med. Chem. 2018, 61, 6140–6152. [Google Scholar] [CrossRef]
- de la Torre-Martínez, R.; Bonache, M.A.; Llabrés-Campaner, P.J.; Balsera, B.; Fernández-Carvajal, A.; Fernández-Ballester, G.; Ferrer-Montiel, A.; Pérez de Vega, M.J.; González-Muñiz, R. Synthesis, high-throughput screening and pharmacological characterization of β–lactam derivatives as TRPM8 antagonists. Sci. Rep. 2017, 7, 10766. [Google Scholar] [CrossRef]
- Kobayashi, J.; Hirasawa, H.; Ozawa, T.; Ozawa, T.; Takeda, H.; Fujimori, Y.; Nakanishi, O.; Kamada, N.; Ikeda, T. Synthesis and optimization of novel α-phenylglycinamides as selective TRPM8 antagonists. Bioorg. Med. Chem. 2017, 25, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Fujimori, Y.; Kobayashi, J.; Nakanishi, O.; Hirasawa, H.; Kume, H.; Homma, Y.; Igawa, Y. KPR-2579, a novel TRPM8 antagonist, inhibits acetic acid-induced bladder afferent hyperactivity in rats. Neurourol. Urodyn. 2018, 37, 1633–1640. [Google Scholar] [CrossRef]
- Hirasawa, H.; Kawamura, N.; Kobayashi, J. TRPM8 inhibitors containing α-substituted glycine amides. Patent Number JP 2016094407, 26 May 2016. [Google Scholar]
- Hirasawa, H.; Tanada, F.; Mutai, Y.; Fushimi, N.; Kobayashi, J.; Kijima, Y. Pharmaceutical composition containing pyrazole derivatives as TRPM8 inhibitors. Patent Number JP2018100269, 28 June 2018. [Google Scholar]
- Hirasawa, H.; Tanada, F.; Mutai, Y.; Fushimi, N.; Kobayashi, J.; Kijima, Y. Method for the preparation of pyrazole derivatives. Patent Number JP 2018108988A, 7 December 2018. [Google Scholar]
- Beccari, A.R.; Gemei, M.; Lo Monte, M.; Menegatti, N.; Fanton, M.; Pedretti, A.; Bovolenta, S.; Nucci, C.; Molteni, A.; Rossignoli, A.; et al. Novel selective, potent naphthyl TRPM8 antagonists identified through a combined ligand- and structure-based virtual screening approach. Sci. Rep. 2017, 7, 10999. [Google Scholar] [CrossRef]
- Kato, T.; Sakamoto, T.; Niwa, Y.; Sawamoto, D.; Otani, N.K.M. Preparation of aromatic carboxylic acida mides having TRPM8 blocking effect. Patent Number JP2017214290, 7 December 2017. [Google Scholar]
- Aramini, A.; Bianchini, G.; Lillini, S. Preparation of 4-hydroxy-2-phenyl-1,3-thiazol-5-yl methanone derivatives as TRPM8 antagonists. Patent Number WO2017108632, 29 June 2017. [Google Scholar]
- Shishido, Y.; Ohmi, M. Preparation of imidazolinone derivatives as TRPM8 antagonist. Patent Number WO2017043092, 16 March 2017. [Google Scholar]
- Aizawa, N.; Ohshiro, H.; Watanabe, S.; Kume, H.; Homma, Y.; Igawa, Y. RQ-00434739, a novel TRPM8 antagonist, inhibits prostaglandin E2-induced hyperactivity of the primary bladder afferent nerves in rats. Life Sci. 2019, 218, 89–95. [Google Scholar] [CrossRef]
- Moriconi, A.; Bianchini, G.; Colagioia, S.; Brandolini, L.; Aramini, A.; Liberati, C.; Bovolenta, S. TRPM8 antagonists. Patent Number WO2013092711, 27 June 2013. [Google Scholar]
- Mistretta, F.A.; Russo, A.; Castiglione, F.; Bettiga, A.; Colciago, G.; Montorsi, F.; Brandolini, L.; Aramini, A.; Bianchini, G.; Allegretti, M.; et al. DFL23448, A Novel Transient Receptor Potential Melastin 8-Selective Ion Channel Antagonist, Modifies Bladder Function and Reduces Bladder Overactivity in Awake Rats. J. Pharmacol. Exp. Ther. 2016, 356, 200–211. [Google Scholar] [CrossRef]
- De Caro, C.; Russo, R.; Avagliano, C.; Cristiano, C.; Calignano, A.; Aramini, A.; Bianchini, G.; Allegretti, M.; Brandolini, L. Antinociceptive effect of two novel transient receptor potential melastatin 8 antagonists in acute and chronic pain models in rat. Br. J. Pharmacol. 2018, 175, 1691–1706. [Google Scholar] [CrossRef] [Green Version]
- Abelson, M.B.; Corcoran, P.; Lnea, K. Transient receptor potential cation channel subfamily M member 8 (TRPM8) Antagonist and methods of use. Patent Number WO2017062570, 13 April 2017. [Google Scholar]
- Palumbo, J.M. Composition for treating or preventing vasomotor symptoms. Patent Number WO 2017217351, 21 December 2017. [Google Scholar]
- Yapa, K.T.D.S.; Deuis, J.; Peters, A.A.; Kenny, P.A.; Roberts-Thomson, S.J.; Vetter, I.; Monteith, G.R. Assessment of the TRPM8 inhibitor AMTB in breast cancer cells and its identification as an inhibitor of voltage gated sodium channels. Life Sci. 2018, 198, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollastro, F.; De Petrocellis, L.; Schiano-Moriello, A.; Chianese, G.; Heyman, H.; Appendino, G.; Taglialatela-Scafati, O. Amorfrutin-type phytocannabinoids from Helichrysum umbraculigerum. Fitoterapia 2017, 123, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues de Carvalho, A.M.; Vasconcelos, L.F.; Rocha, N.F.M.; Vasconcelos Rios, E.R.; Dias, M.L.; Fonteles, M.M.d.F.; Gaspar, D.M.; Barbosa Filho, J.M.; Gutierrez, S.J.C.; Florenco de Sousa, F.C. Antinociceptive activity of Riparin II from Aniba riparia: Further elucidation of the possible mechanisms. Chem. Biol. Interact. 2018, 287, 49–56. [Google Scholar] [CrossRef]
- Yamamoto, S.; Egashira, N.; Tsuda, M.; Masuda, S. Riluzole prevents oxaliplatin-induced cold allodynia via inhibition of overexpression of transient receptor potential melastatin 8 in rats. J. Pharmacol. Sci. 2018, 138, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, Y.; Yang, J. Structural biology of TRP channels. Adv. Exp. Med. Biol. 2011, 704, 1–23. [Google Scholar]
- Bandell, M.; Dubin, A.E.; Petrus, M.J.; Orth, A.; Mathur, J.; Hwang, S.W.; Patapoutian, A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat. Neurosci. 2006, 9, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Malkia, A.; Pertusa, M.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; Viana, F. Differential role of the menthol-binding residue Y745 in the antagonism of thermally gated TRPM8 channels. Mol. Pain 2009, 5, 62. [Google Scholar] [CrossRef]
- Pedretti, A.; Marconi, C.; Bettinelli, I.; Vistoli, G. Comparative modeling of the quaternary structure for the human TRPM8 channel and analysis of its binding features. Biochim. Biophys. Acta Biomembr. 2009, 1788, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Pedretti, A.; Labozzetta, A.; Lo Monte, M.; Beccari, A.R.; Moriconi, A.; Vistoli, G. Exploring the activation mechanism of TRPM8 channel by targeted MD simulations. Biochem. Biophys. Res. Commun. 2011, 414, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bidaux, G.; Sgobba, M.; Lemonnier, L.; Borowiec, A.-S.; Noyer, L.; Jovanovic, S.; Zholos, A.V.; Haider, S. Functional and Modeling Studies of the Transmembrane Region of the TRPM8 Channel. Biophys. J. 2015, 109, 1840–1851. [Google Scholar] [CrossRef] [Green Version]
- Taberner, F.J.; Lopez-Cordoba, A.; Fernandez-Ballester, G.; Korchev, Y.; Ferrer-Montiel, A. The Region Adjacent to the C-end of the Inner Gate in Transient Receptor Potential Melastatin 8 (TRPM8) Channels Plays a Central Role in Allosteric Channel Activation. J. Biol. Chem. 2014, 289, 28579–28594. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Wu, M.; Zubcevic, L.; Borschel, W.F.; Lander, G.C.; Lee, S.-Y. Structure of the cold- and menthol-sensing ion channel TRPM8. Science 2018, 359, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Zubcevic, L.; Herzik, M.A.J.; Chung, B.C.; Liu, Z.; Lander, G.C.; Lee, S.-Y. Cryo-electron microscopy structure of the TRPV2 ion channel. Nat. Struct. Mol. Biol. 2016, 23, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef]
- Yin, Y.; Le, S.C.; Hsu, A.L.; Borgnia, M.J.; Yang, H.; Lee, S.-Y. Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 2019. Ahead of print. [Google Scholar] [CrossRef]
- Pertusa, M.; Rivera, B.; Gonzalez, A.; Ugarte, G.; Madrid, R. Critical role of the pore domain in the cold response of TRPM8 channels identified by ortholog functional comparison. J. Biol. Chem. 2018, 293, 12454–12471. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Zhai, M.; Yan, D.; Li, D.; Li, C.; Zhang, Y.; Xiao, L.; Xiong, D.; Deng, Q.; Sun, W. Dietary menthol-induced TRPM8 activation enhances WAT “browning” and ameliorates diet-induced obesity. Oncotarget 2017, 8, 75114–75126. [Google Scholar] [CrossRef]
- Clemmensen, C.; Jall, S.; Kleinert, M.; Quarta, C.; Gruber, T.; Sachs, S.; Fischer, K.; Grandl, G.; Loher, D.; Sanchez-Quant, E.; et al. Coordinated targeting of cold and nicotinic receptors synergistically improves obesity and type 2 diabetes. Nat. Commun. 2018, 9, 4304. [Google Scholar] [CrossRef]
- Alcalde, I.; Íñigo-Portugués, A.; González-González, O.; Almaraz, L.; Artime, E.; Morenilla-Palao, C.; Gallar, J.; Viana, F.; Merayo-Lloves, J.; Belmonte, C. Morphological and functional changes in TRPM8-expressing corneal cold thermoreceptor neurons during aging and their impact on tearing in mice. J. Comp. Neurol. 2018, 526, 1859–1874. [Google Scholar] [CrossRef]
- Liu, X.; Ong, H.L.; Ambudkar, I. TRP Channel Involvement in Salivary Glands-Some Good, Some Bad. Cells 2018, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Ordas, P.; Hernandez-Ortego, P.; Vara, H.; Fernandez-Pena, C.; Morenilla-Palao, C.; Gomis, A.; Viana, F.; Reimundez, A.; Senaris, R.; Guadano-Ferraz, A.; et al. Expression of the cold thermoreceptor TRPM8 in rodent brain thermoregulatory circuits. J. Comp. Neurol. 2019. Ahead of print. [Google Scholar] [CrossRef]
- Khalil, M.; Babes, A.; Lakra, R.; Försch, S.; Reeh, P.W.; Wirtz, S.; Becker, C.; Neurath, M.F.; Engel, M.A. Transient receptor potential melastatin 8 ion channel in macrophages modulates colitis through a balance-shift in TNF-alpha and interleukin-10 production. Mucosal Immunol. 2016, 9, 1500–1513. [Google Scholar] [CrossRef]
- Kume, H.; Tsukimoto, M. TRPM8 channel inhibitor AMTB suppresses murine T-cell activation induced by T-cell receptor stimulation, concanavalin A, or external antigen re-stimulation. Biochem. Biophys. Res. Commun. 2019, 509, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Bidaux, G.; Gordienko, D.; Shapovalov, G.; Farfariello, V.; Borowiec, A.; Iamshanova, O.; Lemonnier, L.; Gueguinou, M.; Guibon, R.; Fromont, G.; et al. 4TM-TRPM8 channels are new gatekeepers of the ER-mitochondria Ca2+ transfer. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 981–994. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Muñiz, R.; Bonache, M.A.; Martín-Escura, C.; Gómez-Monterrey, I. Recent Progress in TRPM8 Modulation: An Update. Int. J. Mol. Sci. 2019, 20, 2618. https://doi.org/10.3390/ijms20112618
González-Muñiz R, Bonache MA, Martín-Escura C, Gómez-Monterrey I. Recent Progress in TRPM8 Modulation: An Update. International Journal of Molecular Sciences. 2019; 20(11):2618. https://doi.org/10.3390/ijms20112618
Chicago/Turabian StyleGonzález-Muñiz, Rosario, M. Angeles Bonache, Cristina Martín-Escura, and Isabel Gómez-Monterrey. 2019. "Recent Progress in TRPM8 Modulation: An Update" International Journal of Molecular Sciences 20, no. 11: 2618. https://doi.org/10.3390/ijms20112618