Gosha-Jinki-Gan Recovers Spermatogenesis in Mice with Busulfan-Induced Aspermatogenesis
Abstract
:1. Introduction
2. Results
2.1. TJ107 Recovered Body Weight and Reproductive Parameters in BSF-Treated Mice
2.2. TJ107 Normalized Proliferation and Apoptosis in the Testes of BSF-Treated Mice
2.3. TJ107 Suppressed Macrophage Migration via Reduction of the Expressions of TLR2 and TLR4 in BSF-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of a Gosha-Jinki-Gan Diet
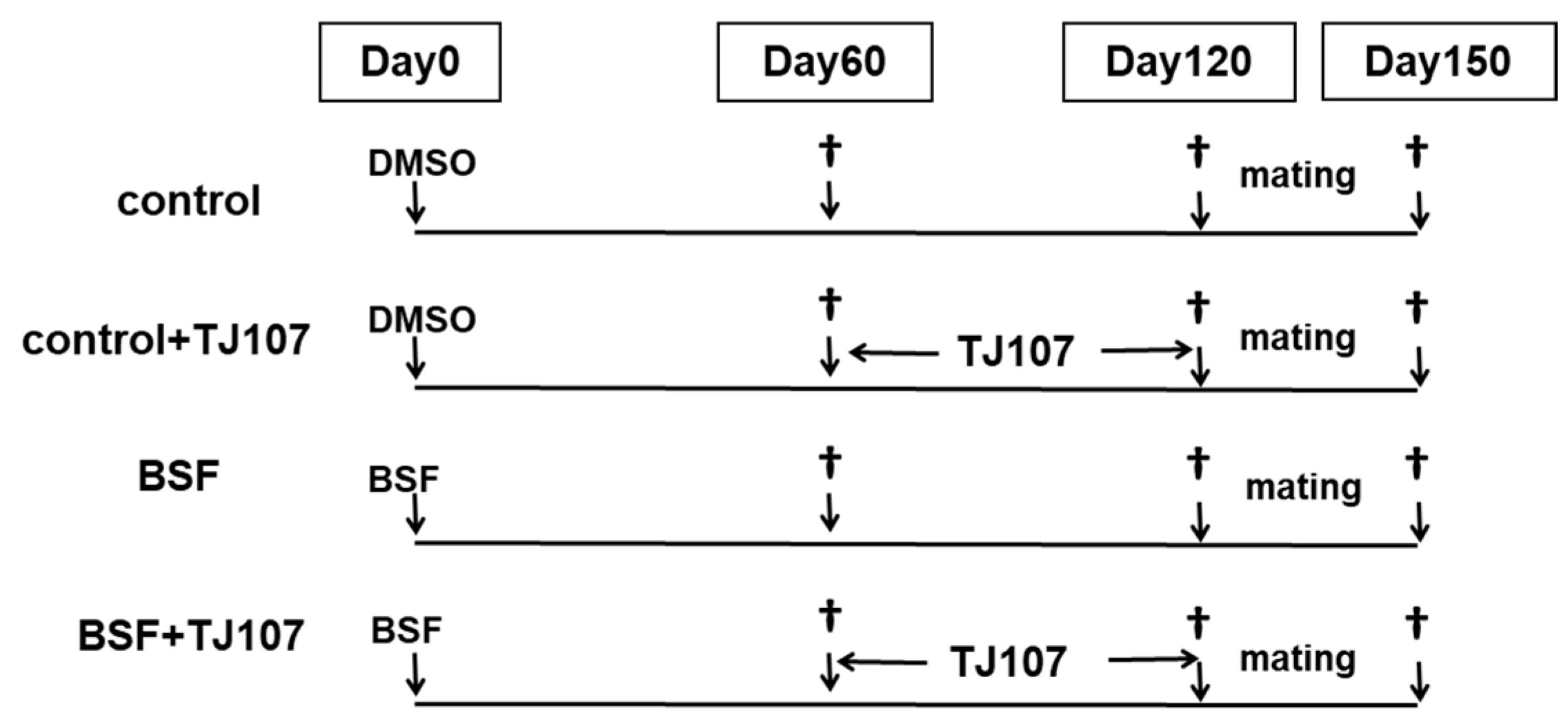
4.3. Experimental Design
4.4. Histological Examination of the Testes
4.5. Histopathological Assessment
4.6. Analysis of the mRNA Expressions of Cytokines Using Real-Time RT-PCR
4.7. Epididymal Spermatozoa Count
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSF | Busulfan |
| TJ107 | Gosha-jinki-gan |
| DMSO | Dimethyl sulfoxide |
| DW | Distilled water |
References
- Ploemacher, R.E.; Johnson, K.W.; Rombouts, E.J.; Etienne, K.; Westerhof, G.R.; Baumgart, J.; White-Scharf, M.E.; Down, J.D. Addition of treosulfan to a nonmyeloablative conditioning regimen results in enhanced chimerism and immunologic tolerance in an experimental allogeneic bone marrow transplant model. Biol. Blood Marrow Transplant. 2004, 10, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.; Rosendaal, M. A cautionary tale: How to delete mouse haemopoietic stem cells with busulphan. Br. J. Haematol. 2001, 113, 970–974. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, M.E.; Huitema, A.D.; Rodenhuis, S.; Beijnen, J.H. Clinical pharmacokinetics of cyclophosphamide. Clin. Pharmacokinet. 2005, 44, 1135–1164. [Google Scholar] [CrossRef] [PubMed]
- Galton, D.A.; Till, M.; Wiltshaw, E. Busulfan (1,4-dimethanesulfonyloxybutane, Myleran): Summary of clinical results. Ann. N. Y. Acad. Sci. 1958, 68, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Kenis, Y.; Dustin, P.; Henry, J.A.; Tagnon, H.J. Action du Myleran dans 22 cas de leucemie myeloide chromique. Rev. Fr. Etud. Clin. Biol. 1956, 1, 435–442. [Google Scholar] [PubMed]
- Kyle, R.A.; Schwartz, R.S.; Oliner, H.L.; Damashek, W. A syndrome resembling adrenal cortical insufficiency associated with long-term busulfan (Myleran) therapy. Blood 1961, 18, 497–510. [Google Scholar] [PubMed]
- Van Keulen, C.J.; de Rooij, D.G. The recovery from various gradations of cell loss in the mouse seminiferous epithelium and its implications for the spermatogonial stem cell renewal theory. Cell Tissue Kinet. 1974, 7, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Van Keulen, C.J.; de Rooij, D.G. Spermatogonic clones developing from repopulating stem cells surviving a high dose of an alkylating agent. Cell Tissue Kinet. 1975, 8, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Carvalho, T.L.; Anselmo-Franci, J.A.; Petenusci, S.O.; Favaretto, A.L. Ultrasound stimulation of rat testes damaged by busulfan. Ultrasound Med. Biol. 1997, 23, 1421–1425. [Google Scholar] [CrossRef]
- Brinster, C.J.; Ryu, B.Y.; Avarbock, M.R.; Karagenc, L.; Brinster, R.L.; Orwig, K.E. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol. Reprod. 2003, 69, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Toyokuni, S.; Morimoto, T.; Matsui, S.; Honjo, T.; Shinohara, T. Functional assessment of self-renewal activity of male germline stem cells following cytotoxic damage and serial transplantation. Biol. Reprod. 2003, 68, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Hirayanagi, Y.; Qu, N.; Hirai, S.; Naito, M.; Terayama, H.; Hayashi, S.; Hatayama, N.; Kuramasu, M.; Ogawa, Y.; Itoh, M. Busulfan pretreatment for transplantation of rat spermatogonia differentially affects immune and reproductive systems in male recipient mice. Anat. Sci. Int. 2015, 90, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Wang, L.G.; Li, H.Y.; Ji, X.J. Induction of differentiation and down-refulation of c-myb gene expression in ML-1 human myeloblastic leukemia cells by the clinically effective anti-leukemia agent meisoindigo. Biol. Pharm. 1996, 51, 1545–1551. [Google Scholar] [CrossRef]
- Ouyang, H.; Wang, P.; Meng, Z.; Chen, Z.; Yu, E.; Jin, H.; Chang, D.Z.; Liao, Z.; Cohen, L.; Liu, L. Multimodality treatment of pancreatic cancer with liver metastases using chemotherapy, radiation therapy, and/or Chinese herbal medicine. Pancreas 2011, 40, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, N.M.; Davis, R.B.; Ettner, S.L.; Appel, S.; Wilkey, S.; van Rompay, M.; Kessler, R.C. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA 1998, 280, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Huh, Y.; Bae, H.; Ahn, D.; Park, S.K. The multi-herbal formula Guibi-tang enhances memory and increases cell proliferation in the rat hippocampus. Neurosci. Lett. 2005, 379, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Usuki, Y.; Usuki, S.; Hommura, S. Successful treatment of a senile diabetic woman with cataract with goshajinkigan. Am. J. Chin. Med. 1991, 19, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.A.; Andoh, T.; Ogura, K.; Hayakawa, Y.; Saiki, I.; Kuraishi, Y. Herbal medicine goshajinkigan prevents paclitaxel-induced mechanical allodynia without impairing antitumor activity of paclitaxel. Evid. Based Complement. Altern. Med. 2013, 2013, 849754. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Hata, T.; Morita, S.; Munemoto, Y.; Matsui, T.; Kojima, H.; Takemoto, H.; Fukunaga, M.; Nagata, N.; Shimada, M.; et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): A phase 2, multicenter, randomized, doubleblind, placebocontrolled trial of goshajinkigan to prevent oxaliplatininduced neuropathy. Cancer Chemother. Pharmacol. 2013, 72, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Hosokawa, T.; Ishikawa, T.; Yagi, N.; Kokura, S.; Naito, Y.; Nakanishi, M.; Kokuba, Y.; Otsuji, E.; Kuroboshi, H.; et al. Efficacy of goshajinkigan for oxaliplatin-induced peripheral neuropathy in colorectal cancer patients. J. Oncol. 2013, 2013, 139740. [Google Scholar] [CrossRef] [PubMed]
- Oki, E.; Emi, Y.; Kojima, H.; Higashijima, J.; Kato, T.; Miyake, Y.; Kon, M.; Ogata, Y.; Takahashi, K.; Ishida, H.; et al. Preventive effect of goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): A placebo-controlled, doubleblind, randomized phase III study. Int. J. Clin. Oncol. 2015, 20, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Tawata, M.; Kurihara, A.; Nitta, K.; Iwase, E.; Gan, N.; Onaya, T. The effects of goshajinkigan, a herbal medicine, on subjective symptoms and vibratory threshold in patients with diabetic neuropathy. Diabetes Res. Clin. Pract. 1994, 26, 121–128. [Google Scholar] [CrossRef]
- Nishizawa, M.; Sutherland, W.H.; Nukada, H. Goshajinki-gan (herbal medicine) in streptozocin-induced diabetic neuropathy. J. Neurol. Sci. 1995, 132, 177–181. [Google Scholar] [CrossRef]
- Wyrobek, A.J.; Schmid, T.E.; Marchetti, F. Relative susceptibilities of male germ cells to genetic defects induced by cancer chemotherapies. J. Natl. Cancer Inst. Mon. 2005, 34, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K. Fertility preservation: An emerging discipline in the care of young patients with cancer. Lancet Oncol. 2005, 6, 192–193. [Google Scholar] [CrossRef]
- Shetty, G.; Meistrech, M.L. Hormonal approaches to preservation and restoration of male fertility after cancer treatment. J. Natl. Cancer Inst. Mon. 2005, 34, 36–39. [Google Scholar] [CrossRef] [PubMed]
- World Health Organizaiton. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Muscus Interaction; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Zhang, E.Q. Basic Theory of Traditional Chinese Medicine (I) in a Practical English-Chinese Library; Publishing House of Shanghai University of Traditional Chinese Medicine: Shanghai, China, 1999; pp. 100–105. [Google Scholar]
- Bucci, L.R.; Meistrich, M.L. Effects of busulfan on murine spermatogenesis: Cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat. Res. 1987, 176, 259–268. [Google Scholar] [CrossRef]
- Choi, Y.J.; Ok, D.W.; Kwon, D.N.; Chung, J.I.; Kim, H.C.; Yeo, S.M.; Kim, T.; Seo, H.G.; Kim, J.H. Murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a Fas/FasL- and p53-independent manner. FEBS Lett. 2004, 575, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, T.; Deng, T.; Xiong, W.; Lui, P.; Li, N.; Chen, Y.; Han, D. Damaged spermatogenic cells induce inflammatory gene expression in mouse Sertoli cells through the activation of Toll-like receptors 2 and 4. Mol. Cell. Endocrinol. 2013, 365, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Jung, D.Y.; Ha, H.; Son, K.H.; Jeon, S.J.; Kim, C. Induction of growth hormone release by dioscin from Dioscorea batatas DECNE. J. Biochem. Mol. Biol. 2007, 40, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Lysiak, J.J.; Turner, S.D.; Turner, T.T. Molecular pathway of germ cell apoptosis following ischemia/reperfusion of the rat testis. Biol. Reprod. 2000, 63, 1465–1472. [Google Scholar] [CrossRef] [PubMed]


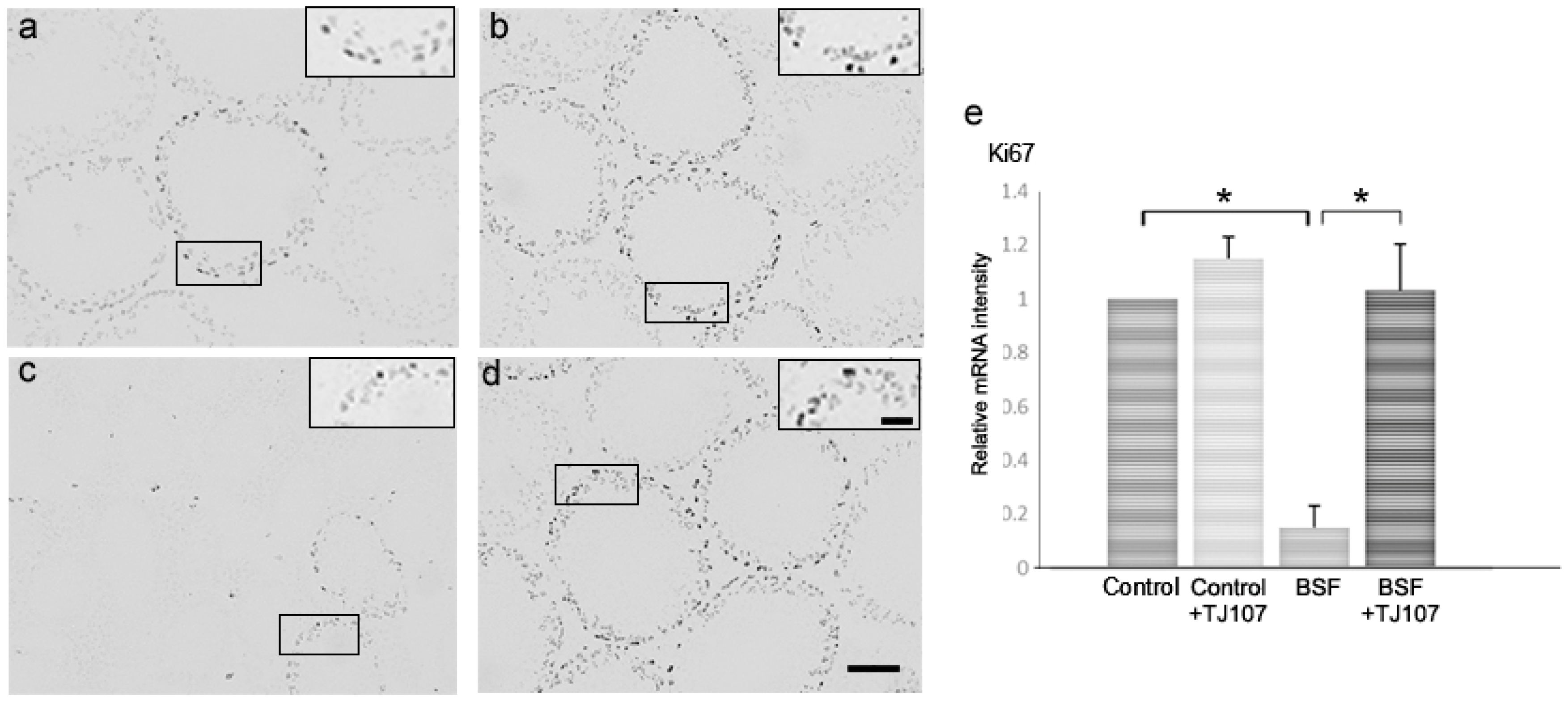


| Groups | Days after Injection | Control | Control+TJ107 | BSF | BSF+TJ107 |
|---|---|---|---|---|---|
| Body weight (g) | Day 60 | 28.301 ± 1.090 | 28.301 ± 1.090 | 25.913 ± 1.362 a | 25.913 ± 1.362 a |
| Day 120 | 34.010 ± 0.917 | 34.005 ± 1.240 | 27.862 ± 2.203 a | 31.008 ± 1.524 a,b | |
| Absolute testis weight (g) | Day 60 | 0.098 ± 0.002 | 0.098 ± 0.002 | 0.041 ± 0.008 a | 0.041 ± 0.008 a |
| Day 120 | 0.099 ± 0.002 | 0.102 ± 0.005 | 0.017 ± 0.002 a | 0.100 ± 0.006 b | |
| Relative testis weight (%) | Day 60 | 0.354 ± 0.013 | 0.354 ± 0.013 | 0.174 ± 0.017 a | 0.174 ± 0.017 a |
| Day 120 | 0.291 ± 0.004 | 0.303 ± 0.008 a | 0.060 ± 0.007 a | 0.334 ± 0.012 b | |
| Epididymal spermatozoa (×105) | Day 60 | 19.800 ± 2.180 | 19.800 ± 2.180 | 1.575 ± 0.308 a | 1.575 ± 0.308 a |
| Day 120 | 20.500 ± 2.462 | 27.468 ± 1.578 a | 0.217 ± 0.019 a | 21.680 ± 1.700 b | |
| Fertility rate | Day 120 | 100% (10/10) | 100% (10/10) | 0% (0/10) a | 100% (10/10) b |
| Number of fetuses/female | Day 150 | 5.875 ± 2.368 | 5.750 ± 1.639 | 0 a | 3.272 ± 2.014 a,b |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Caspase3 | GAGGCTGACTTCCTGTATGCTT | AACCACGACCCGTCCTTT |
| Caspase8 | TTGAACAATGAGATCCCCAAA | CCATTTCTACAAAAATTTCAAGCAG |
| Caspase9 | TGCAGTCCCTCCTTCTCAG | GCTTTTTCCGGAGGAAGTTAAA |
| Fas | GCAGACATGCTGTGGATCTGG | TCACAGCCAGGAGAATCGCAG |
| FasL | TCCAGGGTGGGTCTACTTACTAC | CCCTCTTACTTCTCCGTTAGGA |
| F4/80 | CTTTGGCTATGGGCTTCCAGTC | GCAAGGAGGACAGAGTTTATCGTG |
| Ki67 | GCTGTCCTCAAGACAATCATCA | GGCGTTATCCCAGGAGACT |
| TLR2 | TGCAAGTACGAACTGGACTTCT | CCAGGTAGGTCTTGGTCATT |
| TLR4 | GTGCCATCATTATGAGTGCC | CAAGCCAAGAAATATACCATCGAAG |
| GAPDH | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, N.; Kuramasu, M.; Hirayanagi, Y.; Nagahori, K.; Hayashi, S.; Ogawa, Y.; Terayama, H.; Suyama, K.; Naito, M.; Sakabe, K.; et al. Gosha-Jinki-Gan Recovers Spermatogenesis in Mice with Busulfan-Induced Aspermatogenesis. Int. J. Mol. Sci. 2018, 19, 2606. https://doi.org/10.3390/ijms19092606
Qu N, Kuramasu M, Hirayanagi Y, Nagahori K, Hayashi S, Ogawa Y, Terayama H, Suyama K, Naito M, Sakabe K, et al. Gosha-Jinki-Gan Recovers Spermatogenesis in Mice with Busulfan-Induced Aspermatogenesis. International Journal of Molecular Sciences. 2018; 19(9):2606. https://doi.org/10.3390/ijms19092606
Chicago/Turabian StyleQu, Ning, Miyuki Kuramasu, Yoshie Hirayanagi, Kenta Nagahori, Shogo Hayashi, Yuki Ogawa, Hayato Terayama, Kaori Suyama, Munekazu Naito, Kou Sakabe, and et al. 2018. "Gosha-Jinki-Gan Recovers Spermatogenesis in Mice with Busulfan-Induced Aspermatogenesis" International Journal of Molecular Sciences 19, no. 9: 2606. https://doi.org/10.3390/ijms19092606







