Protective Effects of Caffeic Acid Phenethyl Ester (CAPE) and Novel Cape Analogue as Inducers of Heme Oxygenase-1 in Streptozotocin-Induced Type 1 Diabetic Rats
Abstract
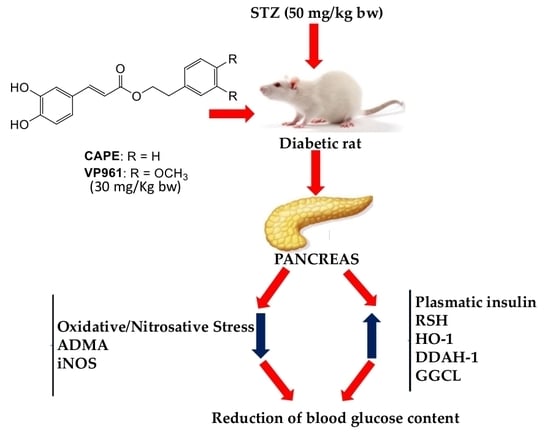
:1. Introduction
2. Results
2.1. Body Weight, Blood Glucose Content, Food Intake, Water Intake, and Volume of Urine Excreted
2.1.1. The Effects of CAPE and VP961 on Animal Body Weight
2.1.2. The Effects of CAPE and VP961 on Blood Glucose Content
2.1.3. The Effects of CAPE and VP961 on Water Intake, Volume of Urine Excreted, and Food Intake
2.2. Plasma Insulin, RSH, LOOH, ADMA, and Nitrite/Nitrate Levels
2.3. Pancreatic RSH, LOOH, ADMA, and Nitrite/Nitrate Levels
2.4. Pancreatic HO-1, DDAH-1, GGCL, iNOS Protein Expressions
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Measurement of Glucose and Insulin in the Plasma
4.3. Plasmatic and Pancreatic Nitrite/Nitrate Determination
4.4. Plasmatic and Pancreatic ADMA Determination
4.5. Determination of Plasmatic and Pancreatic Lipid Hydroperoxide Levels
4.6. Non-Proteic Thiol Groups Determination
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pinto, A.; Tuttolomondo, A.; Di Raimondo, D.; Fernández, P.; La Placa, S.; Di Gati, M.; Licata, G. Cardiovascular risk profile and morbidity in subjects affected by type 2 diabetes mellitus with and without diabetic foot. Metab. Clin. Exp. 2008, 57, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.P. Diabetes and the Endothelium. Acta Clin. Belg. 2007, 62, 97–101. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sobrevia, L.; Mann, G. Dysfunction of the endothelial nitric oxide signalling pathway in diabetes and hyperglycaemia. Exp. Physiol. 1997, 82, 423–452. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, M.; Cagliero, E.; Toledo, S. Glucose Toxicity for Human Endothelial Cells in Culture: Delayed Replication, Disturbed Cell Cycle, and Accelerated Death. Diabetes 1985, 34, 621–627. [Google Scholar] [CrossRef]
- Ceriello, A.; dello Russo, P.; Amstad, P.; Cerutti, P. High Glucose Induces Antioxidant Enzymes in Human Endothelial Cells in Culture. Evidence Linking Hyperglycemia and Oxidative Stress. Diabetes 1996, 45, 471–477. [Google Scholar] [CrossRef]
- Evans, J.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative Stress and Stress-Activated Signaling Pathways: A Unifying Hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [Green Version]
- Lash, J.M.; Nase, G.P.; Bohlen, H.G. Acute hyperglycemia depresses arteriolar NO formation in skeletal muscle. Am. J. Physiol. Circ. Physiol. 1999, 277, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, J.; Sorrenti, V.; Acquaviva, R.; Di Giacomo, C. Dietary Compounds, Epigenetic Modifications and Metabolic Diseases. Chem. Boil. 2017, 11, 17. [Google Scholar]
- Taubert, D.; Rosenkranz, A.; Berkels, R.; Roesen, R. Acute effects of glucose and insulin on vascular endothelium. Diabetologia 2004, 47, 2059–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elahi, M.M.; Kong, Y.X.; Matata, B.M. Oxidative stress as a mediator of cardiovascular disease. Oxidative Med. Cell. Longev. 2009, 2, 259–269. [Google Scholar] [CrossRef]
- Jin, J.-S.; D’Alecy, L.G. Central and Peripheral Effects of Asymmetric Dimethylarginine, an Endogenous Nitric Oxide Synthetase Inhibitor. J. Cardiovasc. Pharmacol. 1996, 28, 439–446. [Google Scholar] [CrossRef]
- Ito, A.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Ogawa, T.; Cooke, J.P. Novel mechanism for endothelial dysfunction: Dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 1999, 99, 3092–3095. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.T.; Leiper, J.M.; Vallance, P. The Ddah/Adma/Nos Pathway. Atheroscler 2003, 4 (Suppl. 4), 33–40. [Google Scholar] [CrossRef]
- Leiper, J.M.; Maria, J.S.; Chubb, A.; MacAllister, R.J.; Charles, I.G.; Whitley, G.S.J.; Vallance, P. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem. J. 1999, 343, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Sorrenti, V.; Mazza, F.; Campisi, A.; Vanella, L.; Volti, G.; Giacomo, C.; Di Giacomo, C. High Glucose-Mediated Imbalance of Nitric Oxide Synthase and Dimethylarginine Dimethylaminohydrolase Expression in Endothelial Cells. Curr. Neurovascular Res. 2006, 3, 49–54. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Kao, Y.-H.; Hsieh, C.-S.; Chen, C.-C.; Sheen, J.-M.; Lin, I.-C.; Huang, L.-T. Melatonin blocks oxidative stress-induced increased asymmetric dimethylarginine. Radic. Boil. Med. 2010, 49, 1088–1098. [Google Scholar] [CrossRef]
- Palm, F.; Onozato, M.L.; Luo, Z.; Wilcox, C.S. Dimethylarginine dimethylaminohydrolase (DDAH): Expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Circ. Physiol. 2007, 293, H3227–H3245. [Google Scholar] [CrossRef]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988, 2, 2557–2568. [Google Scholar] [CrossRef]
- Luo, Z.; Aslam, V.; Welch, W.J.; Wilcox, C.S. Activation of Nuclear Factor Erythroid 2-Related Factor 2 Coordinates Dimethylarginine Dimethylaminohydrolase/Ppar-Gamma/Endothelial Nitric Oxide Synthase Pathways That Enhance Nitric Oxide Generation in Human Glomerular Endothelial Cells. Hypertension 2015, 65, 896–902. [Google Scholar] [CrossRef]
- Wild, A.C.; Moinova, H.R.; Mulcahy, R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Boil. Chem. 1999, 274, 33627–33636. [Google Scholar] [CrossRef]
- Raffaele, M.; Volti, G.L.; Barbagallo, I.A.; Vanella, L. Therapeutic Efficacy of Stem Cells Transplantation in Diabetes: Role of Heme Oxygenase. Front. Cell Dev. Boil. 2016, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csepanyi, E.; Czompa, A.; Szabados-Furjesi, P.; Lekli, I.; Balla, J.; Balla, G.; Tosaki, A.; Bak, I. The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1. Int. J. Mol. Sci. 2018, 19, 1132. [Google Scholar] [CrossRef] [PubMed]
- Farhangkhoee, H.; Khan, Z.A.; Mukherjee, S.; Cukiernik, M.; Barbin, Y.P.; Karmazyn, M.; Chakrabarti, S. Heme oxygenase in diabetes-induced oxidative stress in the heart. J. Mol. Cell. Cardiol. 2003, 35, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Khan, Z.A.; Barbin, Y.; Chakrabarti, S. Pro-oxidant Role of Heme Oxygenase in Mediating Glucose-induced Endothelial Cell Damage. Free. Radic. Res. 2004, 38, 1301–1310. [Google Scholar] [CrossRef]
- Liu, L.; Puri, N.; Raffaele, M.; Schragenheim, J.; Singh, S.P.; Bradbury, J.A.; Bellner, L.; Vanella, L.; Zeldin, D.C.; Cao, J.; et al. Ablation of soluble epoxide hydrolase reprogram white fat to beige-like fat through an increase in mitochondrial integrity, HO-1-adiponectin in vitro and in vivo. Prostaglandins Lipid Mediat. 2018, 138, 1–8. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, I.; Volti, G.; Sorrenti, V.; Giacomo, C.; Acquaviva, R.; Raffaele, M.; Galvano, F.; Vanella, L. Caffeic Acid Phenethyl Ester Restores Adipocyte Gene Profile Expression Following Lipopolysaccharide Treatment. Lett. Drug Discov. 2017, 14, 481–487. [Google Scholar] [CrossRef]
- Kamiya, T.; Izumi, M.; Hara, H.; Adachi, T. Propolis Suppresses CdCl2-Induced Cytotoxicity of COS7 Cells through the Prevention of Intracellular Reactive Oxygen Species Accumulation. Boil. Pharm. 2012, 35, 1126–1131. [Google Scholar] [CrossRef] [Green Version]
- Pittala, V.; Vanella, L.; Salerno, L.; Di Giacomo, C.; Acquaviva, R.; Raffaele, M.; Romeo, G.; Modica, M.N.; Prezzavento, O.; Sorrenti, V. Novel Caffeic Acid Phenethyl Ester (Cape) Analogues as Inducers of Heme Oxygenase-1. Curr. Pharm. Des. 2017, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-C.; Chuang, S.-T.; Lin, Y.-C.; Chuang, C.-F.; Chi, T.-C.; Chiu, H.-L.; Kuo, Y.-H.; Su, M.-J. Caffeic Acid Phenylethyl Amide Protects against the Metabolic Consequences in Diabetes Mellitus Induced by Diet and Streptozocin. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shen, L.; Tong, Y.; Zhang, J.; Wu, G.; He, Q.; Yu, S.; Ye, X.; Zou, L.; Zhang, Z.; et al. Antitumor activity of caffeic acid 3,4-dihydroxyphenethyl ester and its pharmacokinetic and metabolic properties. Phytomedicine 2013, 20, 904–912. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1–44. [Google Scholar] [CrossRef]
- Srivastava, K.K.; Kuma, R. Stress, Oxidative Injury and Disease. Indian J. Clin. Biochem. 2015, 30, 3–10. [Google Scholar] [CrossRef]
- Barbagallo, I.; Galvano, F.; Frigiola, A.; Cappello, F.; Riccioni, G.; Murabito, P.; D’Orazio, N.; Torella, M.; Gazzolo, D.; Volti, G.L. Potential Therapeutic Effects of Natural Heme Oxygenase-1 Inducers in Cardiovascular Diseases. Antioxidants Redox Signal. 2013, 18, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Salerno, L.; Romeo, G.; Acquaviva, R.; Di Giacomo, C.; Sorrenti, V. Therapeutic Potential of Caffeic Acid Phenethyl Ester (Cape) in Diabetes. Curr. Med. Chem. 2018, 25, 4827–4836. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. The potential of antioxidant enzymes as pharmacological agents in vivo. Xenobiotica 1991, 21, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Dinkova-Kostova, A.T.; Dinkova-Kostova, A.T. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, 128–138. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxidative Med. Cell. Longev. 2016, 2016, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Acquaviva, R.; Campisi, A.; Sorrenti, V.; Di Giacomo, C.; Virgata, G.; Barcellona, M.; Vanella, A. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Boil. Toxicol. 2000, 16, 91–98. [Google Scholar] [CrossRef]
- Di Giacomo, C.; Acquaviva, R.; Piva, A.; Sorrenti, V.; Vanella, L.; Piva, G.; Casadei, G.; La Fauci, L.; Ritieni, A.; Bognanno, M.; et al. Protective Effect of Cyanidin 3-O-Beta-d-Glucoside on Ochratoxin a-Mediated Damage in the Rat. Br. J. Nutr. 2007, 98, 937–943. [Google Scholar] [CrossRef]
- Di Giacomo, C.; Acquaviva, R.; Santangelo, R.; Sorrenti, V.; Vanella, L.; Li Volti, G.; D’Orazio, N.; Vanella, A.; Galvano, F. Effect of Treatment with Cyanidin-3-O-Beta-d-Glucoside on Rat Ischemic/Reperfusion Brain Damage. Evid Based Complement. Alternat. Med. 2012, 2012, 285750. [Google Scholar]
- Salerno, L.; Modica, M.N.; Pittalà, V.; Romeo, G.; Siracusa, M.A.; Di Giacomo, C.; Sorrenti, V.; Acquaviva, R. Antioxidant Activity and Phenolic Content of Microwave-Assisted Solanum melongena Extracts. Sci. World. J. 2014, 2014, 719486. [Google Scholar] [CrossRef]
- Acquaviva, R.; Di Giacomo, C.; Vanella, L.; Santangelo, R.; Sorrenti, V.; Barbagallo, I.; Genovese, C.; Mastrojeni, S.; Ragusa, S.; Iauk, L.; et al. Antioxidant Activity of Extracts of Momordica Foetida Schumach. et Thonn. Molecules 2013, 18, 3241–3249. [Google Scholar] [CrossRef] [Green Version]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Bognanno, M.; Grilli, E.; D’Orazio, N.; Galvano, F. Dimethylarginine Dimethylaminohydrolase/Nitric Oxide Synthase Pathway in Liver and Kidney: Protective Effect of Cyanidin 3-O-Beta-d-Glucoside on Ochratoxin-a Toxicity. Toxins 2012, 4, 353–363. [Google Scholar] [CrossRef]
- Di Giacomo, C.; Acquaviva, R.; Sorrenti, V.; Vanella, A.; Grasso, S.; Barcellona, M.L.; Galvano, F.; Vanella, L.; Renis, M. Oxidative and Antioxidant Status in Plasma of Runners: Effect of Oral Supplementation with Natural Antioxidants. J. Med. Food 2009, 12, 145–150. [Google Scholar] [CrossRef]
- Pittala, V.; Vanella, L.; Salerno, L.; Romeo, G.; Marrazzo, A.; Sorrenti, V.; Di Giacomo, C. Effects of Polyphenolic Derivatives on Heme Oxygenase-System in Metabolic Dysfunctions. Med. Chem. 2018, 25, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Randazzo, C.L.; Caggia, C.; Ballistreri, G.; Romeo, F.V.; Fabroni, S.; Timpanaro, N.; Raffaele, M.; Vanella, L. Beneficial Effects of Pomegranate Peel Extract and Probiotics on Pre-adipocyte Differentiation. Front. Microbiol. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Volti, G.L.; Salomone, S.; Sorrenti, V.; Mangiameli, A.; Urso, V.; Siarkos, I.; Galvano, F.; Salamone, F. Effect of silibinin on endothelial dysfunction and ADMA levels in obese diabetic mice. Cardiovasc. Diabetol. 2011, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E. Type 1 Diabetes and Autoimmunity. Clin. Pediatr. Endocrinol. 2014, 23, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, L.H.; Badr, G.; Omar, H.M.; El-Rahim, A.M.A.; Mahmoud, M.H. Camel whey protein improves oxidative stress and histopathological alterations in lymphoid organs through Bcl-XL/Bax expression in a streptozotocin-induced type 1 diabetic mouse model. Biomed. Pharmacother. 2017, 88, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Al Dubayee, M.S.; AlAyed, H.; Almansour, R.; Alqaoud, N.; Alnamlah, R.; Obeid, D.; Alshahrani, A.; Zahra, M.M.; Nasr, A.; Al-Bawab, A.; et al. Differential Expression of Human Peripheral Mononuclear Cells Phenotype Markers in Type 2 Diabetic Patients and Type 2 Diabetic Patients on Metformin. Front. Endocrinol. 2018, 9, 537. [Google Scholar] [CrossRef]
- Roh, S.S.; Kwon, O.J.; Yang, J.H.; Kim, Y.S.; Lee, S.H.; Jin, J.S.; Jeon, Y.D.; Yokozawa, T.; Kim, H.J. Allium Hookeri Root Protects Oxidative Stress-Induced Inflammatory Responses and Beta-Cell Damage in Pancreas of Streptozotocin-Induced Diabetic Rats. BMC Complement. Altern Med. 2016, 16, 63. [Google Scholar] [CrossRef]
- Bruce, C.R.; Carey, A.L.; Hawley, J.A.; Febbraio, M.A. Intramuscular Heat Shock Protein 72 and Heme Oxygenase-1 mRNA Are Reduced in Patients with Type 2 Diabetes: Evidence That Insulin Resistance Is Associated with a Disturbed Antioxidant Defense Mechanism. Diabetes 2003, 52, 2338–2345. [Google Scholar] [CrossRef]
- Tiwari, S.; Ndisang, J. The Heme Oxygenase System and Type-1 Diabetes. Curr. Pharm. Des. 2014, 20, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Abduljawad, S.H.; El-Refaei, M.F.; El-Nashar, N.N. Protective and anti-angiopathy effects of caffeic acid phenethyl ester against induced type 1 diabetes in vivo. Int. Immunopharmacol. 2013, 17, 408–414. [Google Scholar] [CrossRef]
- Okutan, H.; Özçelik, N.; Yilmaz, H.R.; Uz, E. Effects of caffeic acid phenethyl ester on lipid peroxidation and antioxidant enzymes in diabetic rat heart. Clin. Biochem. 2005, 38, 191–196. [Google Scholar] [CrossRef]
- Celik, S.; Erdogan, S. Caffeic acid phenethyl ester (CAPE) protects brain against oxidative stress and inflammation induced by diabetes in rats. Mol. Cell. Biochem. 2008, 312, 39–46. [Google Scholar] [CrossRef]
- Yilmaz, H.R.; Uz, E.; Yucel, N.; Altuntas, I.; Özçelik, N.; Yılmaz, H.R. Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J. Biochem. Mol. Toxicol. 2004, 18, 234–238. [Google Scholar] [CrossRef]
- Park, S.H.; Min, T.S. Caffeic acid phenethyl ester ameliorates changes in IGFs secretion and gene expression in streptozotocin-induced diabetic rats. Life Sci. 2006, 78, 1741–1747. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Gao, L.; Zhang, J.; Xie, X.; Tong, Z.; Li, B.; Li, G.; Lu, G.; Li, W. Naringenin Protects against Acute Pancreatitis in Two Experimental Models in Mice by NLRP3 and Nrf2/HO-1 Pathways. Mediat. Inflamm. 2018, 2018, 1–13. [Google Scholar] [Green Version]
- Ye, J.; Laychock, S.G. A Protective Role for Heme Oxygenase Expression in Pancreatic Islets Exposed to Interleukin-1beta. Endocrinology 1998, 139, 4155–4163. [Google Scholar] [CrossRef]
- Ferrigno, A.; Di Pasqua, L.G.; Berardo, C.; Richelmi, P.; Vairetti, M. Liver plays a central role in asymmetric dimethylarginine-mediated organ injury. World J. Gastroenterol. 2015, 21, 5131–5137. [Google Scholar] [CrossRef]
- Hu, T.; Chouinard, M.; Cox, A.L.; Sipes, P.; Marcelo, M.; Ficorilli, J.; Li, S.; Gao, H.; Ryan, T.P.; Michael, M.D.; et al. Farnesoid X Receptor Agonist Reduces Serum Asymmetric Dimethylarginine Levels through Hepatic Dimethylarginine Dimethylaminohydrolase-1 Gene Regulation. J. Boil. Chem. 2006, 281, 39831–39838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanteri, R.; Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Destri, G.L.; Santangelo, M.; Vanella, L.; Di Cataldo, A. Rutin in rat liver ischemia/reperfusion injury: Effect on DDAH/NOS pathway. Microsurgery 2007, 27, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Homem De Bittencourt, P.I.; O’ Hagan, C.; De Vito, G.; Murphy, C.; Krause, M.S. Exercise and Possible Molecular Mechanisms of Protection from Vascular Disease and Diabetes: The Central Role of Ros and Nitric Oxide. Clin. Sci. 2009, 118, 341–349. [Google Scholar] [CrossRef]
- Acquaviva, R.; Lanteri, R.; Destri, G.L.; Caltabiano, R.; Vanella, L.; Lanzafame, S.; Di Cataldo, A.; Volti, G.L.; Di Giacomo, C. Beneficial effects of rutin and l-arginine coadministration in a rat model of liver ischemia-reperfusion injury. Am. J. Physiol. Liver Physiol. 2009, 296, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Russo, G.I.; Cimino, S.; Fragalà, E.; Favilla, V.; Volti, G.L.; Barbagallo, I.; Sorrenti, V.; Morgia, G. Correlation Between Lipid Profile and Heme Oxygenase System in Patients with Benign Prostatic Hyperplasia. Urology 2014, 83, 1444.e7–1444.e13. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, I.; Giallongo, C.; Volti, G.L.; Distefano, A.; Camiolo, G.; Raffaele, M.; Salerno, L.; Pittala, V.; Sorrenti, V.; Avola, R.; et al. Heme Oxygenase Inhibition Sensitizes Neuroblastoma Cells to Carfilzomib. Mol. Neurobiol. 2019, 56, 1451–1460. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]






| Groups | T0 | 8 Days | 15 Days | 21 Days |
|---|---|---|---|---|
| Body Weight (g) | Body Weight (g) | Body Weight (g) | Body Weight (g) | |
| Control | 231 ± 3 | 265 ± 5 | 300 ± 7 | 335 ± 11 |
| STZ | 228 ± 5 | 238 ± 7* | 262 ± 5 * | 280 ± 3 * |
| STZ/CAPE | 220 ± 3 | 256 ± 9 | 291 ± 3 | 329 ± 4 |
| STZ/VP961 | 226 ± 5 | 253 ± 8 | 291 ± 4 | 318 ± 6 |
| PLASMA | Insulin (ng/mL) | RSH (nmoles/mL) | LOOH (nmoles/mL) | ADMA (nmoles/mL) | NO2−/NO3− (nmoles/mL) |
|---|---|---|---|---|---|
| Control | 1.0 ± 0.03 | 140 ± 10 | 15 ± 2 | 0.1 ± 0.02 | 0.75 ± 0.03 |
| STZ | 0.45 ± 0.05 * | 80 ± 7 * | 30 ± 5 * | 0.9 ± 0.03 * | 1.5 ± 0.05 * |
| STZ/CAPE | 0.82 ± 0.03 ** | 130 ± 9 ** | 18 ± 3 ** | 0.6 ± 0.02 ** | 0.8 ± 0.03 ** |
| STZ/VP961 | 0.78 ± 0.07 ** | 120 ± 8 ** | 17 ± 4 ** | 0.3 ± 0.02 **± | 0.7 ± 0.04 ** |
| PANCREAS | RSH (nmoles/mg prot.) | LOOH (nmoles/mg prot.) | ADMA (nmoles/mg prot.) | NO2−/NO3− (nmoles/mg prot.) |
|---|---|---|---|---|
| Control | 28 ± 2 | 0.2 ± 0.03 | 20 ± 0.8 | 4 ± 0.9 |
| STZ | 12 ± 1 * | 1 ± 0.04 * | 200 ± 5 * | 12 ± 2 * |
| STZ/CAPE | 27 ± 2 ** | 0.4 ± 0.02 ** | 50 ± 4 ** | 6 ± 0.8 ** |
| STZ/VP961 | 26 ± 3 ** | 0.3 ± 0.03 ** | 53 ± 3 ** | 5 ± 0.9 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrenti, V.; Raffaele, M.; Vanella, L.; Acquaviva, R.; Salerno, L.; Pittalà, V.; Intagliata, S.; Di Giacomo, C. Protective Effects of Caffeic Acid Phenethyl Ester (CAPE) and Novel Cape Analogue as Inducers of Heme Oxygenase-1 in Streptozotocin-Induced Type 1 Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 2441. https://doi.org/10.3390/ijms20102441
Sorrenti V, Raffaele M, Vanella L, Acquaviva R, Salerno L, Pittalà V, Intagliata S, Di Giacomo C. Protective Effects of Caffeic Acid Phenethyl Ester (CAPE) and Novel Cape Analogue as Inducers of Heme Oxygenase-1 in Streptozotocin-Induced Type 1 Diabetic Rats. International Journal of Molecular Sciences. 2019; 20(10):2441. https://doi.org/10.3390/ijms20102441
Chicago/Turabian StyleSorrenti, Valeria, Marco Raffaele, Luca Vanella, Rosaria Acquaviva, Loredana Salerno, Valeria Pittalà, Sebastiano Intagliata, and Claudia Di Giacomo. 2019. "Protective Effects of Caffeic Acid Phenethyl Ester (CAPE) and Novel Cape Analogue as Inducers of Heme Oxygenase-1 in Streptozotocin-Induced Type 1 Diabetic Rats" International Journal of Molecular Sciences 20, no. 10: 2441. https://doi.org/10.3390/ijms20102441