Tracking of Adipose-Derived Mesenchymal Stromal/Stem Cells in a Model of Cisplatin-Induced Acute Kidney Injury: Comparison of Bioluminescence Imaging versus qRT-PCR
Abstract
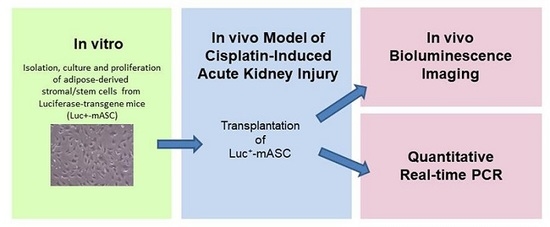
:1. Introduction
2. Results
2.1. Characterization of mASC
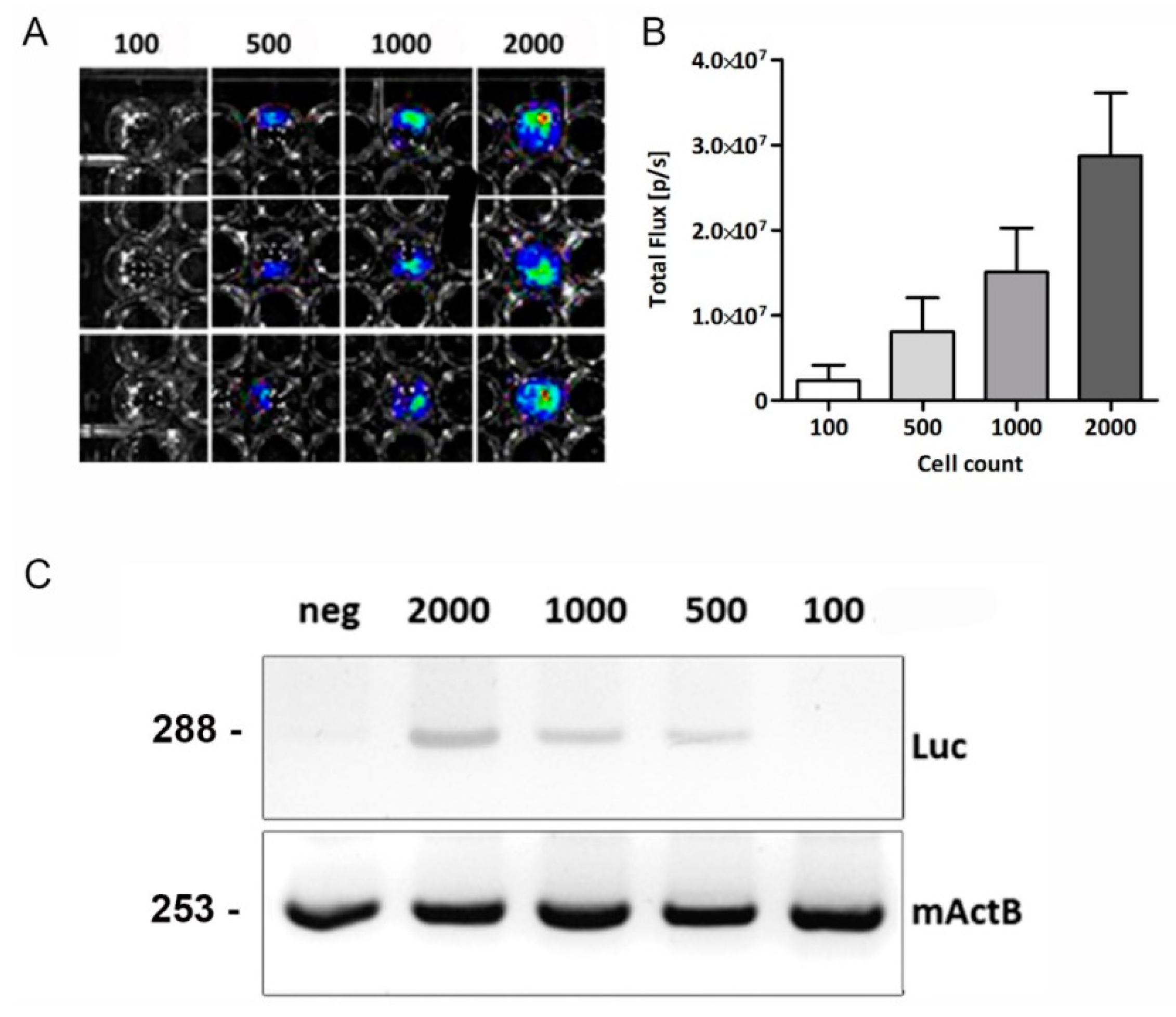
2.2. In Vitro Bioluminescence and PCR Analysis
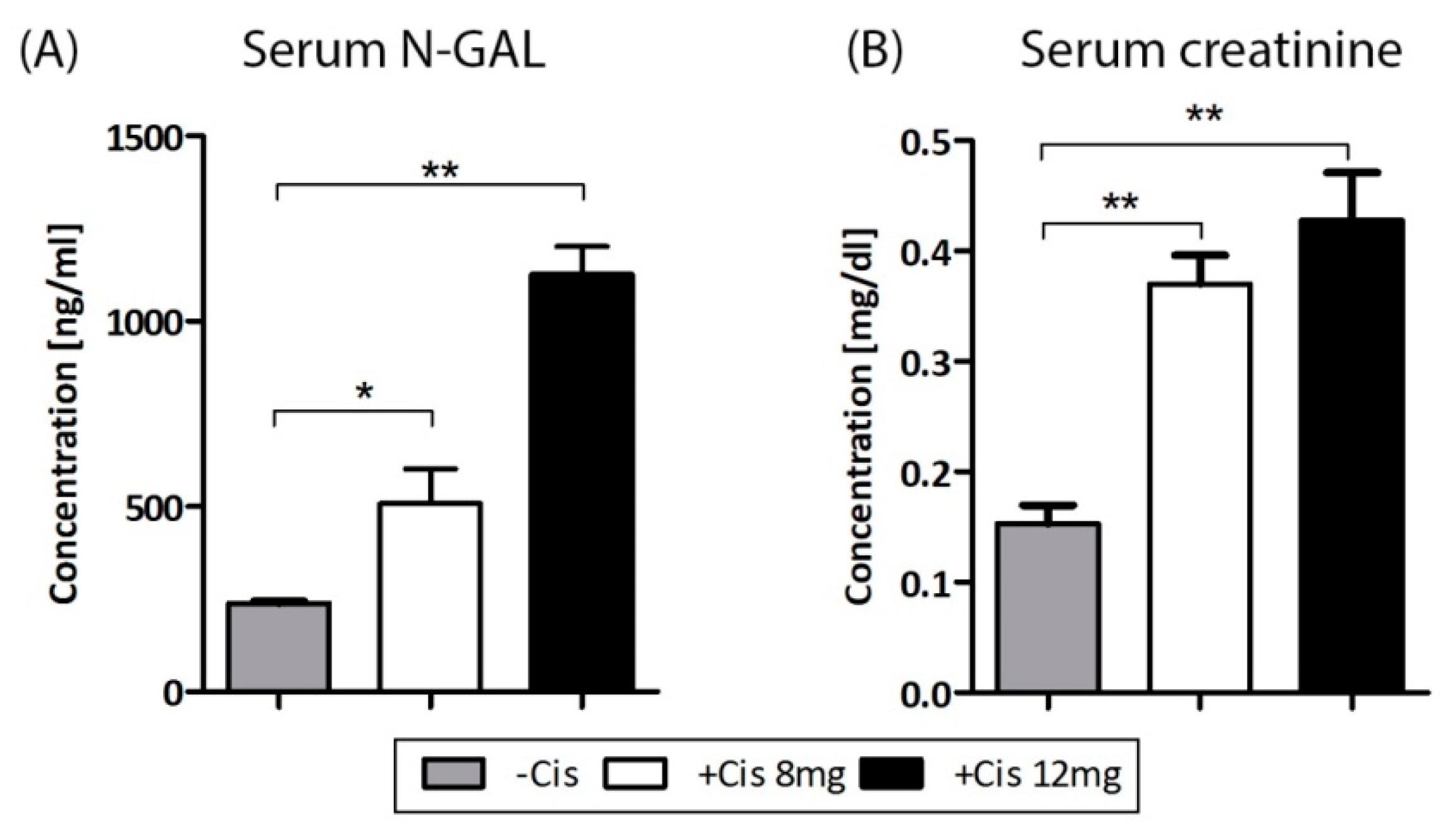
2.3. Cisplatin-Induced AKI
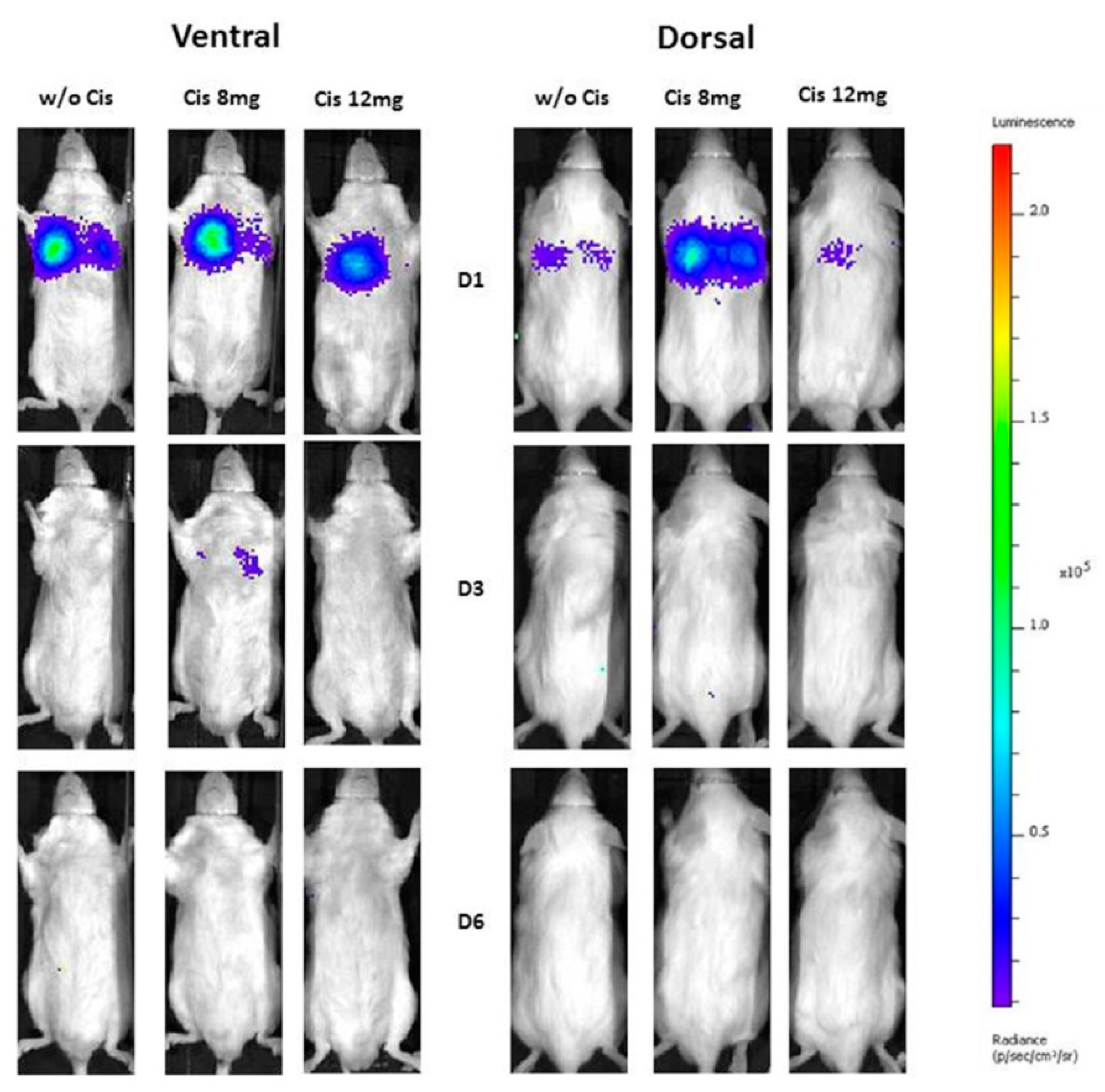
2.4. In Vivo Biolumunescence Imaging
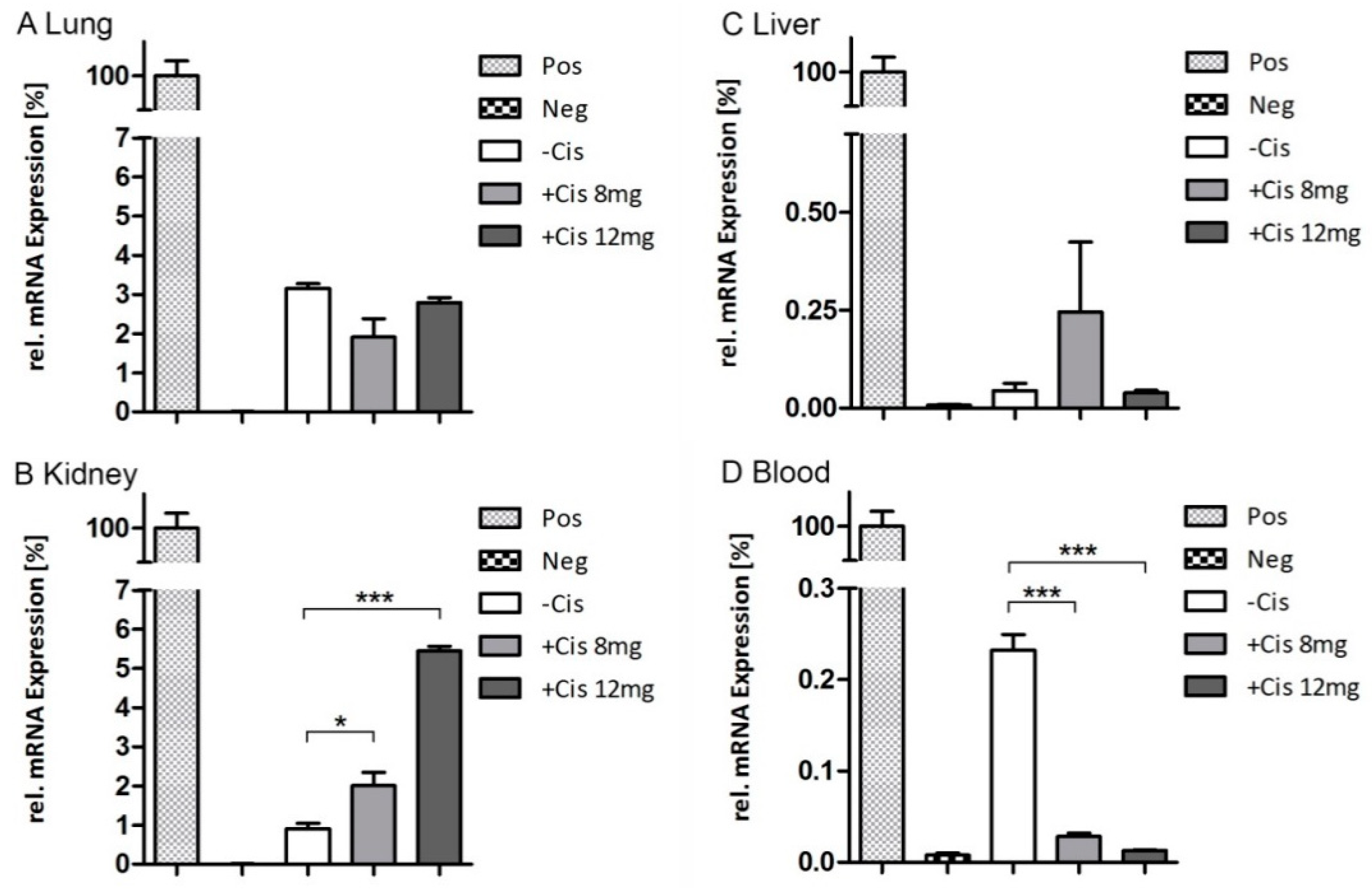
2.5. Endpoint qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Isolation, Culture, and Characterization
4.3. In Vivo Model of Cisplatin-Induced Acute Kidney Injury
4.4. Polymerase Chain Reaction
4.5. In Vitro Imaging of Luc+-mASCs
4.6. In Vivo Bioluminescence Imaging
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| BLI | bioluminescence imaging |
| Cis | Cisplatin |
| i.p. | intraperitoneal |
| IV | intravenous |
| Luc+-mASCs | Luciferase transgenic adipose-derived stromal/stem cells |
| mASCs | adipose-derived stromal/stem cells |
| MSCs | mesenchymal stromal/stem cells |
| N-GAL | neutrophil gelatinase-associated Lipocalin |
| PCR | polymerase chain reaction |
| qRT | quantitative real-time reverse transcriptase |
| WT | wild type |
References
- Creane, M.; Howard, L.; O’Brien, T.; Coleman, C.M. Biodistribution and retention of locally administered human mesenchymal stromal cells: Quantitative polymerase chain reaction-based detection of human DNA in murine organs. Cytotherapy 2017, 19, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs Improve Myocardial Infarction in Mice because Cells Embolized in Lung Are Activated to Secrete the Anti-inflammatory Protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Yang, Y.; Zhang, P.; Hu, Z.; Westenfelder, C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008, 295, F315–F321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Almeida, P.E.; van Rappard, J.R.M.; Wu, J.C. In vivo bioluminescence for tracking cell fate and function. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H663–H671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kircher, M.F.; Gambhir, S.S.; Grimm, J. Noninvasive cell-tracking methods. Nat. Rev. Clin. Oncol. 2011, 8, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Kim, J.-Y.; Shin, D.-M.; Huh, J.W.; Lee, S.W.; Oh, Y.-M. Tracking Intravenous Adipose-Derived Mesenchymal Stem Cells in a Model of Elastase-Induced Emphysema. Tuberc. Respir. Dis. 2014, 77, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C.; Geiger, H. Adipose-Derived Mesenchymal Stromal/Stem Cells: Tissue Localization, Characterization, and Heterogeneity. Stem Cells Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, I.R.; Péault, B. Q&A: Mesenchymal stem cells―Where do they come from and is it important? BMC Biol. 2015, 13, 99. [Google Scholar] [CrossRef]
- Caplan, A.I. All MSCs are pericytes? Cell Stem Cell 2008, 3, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.B.; Bussolati, B.; Bruno, S.; Morando, L.; Mauriello-Romanazzi, G.; Sanavio, F.; Stamenkovic, I.; Biancone, L.; Camussi, G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007, 72, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Rota, C.; Remuzzi, G. Mesenchymal Stem Cells in Kidney Repair. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1416, pp. 89–107. [Google Scholar]
- Bi, B.; Schmitt, R.; Israilova, M.; Nishio, H.; Cantley, L.G. Stromal cells protect against acute tubular injury via an endocrine effect. J. Am. Soc. Nephrol. 2007, 18, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Behr, L.; Hekmati, M.; Fromont, G.; Borenstein, N.; Noel, L.-H.; Lelievre-Pegorier, M.; Laborde, K. Intra renal arterial injection of autologous mesenchymal stem cells in an ovine model in the postischemic kidney. Nephron Physiol. 2007, 107, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Overath, J.M.; Gauer, S.; Obermüller, N.; Schubert, R.; Schäfer, R.; Geiger, H.; Baer, P.C. Short-term preconditioning enhances the therapeutic potential of adipose-derived stromal/stem cell-conditioned medium in cisplatin-induced acute kidney injury. Exp. Cell Res. 2016, 342, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C.; Overath, J.M.; Urbschat, A.; Schubert, R.; Koch, B.; Bohn, A.A.; Geiger, H. Effect of Different Preconditioning Regimens on the Expression Profile of Murine Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 1719. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Yang, H.-M.; Park, J.B.; Choi, G.-S.; Joh, J.-W.; Kwon, C.H.; Chun, J.M.; Lee, S.-K.; Kim, S.-J. Isolation and characterization of mouse mesenchymal stem cells. Transplant. Proc. 2008, 40, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Fleige, S.; Walf, V.; Huch, S.; Prgomet, C.; Sehm, J.; Pfaffl, M.W. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol. Lett. 2006, 28, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Prigent, J.; Herrero, A.; Ambroise, J.; Smets, F.; Deblandre, G.A.; Sokal, E.M. Human Progenitor Cell Quantification After Xenotransplantation in Rat and Mouse Models by a Sensitive qPCR Assay. Cell Transplant. 2015, 24, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K.; Riegler, J.; Wu, J.C. Stem cell imaging: From bench to bedside. Cell Stem Cell 2014, 14, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Terrovitis, J.V.; Smith, R.R.; Marbán, E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ. Res. 2010, 106, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Contag, C.H.; Contag, P.R.; Mullins, J.I.; Spilman, S.D.; Stevenson, D.K.; Benaron, D.A. Photonic detection of bacterial pathogens in living hosts. Mol. Microbiol. 1995, 18, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jang, H.R.; Kim, D.H.; Kwon, G.Y.; Lee, J.E.; Huh, W.; Choi, S.J.; Oh, W.; Oh, H.Y.; Kim, Y.-G. Early, but not late, treatment with human umbilical cord blood-derived mesenchymal stem cells attenuates cisplatin nephrotoxicity through immunomodulation. Am. J. Physiol. Ren. Physiol. 2017, 313, F984–F996. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, F.E.; Sobh, M.-A.; Abouelkheir, M.; Khater, Y.; Mahmoud, K.; Saad, M.-A.; Sobh, M.A. Study of the Effect of Route of Administration of Mesenchymal Stem Cells on Cisplatin-Induced Acute Kidney Injury in Sprague Dawley Rats. Int. J. Stem Cells 2016, 9, 79–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Rocco, G.; Gentile, A.; Antonini, A.; Truffa, S.; Piaggio, G.; Capogrossi, M.C.; Toietta, G. Analysis of biodistribution and engraftment into the liver of genetically modified mesenchymal stromal cells derived from adipose tissue. Cell Transplant. 2012, 21, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, R.L.; Karpetsky, T.P.; Neuwelt, E.A.; Levy, C.C. Levels of serum ribonuclease as an indicator of renal insufficiency in patients with leukemia. Cancer Res. 1977, 37, 2015–2022. [Google Scholar] [PubMed]
- Akagi, K.; Tsuji, H.; Kajiwara, E.; Murai, K.; Shikata, T. Activities of serum acid ribonuclease in patients with malignant neoplasms or with renal failure. Clin. Chim. Acta 1983, 135, 83–87. [Google Scholar] [PubMed]
- Morigi, M.; Benigni, A. Mesenchymal stem cells and kidney repair. Nephrol. Dial. Transplant. 2013, 28, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, F.; Ezquer, M.; Simon, V.; Pardo, F.; Yañez, A.; Carpio, D.; Conget, P. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol. Blood Marrow Transplant. 2009, 15, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Imberti, B.; Morigi, M.; Tomasoni, S.; Rota, C.; Corna, D.; Longaretti, L.; Rottoli, D.; Valsecchi, F.; Benigni, A.; Wang, J.; et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J. Am. Soc. Nephrol. 2007, 18, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Imberti, B.; Zoja, C.; Corna, D.; Tomasoni, S.; Abbate, M.; Rottoli, D.; Angioletti, S.; Benigni, A.; Perico, N.; et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Bonventre, J.V. Mesenchymal stem cells in acute kidney injury. Annu. Rev. Med. 2008, 59, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Beilhack, A.; Schulz, S.; Baker, J.; Beilhack, G.F.; Wieland, C.B.; Herman, E.I.; Baker, E.M.; Cao, Y.-A.; Contag, C.H.; Negrin, R.S. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 2005, 106, 1113–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.-A.; Wagers, A.J.; Beilhack, A.; Dusich, J.; Bachmann, M.H.; Negrin, R.S.; Weissman, I.L.; Contag, C.H. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, R.; Sann, J.; Frueh, J.T.; Ullrich, E.; Geiger, H.; Baer, P.C. Tracking of Adipose-Derived Mesenchymal Stromal/Stem Cells in a Model of Cisplatin-Induced Acute Kidney Injury: Comparison of Bioluminescence Imaging versus qRT-PCR. Int. J. Mol. Sci. 2018, 19, 2564. https://doi.org/10.3390/ijms19092564
Schubert R, Sann J, Frueh JT, Ullrich E, Geiger H, Baer PC. Tracking of Adipose-Derived Mesenchymal Stromal/Stem Cells in a Model of Cisplatin-Induced Acute Kidney Injury: Comparison of Bioluminescence Imaging versus qRT-PCR. International Journal of Molecular Sciences. 2018; 19(9):2564. https://doi.org/10.3390/ijms19092564
Chicago/Turabian StyleSchubert, Ralf, Julia Sann, Jochen T. Frueh, Evelyn Ullrich, Helmut Geiger, and Patrick C. Baer. 2018. "Tracking of Adipose-Derived Mesenchymal Stromal/Stem Cells in a Model of Cisplatin-Induced Acute Kidney Injury: Comparison of Bioluminescence Imaging versus qRT-PCR" International Journal of Molecular Sciences 19, no. 9: 2564. https://doi.org/10.3390/ijms19092564
APA StyleSchubert, R., Sann, J., Frueh, J. T., Ullrich, E., Geiger, H., & Baer, P. C. (2018). Tracking of Adipose-Derived Mesenchymal Stromal/Stem Cells in a Model of Cisplatin-Induced Acute Kidney Injury: Comparison of Bioluminescence Imaging versus qRT-PCR. International Journal of Molecular Sciences, 19(9), 2564. https://doi.org/10.3390/ijms19092564