Glycocalyx Preservation and NO Production in Fatty Livers—The Protective Role of High Molecular Polyethylene Glycol in Cold Ischemia Injury
Abstract
:1. Introduction
2. Results
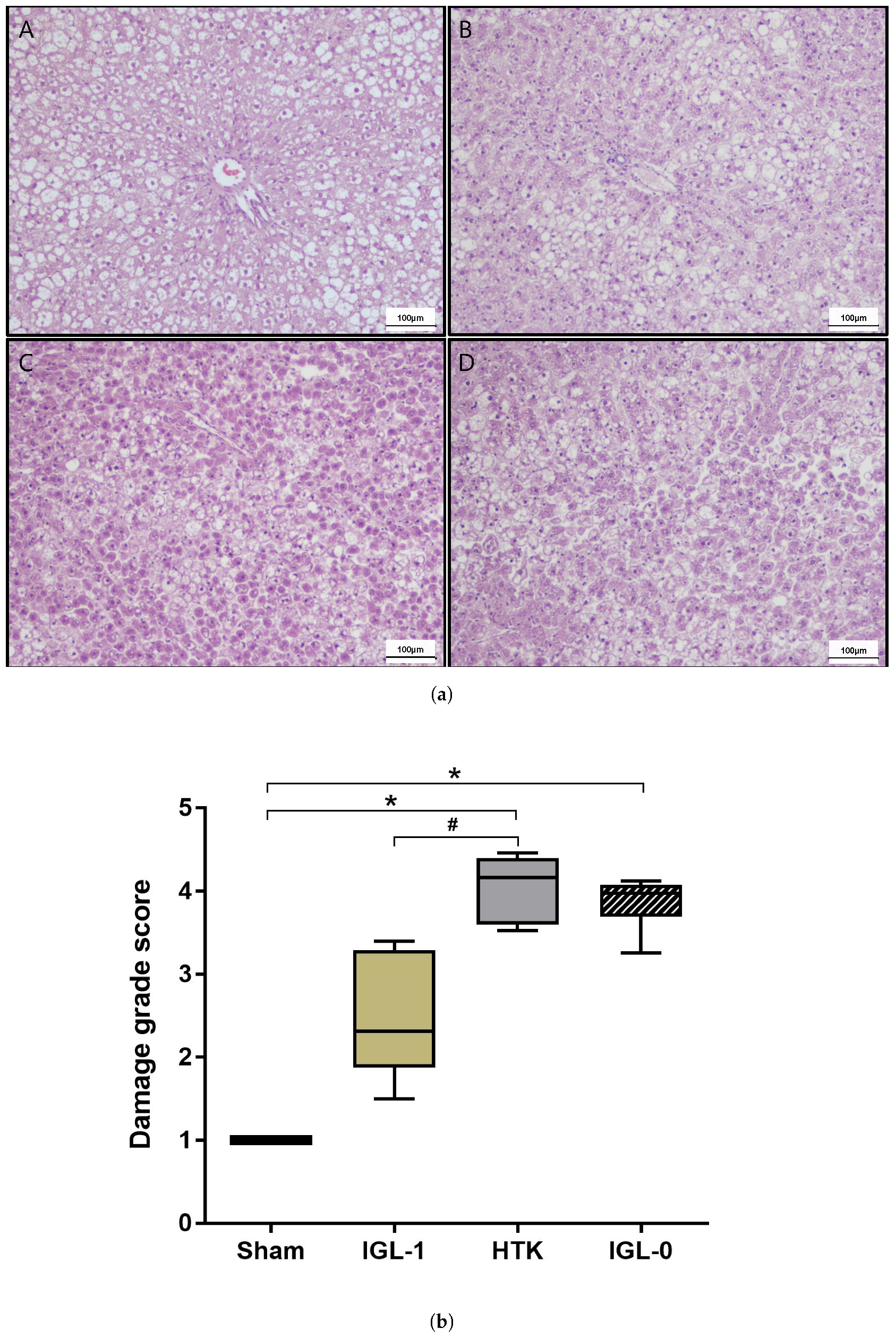
2.1. Fatty Livers Injury and Histology after Cold Ischemia
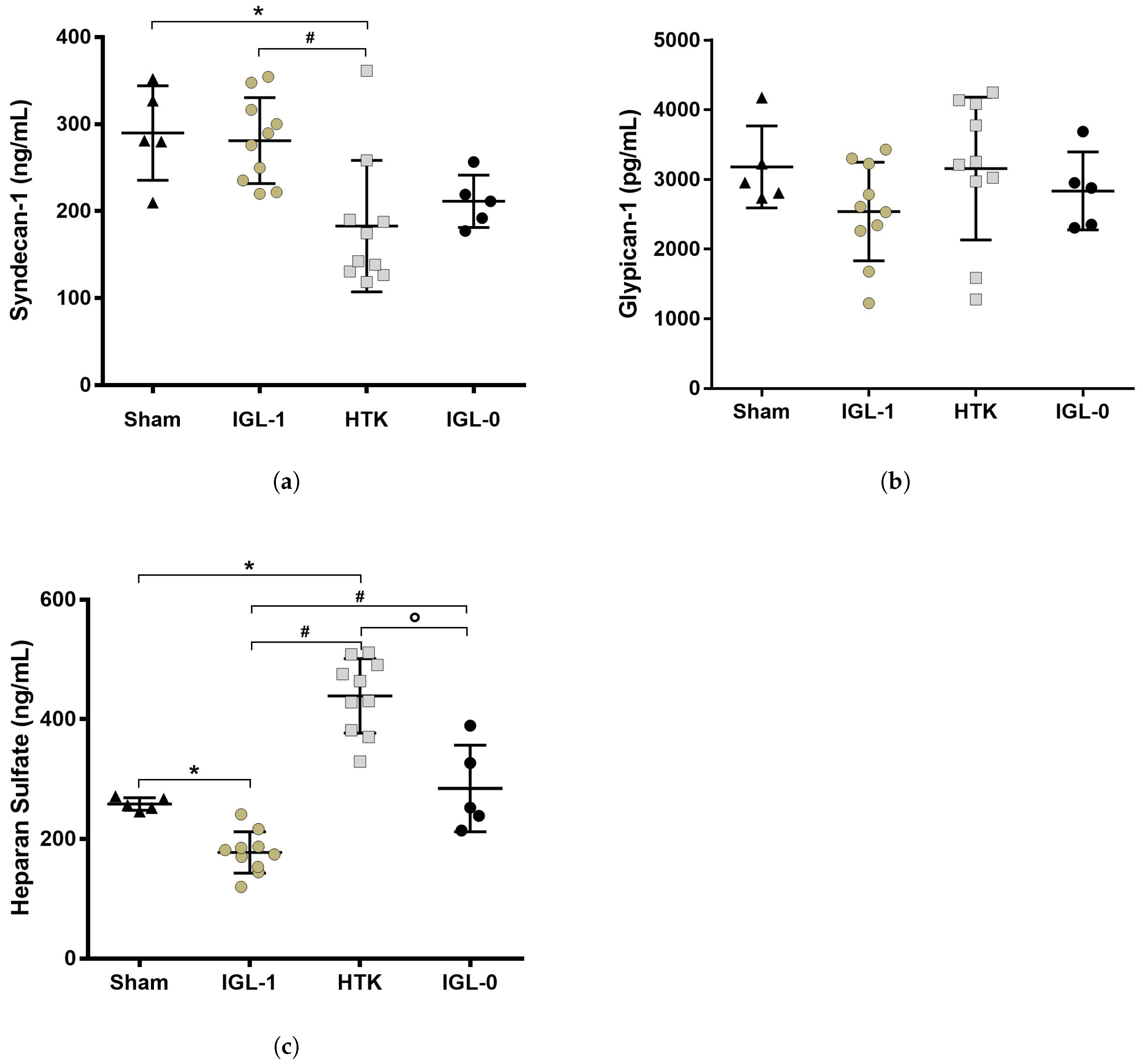
2.2. Syndecan-1, HS and Glypican-1 Preservation during Static Cold Storage
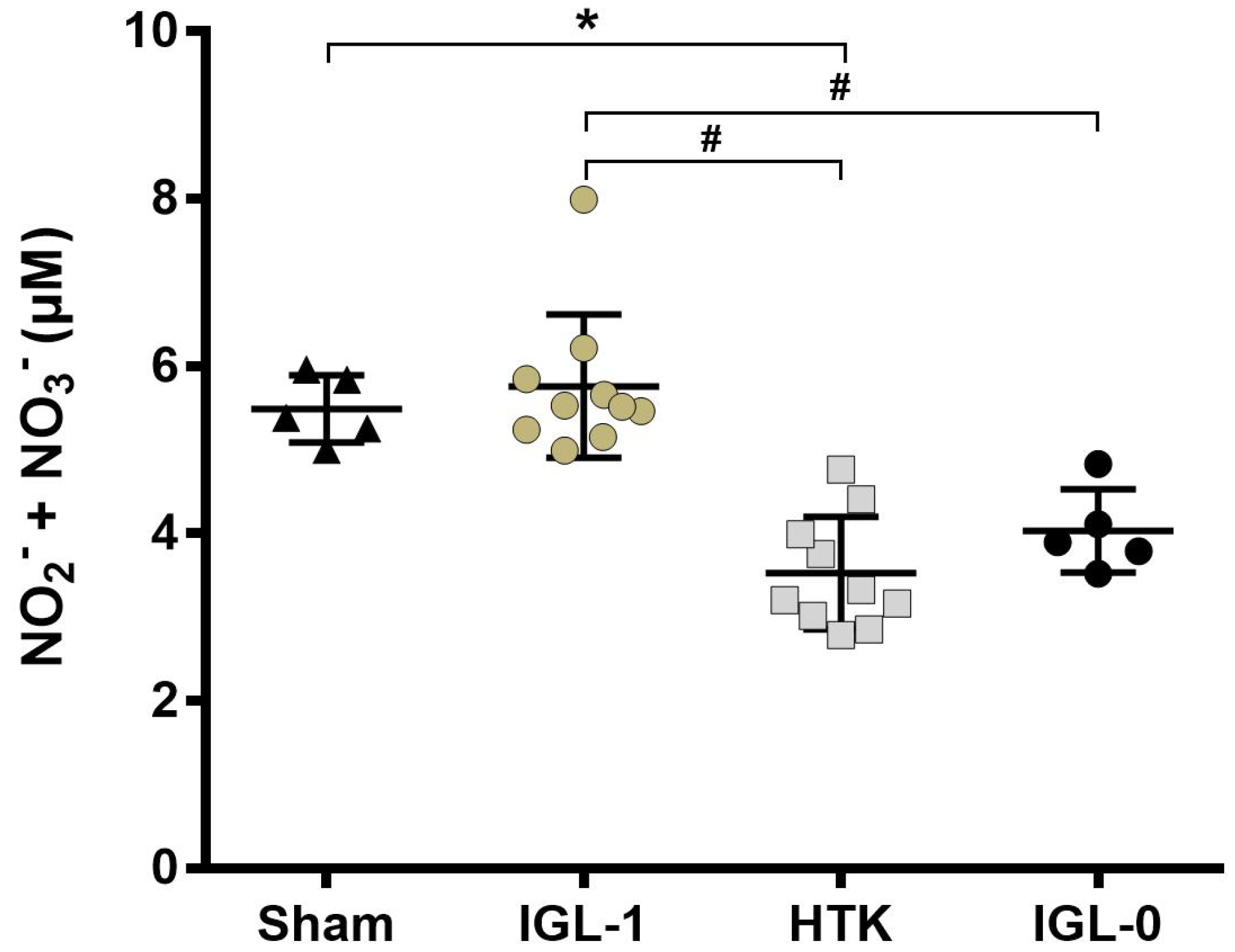
2.3. IGL-1 Protection through NO
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Liver Procurement and Experimental Groups
4.3. Biochemical Determination
4.3.1. Histology
4.3.2. Transaminases Assay
4.3.3. Nitric Oxide Assay
4.3.4. ELISA
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| CIT | Cold Ischemia Time |
| ELTR | European Liver Transplant Registry |
| eNOS | endothelial Nitric Oxide Synthase |
| HTK | Histidine–Tryptophane–Ketoglutarate |
| HS | Heparan Sulfate |
| IGL-1 | Institut Georges Lopez-1 |
| iNOS | inducible NOS |
| IRI | Ischemia–Reperfusion Injury |
| NO | Nitric Oxide |
| OLT | Orthotopic Liver Transplantation |
| PEG35 | PolyEthyene Glycol 35 kDa |
| SCS | Static Cold Storage |
| UNOS | United Network for Organ Sharing |
References
- Sammut, I.A.; Burton, K.; Balogun, E.; Sarathchandra, P.; Brooks, K.J.; Bates, T.E.; Green, C.J. Time-dependent impairment of mitochondrial function after storage and transplantation of rabbit kidneys. Transplantation 2000, 69, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Anavi, S.; Madar, Z.; Tirosh, O. Non-alcoholic fatty liver disease, to struggle with the strangle: Oxygen availability in fatty livers. Redox Biol. 2017, 13, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol. Cell Physiol. 2017, 312, C254–C262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.W.; Kong, L.B.; Li, G.Q.; Wang, X.H. Expression of iNOS in early injury in a rat model of small-for-size liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 146–151. [Google Scholar] [PubMed]
- Abu-Amara, M.; Yang, S.Y.; Seifalian, A.; Davidson, B.; Fuller, B. The nitric oxide pathway—Evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver Int. 2012, 32, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Duranski, M.R.; Elrod, J.W.; Calvert, J.W.; Bryan, N.S.; Feelisch, M.; Lefer, D.J. Genetic overexpression of eNOS attenuates hepatic ischemia–reperfusion injury. AJP Heart Circ. Physiol. 2006, 291, H2980–H2986. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Nishikawa, M.; Yamashita, F.; Hashida, M. Prevention of Hepatic Ischemia/Reperfusion Injury by Prolonged Delivery of Nitric Oxide to the Circulating Blood in Mice. Transplantation 2008, 85, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varadarajan, R.; Golden-Mason, L.; Young, L.; McLoughlin, P.; Nolan, N.; McEntee, G.; Traynor, O.; Geoghegan, J.; Hegarty, J.E.; O’Farrelly, C. Nitric oxide in early ischaemia reperfusion injury during human orthotopic liver transplantation. Transplantation 2004, 78, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Hines, I.; Zibari, G.; Grisham, M.B. Hepatocellular protection by nitric oxide or nitrite in ischemia and reperfusion injury. Arch. Biochem. Biophys. 2009, 484, 232–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, F.; Fuller, B.; Davidson, B. An Evaluation of Ischaemic Preconditioning as a Method of Reducing Ischaemia Reperfusion Injury in Liver Surgery and Transplantation. J. Clin. Med. 2017, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, B.M.; Spaan, J.A.E.; Rolf, T.M.; Vink, H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, J.; Lebherz-Eichinger, D.; Erdoes, G.; Berlakovich, G.; Bacher, A.; Krenn, C.G.; Faybik, P. Alterations of Endothelial Glycocalyx During Orthotopic Liver Transplantation in Patients With End-Stage Liver Disease. Transplantation 2015, 99, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Ebong, E.E.; Lopez-Quintero, S.V.; Rizzo, V.; Spray, D.C.; Tarbell, J.M. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr. Biol. 2014, 6, 338–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosch, A.M.W.; Mathews, R.; Tarbell, J.M. Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling. Biophys. J. 2017, 113, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, I.B.; Rosello-Catafau, J.; Franco-Gou, R.; Ben Abdennebi, H.; Saidane-Mosbahi, D.; Ramella-Virieux, S.; Boillot, O.; Peralta, C. Preservation of Steatotic Livers in IGL-1 Solution. Liver Transplant. 2006, 12, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Ben Mosbah, I.; Franco-Gou, R.; Ben Abnennebi, H.; Hernandez, R.; Escolar, G.; Saidane-Mosbahi, D.; Roselló-Catafau, J.; Peralta, C. Effects of polyethylene glycol and hydroxyethyl starch in University of Wisconsin preservation solution on human red blood cell aggregation and viscosity. Transpl. Proc. 2006, 38, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Panisello-Roselló, A.; Folch-Puy, E.; Lopez, A.; Castro Benítez, C.; Calvo, M.; Carbonell, T.; García-Gil, F.A.; Adam, R.; Roselló-Catafau, J. Polyethylene glycols: An effective strategy for limiting liver ischemia reperfusion injury. World J. Gastroenterol. 2016, 22, 6501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, Z.A.; Cameron, A.M.; Singer, A.L.; Montgomery, R.A.; Segev, D.L. Histidine-tryptophan-ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am. J. Transp. 2009, 9, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Delvart, V.; Karam, V.; Ducerf, C.; Navarro, F.; Letoublon, C.; Belghiti, J.; Pezet, D.; Castaing, D.; Le Treut, Y.P.; et al. Compared efficacy of preservation solutions in liver transplantation: A long-term graft outcome study from the European Liver Transplant Registry. Am. J. Transp. 2015, 15, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.T.; Camp, S.M.; Dudek, S.M.; Brown, M.E.; Usatyuk, P.V.; Zaborina, O.; Alverdy, J.C.; Garcia, J.G.N. Protective effects of high-molecular weight Polyethylene Glycol (PEG) in human lung endothelial cell barrier regulation: Role of actin cytoskeletal rearrangement. Microvasc. Res. 2009, 77, 174–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaouali, M.A.; Ben Mosbah, I.; Ben Abnennebi, H.; Calvo, M.; Boncompagni, E.; Boillot, O.; Peralta, C.; Roselló-Catafau, J. New Insights Into Fatty Liver Preservation Using Institute Georges Lopez Preservation Solution. Transplant. Proc. 2010, 42, 159–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, D.; Dörfler, N.; Jacob, M.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 2010, 34, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Rehm, M.; Bruegger, D.; Christ, F.; Conzen, P.; Thiel, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Reichart, B.; Peter, K.; Becker, B.F. Shedding of the Endothelial Glycocalyx in Patients Undergoing Major Vascular Surgery with Global and Regional Ischemia. Circulation 2007, 116, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Bruegger, D.; Brettner, F.; Rossberg, I.; Nussbaum, C.; Kowalski, C.; Januszewska, K.; Becker, B.F.; Chappell, D. Acute Degradation of the Endothelial Glycocalyx in Infants Undergoing Cardiac Surgical Procedures. Ann. Thorac. Surg. 2015, 99, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Beijert, I.; Mert, S.; Huang, V.; Karimian, N.; Geerts, S.; Hafiz, E.O.A.; Markmann, J.F.; Yeh, H.; Porte, R.J.; Uygun, K. Endothelial Dysfunction in Steatotic Human Donor Livers: A Pilot Study of the Underlying Mechanism During Subnormothermic Machine Perfusion. Transplant. Direct 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Rosello, A.; Castro-Benítez, C.; Lopez, A.; Balloji, S.; Folch-Puy, E.; Adam, R.; Roselló-Catafau, J. Graft Protection Against Cold Ischemia Preservation: An Institute George Lopez 1 and Histidine-tryptophan-ketoglutarate Solution Appraisal. Transplant. Proc. 2018, 50, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Chaumel, E.; Roselló-Catafau, J.; Bartrons, R.; Franco-Gou, R.; Xaus, C.; Casillas, A.; Gelpí, E.; Rodés, J.; Peralta, C. Adenosine monophosphate-activated protein kinase and nitric oxide in rat steatotic liver transplantation. J. Hepatol. 2005, 43, 997–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejaoui, M.; Pantazi, E.; Folch-Puy, E.; Panisello-Roselló, A.; Calvo, M.; Pasut, G.; Rimola, A.; Navasa, M.; Adam, R.; Roselló-Catafau, J. Protective Effect of Intravenous High Molecular Weight Polyethylene Glycol on Fatty Liver Preservation. BioMed Res. Int. 2015, 2015, 794287. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, K.; Lang, J.D. Role of nitric oxide in liver transplantation: Should it be routinely used? World J. Hepatol. 2016, 8, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Pahakis, M.Y.; Kosky, J.R.; Dull, R.O.; Tarbell, J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 2007, 355, 228–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mineo, C.; Shaul, P.W. Regulation of eNOS in Caveolae. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; Volume 729, pp. 51–62. [Google Scholar]
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.; Mani, K.; Van den Born, J.; Ding, K.; Belting, M.; Fransson, L.Å. Nitric oxide-dependent processing of heparan sulfate in recycling S-nitrosylated glypican-1 takes place in caveolin-1-containing endosomes. J. Biol. Chem. 2002, 277, 44431–44439. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liu, J. Role of glypican-1 in endothelial NOS activation under various steady shear stress magnitudes. Exp. Cell Res. 2016, 348, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Svensson, G.; Mani, K. S-Nitrosylation of secreted recombinant human glypican-1. Glycoconj. J. 2009, 26, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Valuckaite, V.; Zaborina, O.; Long, J.; Hauer-Jensen, M.; Wang, J.; Holbrook, C.; Zaborin, A.; Drabik, K.; Katdare, M.; Mauceri, H.; et al. Oral PEG 15–20 protects the intestine against radiation: Role of lipid rafts. AJP Gastrointest. Liver Physiol. 2009, 297, G1041–G1052. [Google Scholar] [CrossRef] [PubMed]
- Ben Abnennebi, H.; Steghens, J.P.; Margonari, J.; Ramella-Virieux, S.; Barbieux, A.; Boulot, O. High-Na low-H UW cold storage solution reduces reperfusion injuries of the rat liver graft. Transpl. Int. 1998, 11, 223–230. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, A.; Panisello-Rosello, A.; Castro-Benitez, C.; Adam, R. Glycocalyx Preservation and NO Production in Fatty Livers—The Protective Role of High Molecular Polyethylene Glycol in Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2375. https://doi.org/10.3390/ijms19082375
Lopez A, Panisello-Rosello A, Castro-Benitez C, Adam R. Glycocalyx Preservation and NO Production in Fatty Livers—The Protective Role of High Molecular Polyethylene Glycol in Cold Ischemia Injury. International Journal of Molecular Sciences. 2018; 19(8):2375. https://doi.org/10.3390/ijms19082375
Chicago/Turabian StyleLopez, Alexandre, Arnau Panisello-Rosello, Carlos Castro-Benitez, and René Adam. 2018. "Glycocalyx Preservation and NO Production in Fatty Livers—The Protective Role of High Molecular Polyethylene Glycol in Cold Ischemia Injury" International Journal of Molecular Sciences 19, no. 8: 2375. https://doi.org/10.3390/ijms19082375




