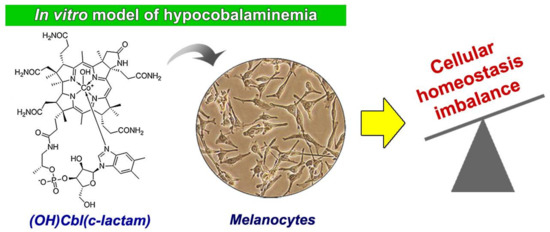
Vitamin B12 Deficiency Induces Imbalance in Melanocytes Homeostasis—A Cellular Basis of Hypocobalaminemia Pigmentary Manifestations
Abstract
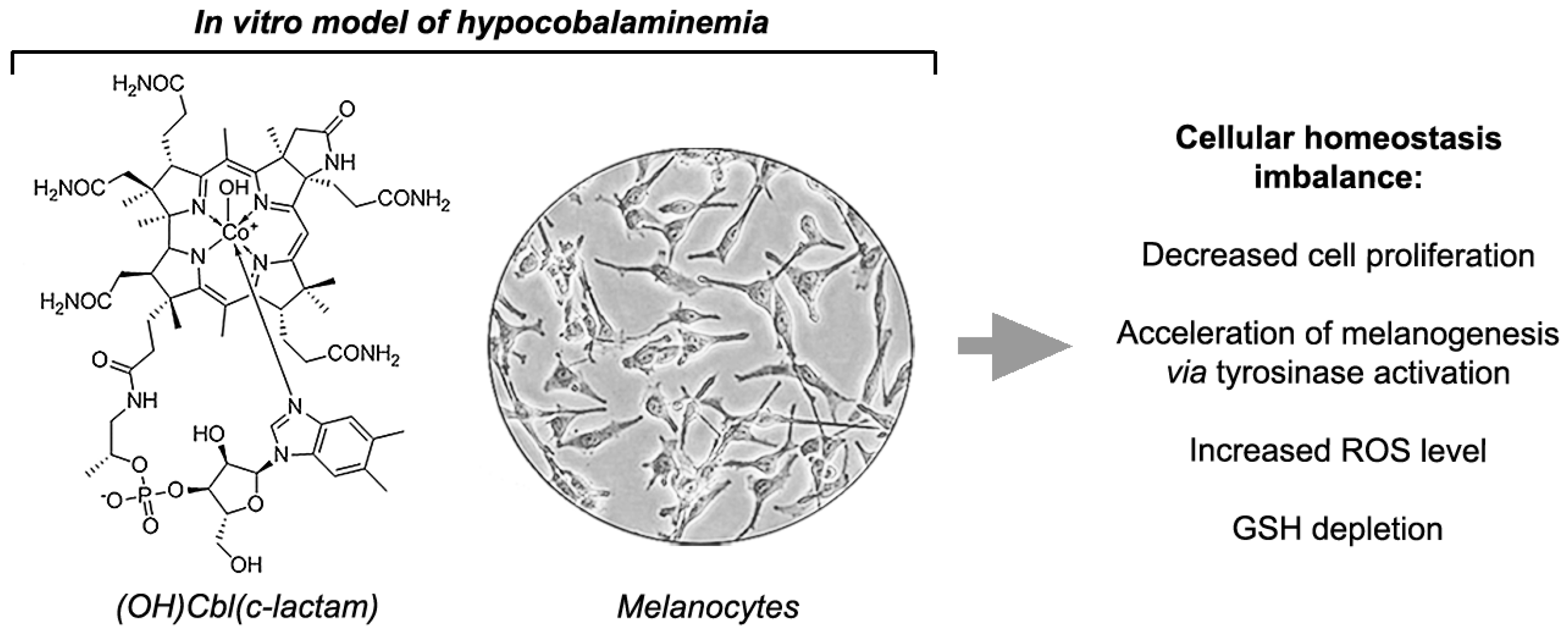
:1. Introduction
2. Results
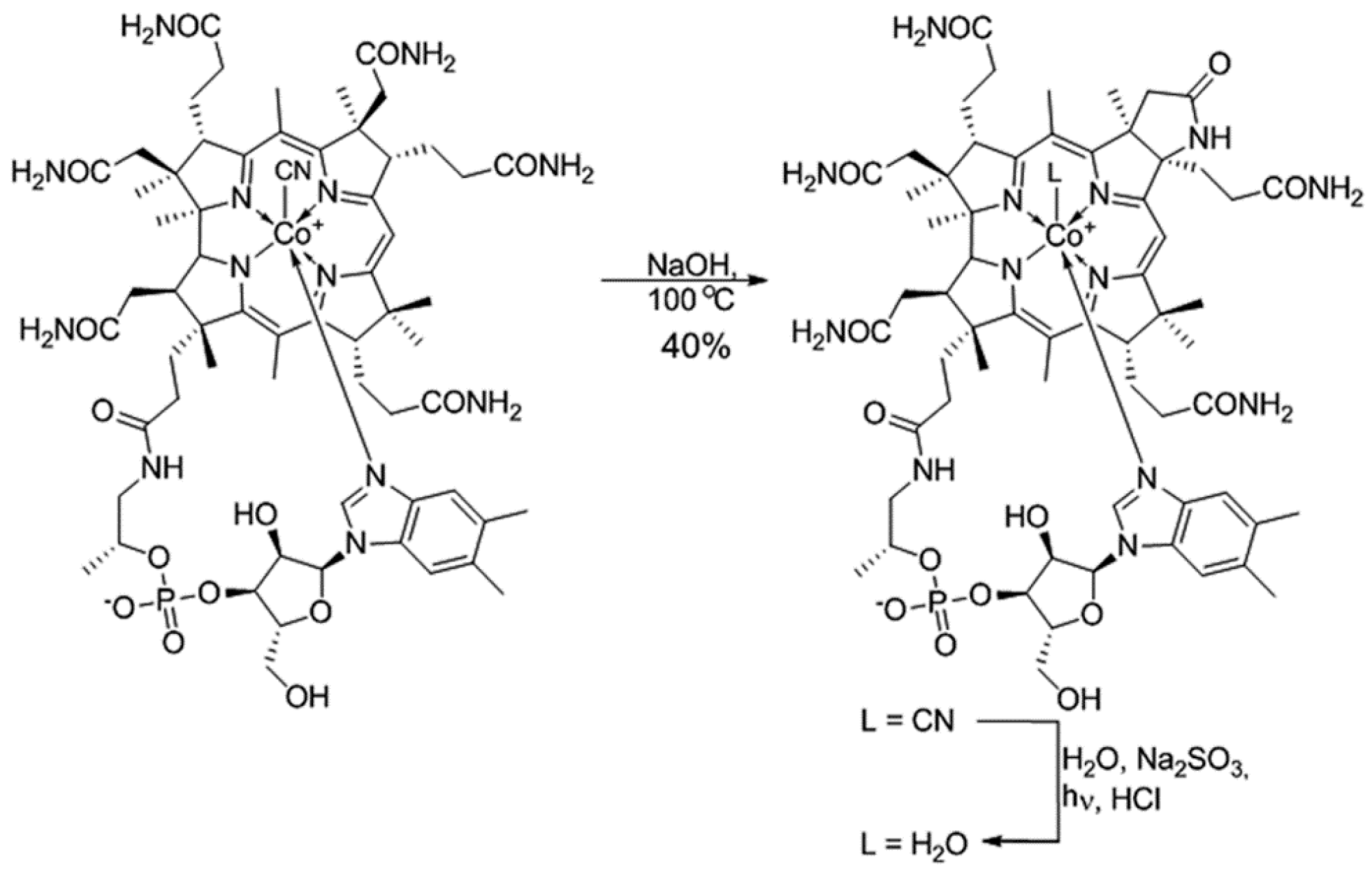
2.1. Synthesis and Identification of (OH)Cbl(c-lactam)
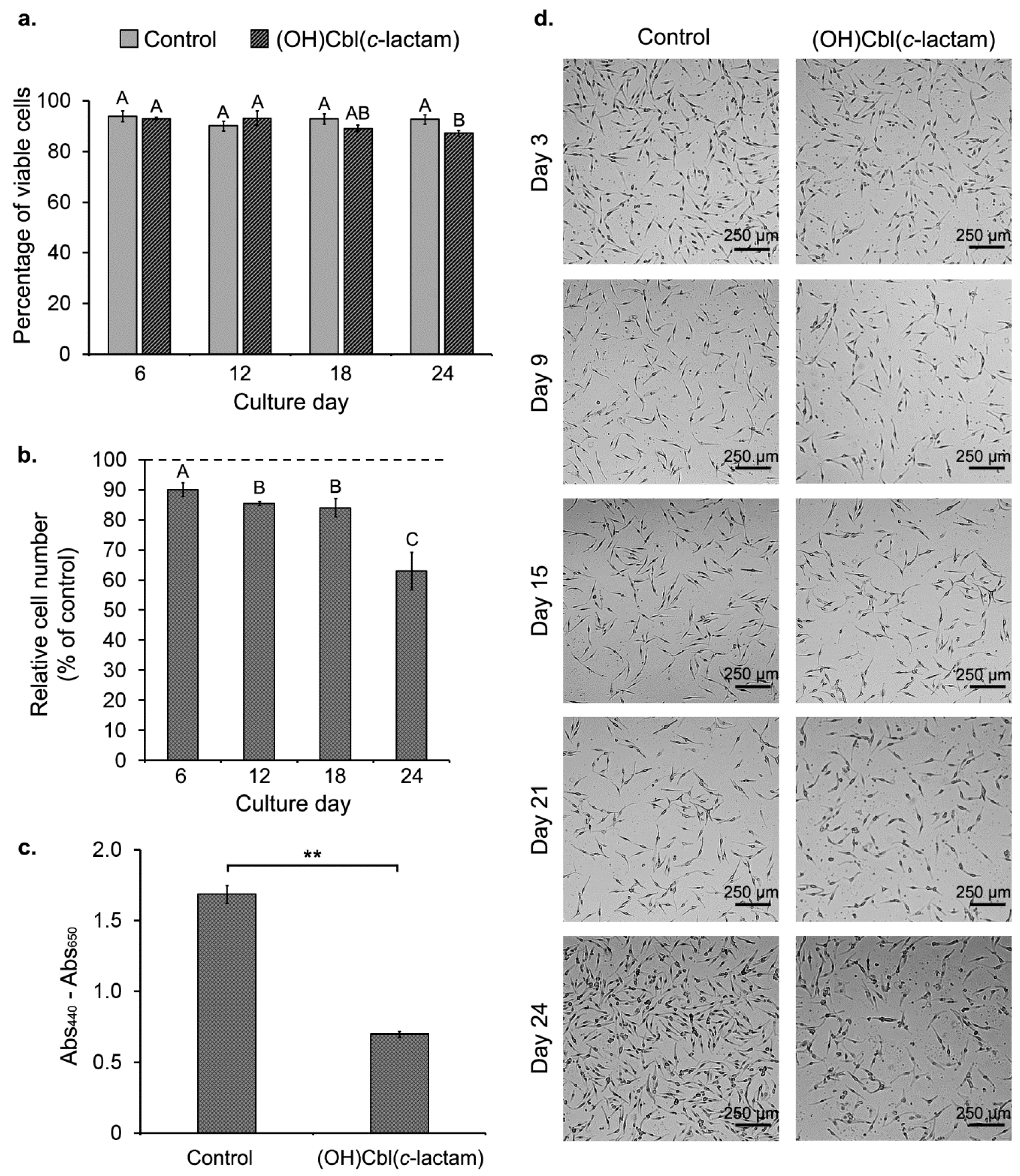
2.2. Effect of (OH)Cbl(c-lactam) on Cell Growth
2.3. Changes in Extracellular Hcy Concentration during Culture with (OH)Cbl(c-lactam)
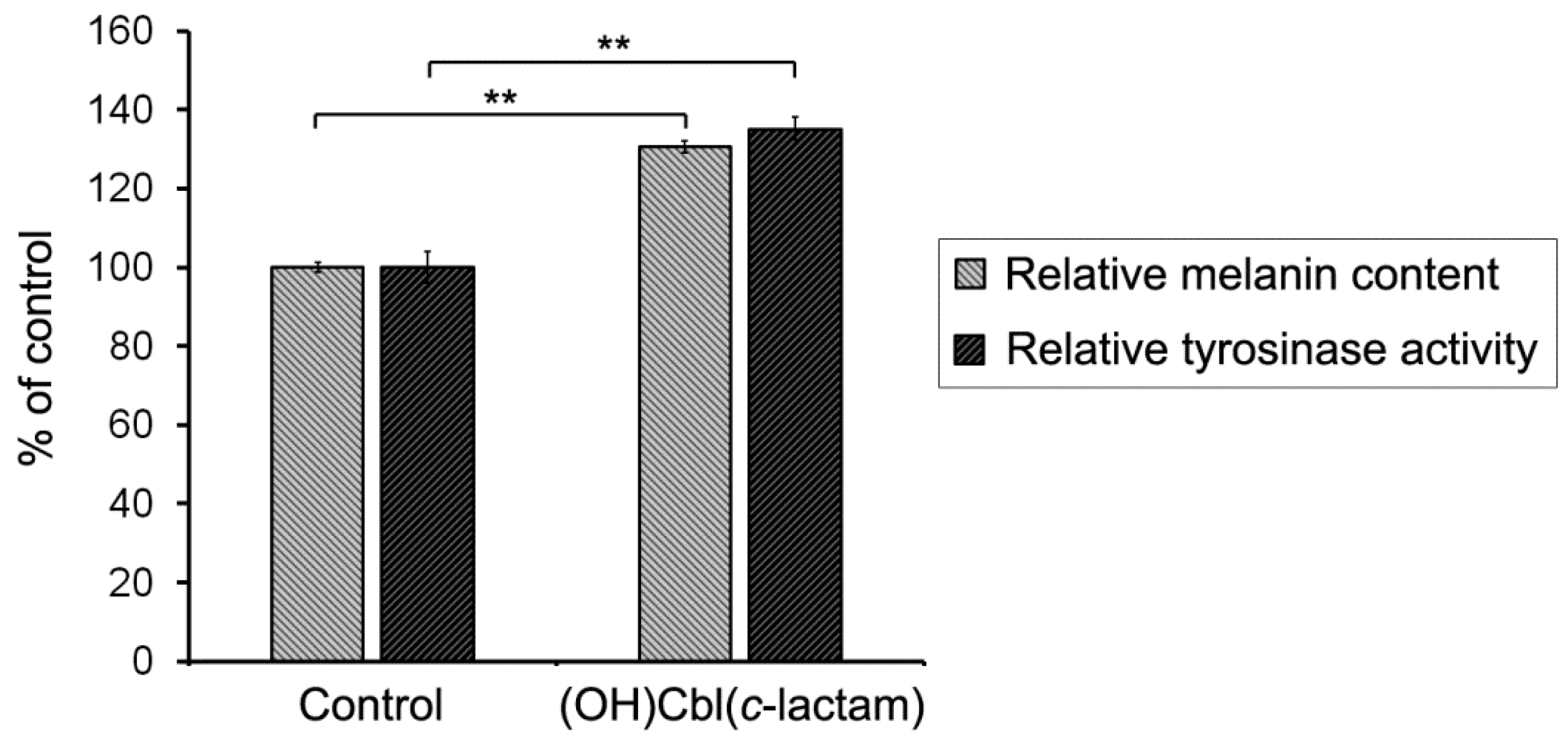
2.4. The Effectiveness of Melanogenesis under Conditions of Cobalamin Deficiency
2.5.Disruption of Redox Homeostasis in Cobalamin-Deficient Melanocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis and Identification of (H2O)Cbl(c-lactam)Cl
4.3. Cell Culture
4.4. The Induction of Vitamin B12 Deficiency
4.5. Viability and Cell Count Assay
4.6. Cell Proliferation Assay
4.7. Quantitative Analysis of Extracellular Hcy Levels
4.8. Melanin Content Assay
4.9. Intracellular Tyrosinase Activity Assay
4.10. Intracellular ROS Level Assay
4.11. Examination of Intracellular Thiol Status
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AO | Acridine orange |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DPBS | Dulbecco’s phosphate-buffered saline |
| GSH | Reduced glutathione |
| GSSG | Glutathione disulfide |
| H2DCF | 2′,7′-dichlorodihydrofluorescein |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| Hcy | Homocysteine |
| HEMn-DP | Human epidermal melanocytes, neonatal, dark pigmented |
| HMGS-2 | Human melanocyte growth supplement-2 |
| HRP | Horseradish peroxidase |
| L-DOPA | 3,4-dihydroxy-L-phenylalanine |
| (OH)Cbl(c-lactam) | Hydroxycobalamin(c-lactam) |
| PI | Propidium iodide |
| ROS | Reactive oxygen species |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| VB-48™ | VitaBright 48™ |
References
- Briani, C.; Dalla Torre, C.; Citton, V.; Manara, R.; Pompanin, S.; Binotto, G.; Adami, F. Cobalamin deficiency: Clinical picture and radiological findings. Nutrients 2013, 5, 4521–4539. [Google Scholar] [CrossRef] [PubMed]
- Zdilla, M.J. Metformin with either histamine H2-receptor antagonists or proton pump inhibitors: A polypharmacy recipe for neuropathy via vitamin B12 depletion. Clin. Diabetes 2015, 33, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, Y.M.; Ruan, M.H.; Zhou, A.H.; Qian, Y.; Chen, C. Homocysteine inhibits neural stem cells survival by inducing DNA interstrand cross-links via oxidative stress. Neurosci. Lett. 2016, 635, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Sedoris, K.C.; Steed, M.; Ovechkin, A.V.; Moshal, K.S.; Tyagi, S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2649–H2656. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.; Pawelek, J. L-tyrosine and L-DOPA as hormone-like regulators of melanocytes functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Rusher, D.R.; Pawlak, R. A review of 89 published case study of vitamin B12 deficiency. J. Hum. Nutr. Food Sci. 2013, 1, 1008. [Google Scholar]
- Hoffman, C.F.; Palmer, D.M.; Papadopoulos, D. Vitamin B12 deficiency: A case report of ongoing cutaneous hyperpigmentation. Cutis 2003, 71, 127–130. [Google Scholar] [PubMed]
- Agrawala, R.K.; Sahoo, S.K.; Choudhury, A.K.; Mohanty, B.K.; Baliarsinha, A.K. Pigmentation in vitamin B12 deficiency masquerading Addison’s pigmentation: A rare presentation. Indian J. Endocrinol. Metab. 2013, 17, S254–S256. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.T.; Hung, C.T.; Wang, W.M.; Lee, J.T.; Yang, F.C. Recreational nitrous oxide abuse-induced vitamin B12 deficiency in a patient presenting with hyperpigmentation of the skin. Case Rep. Dermatol. 2013, 5, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Doğan, M.; Koç, A.; Kaba, S.; Bulan, K.; Ozkol, H.U.; Doğan, S.Z. Dermatological findings of vitamin B12 deficiency and resolving time of these symptoms. Cutan. Ocul. Toxicol. 2014, 33, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Ando, I.; Kukita, A. Generalized hyperpigmentation of the skin due to vitamin B12 deficiency. J. Dermatol. 2001, 28, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, S.; Mukai, H. Reversible cutaneous hyperpigmentation and nails with white hair due to vitamin B12 deficiency. Eur. J. Dermatol. 2007, 17, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Yasui, M.; Iwaki, T.; Yabuta, Y.; Ichiyanagi, T.; Yamaji, R.; Nakano, Y.; Inui, H.; Watanabe, F. Dodecylamine derivative of hydroxocobalamin acts as a potent inhibitor of cobalamin-dependent methionine synthase in mammalian cultured COS-7 cells. Food Nutr. Sci. 2014, 5, 1318–1325. [Google Scholar] [CrossRef]
- Stabler, S.P.; Brass, E.P.; Marcell, P.D.; Allen, R.H. Inhibition of cobalamin-dependent enzymes by cobalamin analogues in rats. J. Clin. Investig. 1991, 87, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Próinsias, K.; Giedyk, M.; Gryko, D. Vitamin B12: Chemical modifications. Chem. Soc. Rev. 2013, 42, 6605–6619. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Wrześniok, D.; Otręba, M.; Miliński, M.; Rok, J.; Buszman, E. Effect of norfloxacin and moxifloxacin on melanin synthesis and antioxidant enzymes activity in normal human melanocytes. Mol. Cell. Biochem. 2015, 401, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Wrześniok, D.; Rzepka, Z.; Rok, J.; Delijewski, M.; Otręba, M.; Respondek, M.; Buszman, E. Effect of fluoroquinolones on melanogenesis in normal human melanocytes HEMn-DP: A comparative in vitro study. Cutan. Ocul. Toxicol. 2017, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wrześniok, D.; Beberok, A.; Otręba, M.; Buszman, E. Modulation of melanogenesis and antioxidant defense system in melanocytes by amikacin. Toxicol. In Vitro 2013, 27, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Wrześniok, D.; Beberok, A.; Otręba, M.; Buszman, E. Effect of streptomycin on melanogenesis and antioxidant status in melanocytes. Mol. Cell. Biochem. 2013, 383, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wrześniok, D.; Otręba, M.; Beberok, A.; Buszman, E. Impact of kanamycin on melanogenesis and antioxidant enzymes activity in melanocytes—An in vitro study. J. Cell. Biochem. 2013, 114, 2746–2752. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Buszman, E.; Delijewski, M.; Otręba, M.; Beberok, A.; Wrześniok, D. Effect of tetracycline and UV radiation on melanization and antioxidant status of melanocytes. J. Photochem. Photobiol. B 2015, 148, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Wrześniok, D.; Beberok, A.; Otręba, M.; Delijewski, M.; Buszman, E. Phototoxic effect of oxytetracycline on normal human melanocytes. Toxicol. In Vitro 2018, 48, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Delijewski, M.; Wrześniok, D.; Beberok, A.; Rok, J.; Otręba, M.; Buszman, E. The effect of simultaneous exposure of HEMn-DP and HEMn-LP melanocytes to nicotine and UV-radiation on the cell viability and melanogenesis. Environ. Res. 2016, 151, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, R.; Cannon, R.J.; Clark, V.M.; Johnson, A.W.; Parker, L.F.J.; Smith, E.L.; Todd, A. Chemistry of vitamin B12 group. Part V, The structure of the chromophoric grouping. J. Chem. Soc. 1957, 0, 1158–1168. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Brandes, N.; Schmitt, S.; Jakob, U. Thiol-based redox switches in Eukaryotic proteins. Antioxid. Redox Signal. 2009, 11, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Ng, M.J. Cutaneous lesions and vitamin B12 deficiency: An often-forgotten link. Can. Fam. Physician 2008, 54, 529–532. [Google Scholar] [PubMed]
- Cherqaoui, R.; Husain, M.; Madduri, S.; Okolie, P.; Nunlee-Bland, G.; Williams, J. A reversible cause of skin hyperpigmentation and postural hypotension. Case Rep. Hematol. 2013, 2013, 680459. [Google Scholar] [CrossRef] [PubMed]
- Sponne, I.E.; Gaire, D.; Stabler, S.P.; Droesch, S.; Barbé, F.M.; Allen, R.H.; Lambert, D.A.; Nicolas, J.P. Inhibition of vitamin B12 metabolism by OH-cobalamin c-lactam in rat oligodendrocytes in culture: A model for studying neuropathy due to vitamin B12 deficiency. Neurosci. Lett. 2000, 288, 191–194. [Google Scholar] [CrossRef]
- Hultberg, B.; Andersson, A.; Isaksson, A. Metabolism of homocysteine, its relation to the other cellular thiols and its mechanism of cell damage in a cell culture line (human histiocytic cell line U-937). Biochim. Biophys. Acta 1995, 1269, 6–12. [Google Scholar] [CrossRef]
- Antoniades, C.; Antonopoulos, A.S.; Tousoulis, D.; Marinou, K.; Stefanadis, C. Homocysteine and coronary atherosclerosis: From folate fortification to the recent clinical trials. Eur. Heart J. 2009, 30, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Jorge-Finnigan, A.; Garcia-Villoria, J.; Merinero, B.; Desviat, L.R.; Gort, L.; Briones, P.; Leal, F.; Pérez-Cerdá, C.; Ribes, A.; et al. Genetic and cellular studies of oxidative stress in methylmalonic aciduria (MMA) cobalamin deficiency type C (cblC) with homocystinuria (MMACHC). Hum. Mutat. 2009, 30, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.W.; Opp, S.; Haarmann, A.; Okun, J.G.; Kölker, S.; Morath, M.A. Long-term exposure of human proximal tubule cells to hydroxycobalamin[c-lactam] as a possible model to study renal disease in methylmalonic acidurias. J. Inherit. Metab. Dis. 2009, 32, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Ingrosso, D.; Lombardi, C.; Acanfora, F.; Satta, E.; Cesare, C.M.; Violetti, E.; Romano, M.M.; de Santo, N.G. Possible mechanisms of homocysteine toxicity. Kidney Int. Suppl. 2003, 63, S137–S140. [Google Scholar] [CrossRef] [PubMed]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic colorimetric proliferation assays: MTT, WST, and resazurin. In Cell Viability Assays. Methods in Molecular Biology; Gilbert, D., Friedrich, O., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1601, pp. 1–17. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef] [PubMed]


| Time (min) | H2O | (0.03% TFA) MeCN |
|---|---|---|
| 0 | 90% | 10 |
| 15 | 30% | 70 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzepka, Z.; Respondek, M.; Rok, J.; Beberok, A.; Ó Proinsias, K.; Gryko, D.; Wrześniok, D. Vitamin B12 Deficiency Induces Imbalance in Melanocytes Homeostasis—A Cellular Basis of Hypocobalaminemia Pigmentary Manifestations. Int. J. Mol. Sci. 2018, 19, 2845. https://doi.org/10.3390/ijms19092845
Rzepka Z, Respondek M, Rok J, Beberok A, Ó Proinsias K, Gryko D, Wrześniok D. Vitamin B12 Deficiency Induces Imbalance in Melanocytes Homeostasis—A Cellular Basis of Hypocobalaminemia Pigmentary Manifestations. International Journal of Molecular Sciences. 2018; 19(9):2845. https://doi.org/10.3390/ijms19092845
Chicago/Turabian StyleRzepka, Zuzanna, Michalina Respondek, Jakub Rok, Artur Beberok, Keith Ó Proinsias, Dorota Gryko, and Dorota Wrześniok. 2018. "Vitamin B12 Deficiency Induces Imbalance in Melanocytes Homeostasis—A Cellular Basis of Hypocobalaminemia Pigmentary Manifestations" International Journal of Molecular Sciences 19, no. 9: 2845. https://doi.org/10.3390/ijms19092845
APA StyleRzepka, Z., Respondek, M., Rok, J., Beberok, A., Ó Proinsias, K., Gryko, D., & Wrześniok, D. (2018). Vitamin B12 Deficiency Induces Imbalance in Melanocytes Homeostasis—A Cellular Basis of Hypocobalaminemia Pigmentary Manifestations. International Journal of Molecular Sciences, 19(9), 2845. https://doi.org/10.3390/ijms19092845