Verapamil Inhibits TRESK (K2P18.1) Current in Trigeminal Ganglion Neurons Independently of the Blockade of Ca2+ Influx
Abstract
:1. Introduction
2. Results
2.1. Inhibitory Effect of Ca2+ Channel Blockers on TRESK Channel Overexpressed in HEK-293 Cells
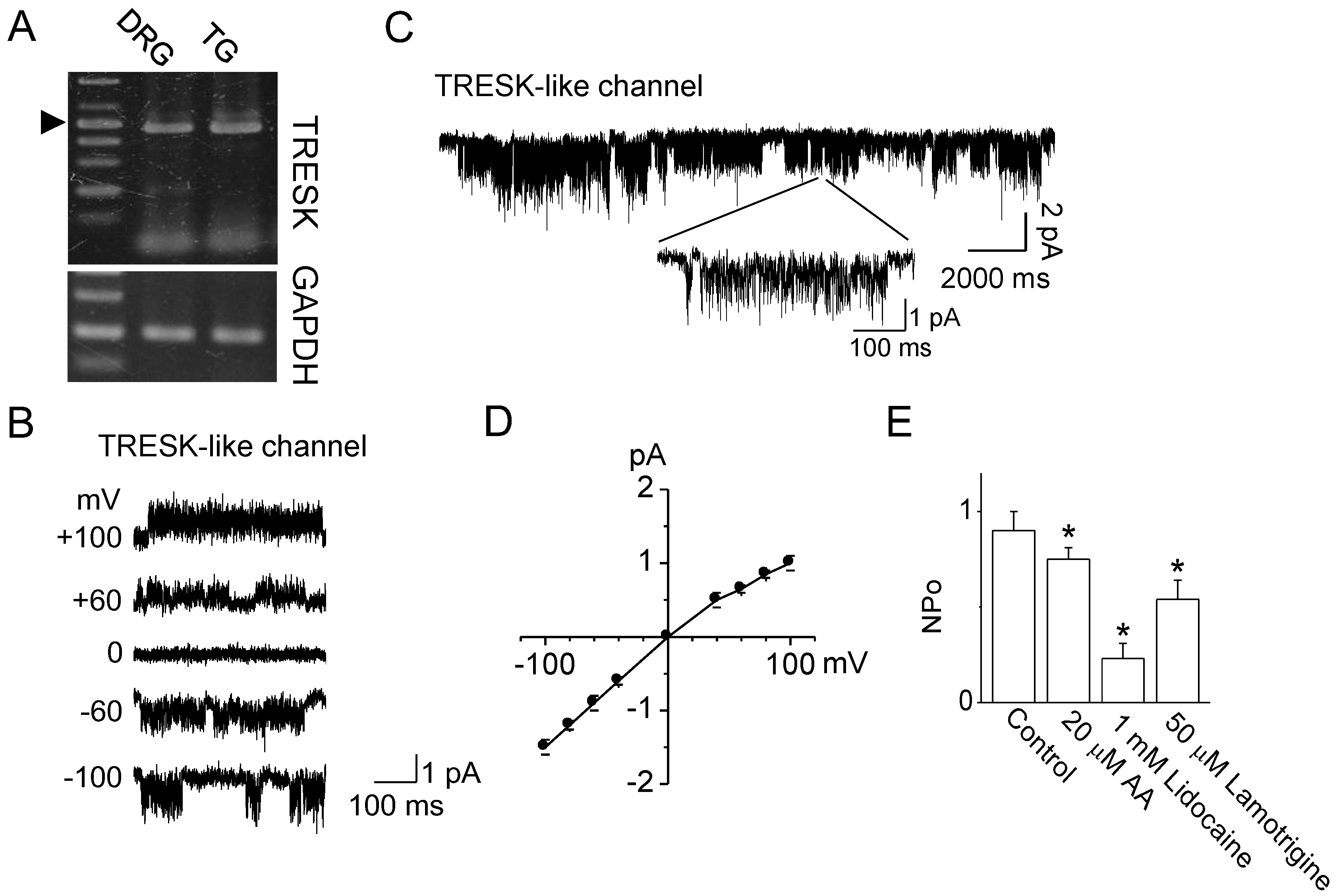
2.2. Verapamil Inhibits TRESK-Like Currents in TG Neurons Independently of the Blockade of Ca2+ Influx
3. Discussion
3.1. Direct Inhibition of TRESK Currents by Verapamil
3.2. Functional Expression of TRESK in TG Neurons
3.3. Physiological Role of TRESK Inhibition by Verapamil
4. Materials and Methods
4.1. Ethical Approval
4.2. Chemicals
4.3. Animal Care
4.4. Culture of TG Neurons
4.5. Transfection
4.6. Electrophysiological Studies
4.7. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.8. Measurement of [Ca2+]i
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DRG | Dorsal root ganglion |
| TG | Trigeminal ganglion neuron |
| TRESK | TWIK-related spinal cord K+ channel |
References
- Czirjak, G.; Toth, Z.E.; Enyedi, P. The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. J. Biol. Chem. 2004, 279, 18550–18558. [Google Scholar] [CrossRef] [PubMed]
- Czirjak, G.; Enyedi, P. Targeting of calcineurin to an NFAT-like docking site is required for the calcium-dependent activation of the background K+ channel, TRESK. J. Biol. Chem. 2006, 281, 14677–14682. [Google Scholar] [CrossRef] [PubMed]
- Czirjak, G.; Enyedi, P. The LQLP calcineurin docking site is a major determinant of the calcium-dependent activation of human TRESK background K+ channel. J. Biol. Chem. 2014, 289, 29506–29518. [Google Scholar] [CrossRef] [PubMed]
- Braun, G.; Nemcsics, B.; Enyedi, P.; Czirjak, G. TRESK background K+ channel is inhibited by PAR-1/MARK microtubule affinity-regulating kinases in Xenopus oocytes. PLoS ONE 2011, 6, e28119. [Google Scholar] [CrossRef] [PubMed]
- Czirjak, G.; Enyedi, P. Zinc and mercuric ions distinguish TRESK from the other two-pore-domain K+ channels. Mol. Pharmacol. 2006, 69, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Bruner, J.K.; Zou, B.; Zhang, H.; Zhang, Y.; Schmidt, K.; Li, M. Identification of novel small molecule modulators of K2P18.1 two-pore potassium channel. Eur. J. Pharmacol. 2014, 740, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Richter, J.A.; Hurley, J.H. Release of glutamate and CGRP from trigeminal ganglion neurons: Role of calcium channels and 5-HT1 receptor signaling. Mol. Pain 2008, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Dussor, G. Ion channels and migraine. Headache 2014, 54, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tsuboi, Y.; Kitagawa, J.; Nakagawa, K.; Iwata, K.; Matsumoto, S. Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol. Pain 2011, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Thalakoti, S.; Patil, V.V.; Damodaram, S.; Vause, C.V.; Langford, L.E.; Freeman, S.E.; Durham, P.L. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache 2007, 47, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; D’Arrigo, A.; Dalle Carbonare, M.; Leon, A. Pathogenesis of migraine: Role of neuromodulators. Headache 2012, 52, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.; McKay, N.G. Modulation of potassium channels as a therapeutic approach. Curr. Pharm. Des. 2006, 12, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Tsantoulas, C.; McMahon, S.B. Opening paths to novel analgesics: The role of potassium channels in chronic pain. Trends Neurosci. 2014, 37, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, Q.; Yan, L.Y.; Wu, W.H.; Liu, S.; Xiao, H.; Wan, Q. Flunarizine inhibits sensory neuron excitability by blocking voltage-gated Na+ and Ca2+ currents in trigeminal ganglion neurons. Chin. Med. J. 2011, 124, 2649–2655. [Google Scholar] [PubMed]
- Waeber, C.; Moskowitz, M.A. Migraine as an inflammatory disorder. Neurology 2005, 64, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Lafreniere, R.G.; Cader, M.Z.; Poulin, J.F.; Andres-Enguix, I.; Simoneau, M.; Gupta, N.; Boisvert, K.; Lafreniere, F.; McLaughlan, S.; Dube, M.P.; et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 2010, 16, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Albury, C.L.; Stuart, S.; Haupt, L.M.; Griffiths, L.R. Ion channelopathies and migraine pathogenesis. Mol. Genet. Genom. 2017, 292, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Cao, Y.Q. Over-expression of TRESK K+ channels reduces the excitability of trigeminal ganglion nociceptors. PLoS ONE 2014, 9, e87029. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, P.; Ren, F.; Cao, Y.Q. Nonmigraine-associated TRESK K+ channel variant C110R does not increase the excitability of trigeminal ganglion neurons. J. Neurophysiol. 2014, 112, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Shi, L.; Zhou, H.; Hou, G.; Cao, T.; Zhao, C. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int. Immunopharmacol. 2015, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Liu, G.Y.; Li, Y.; Yan, W.J.; Wang, Q.; Yang, J.; Lu, D.L.; Ding, D.J.; Lin, D.; Zhou, B. Insights into the importance for designing curcumin-inspired anticancer agents by a prooxidant strategy: The case of diarylpentanoids. Free Radic. Biol. Med. 2015, 85, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Hogan, J.O.; Kim, D. THIK-1 (K2P13.1) is a small-conductance background K+ channel in rat trigeminal ganglion neurons. Pflugers Arch. 2014, 466, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fan, C.; Tian, N. Effects of curcumin on the gene expression profile of L-02 cells. Biomed. Rep. 2015, 3, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebrun, P.; Antoine, M.H.; Ouedraogo, R.; Pirotte, B.; Herchuelz, A.; Cosgrove, K.E.; Kane, C.; Dunne, M.J. Verapamil, a phenylalkylamine Ca2+ channel blocker, inhibits ATP-sensitive K+ channels in insulin-secreting cells from rats. Diabetologia 1997, 40, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- DeCoursey, T.E. Mechanism of K+ channel block by verapamil and related compounds in rat alveolar epithelial cells. J. Gen. Physiol. 1995, 106, 745–779. [Google Scholar] [CrossRef] [PubMed]
- Waldegger, S.; Niemeyer, G.; Morike, K.; Wagner, C.A.; Suessbrich, H.; Busch, A.E.; Lang, F.; Eichelbaum, M. Effect of verapamil enantiomers and metabolites on cardiac K+ channels expressed in Xenopus oocytes. Cell. Physiol. Biochem. 1999, 9, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.; Tachi, M.; Maruyama, Y.; Kazama, I. Suppressive effects of diltiazem and verapamil on delayed rectifier K+-channel currents in murine thymocytes. Pharmacol. Rep. 2015, 67, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Z.; Gong, Q.; Makielski, J.C.; January, C.T. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ. Res. 1999, 84, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.J.; Ma, J.H.; Zhang, P.H.; Wang, X.P.; Zou, A.R.; Tu, D.N. Verapamil blocks HERG channel by the helix residue Y652 and F656 in the S6 transmembrane domain. Acta Pharmacol. Sin. 2007, 28, 959–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opie, L.H. Pharmacological differences between calcium antagonists. Eur. Heart J. 1997, 18, A71–A79. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Jiang, T.; Long, M.; Chen, J.; Ren, D.M.; Wong, P.K.; Chapman, E.; Zhou, B.; Zhang, D.D. A Curcumin Derivative That Inhibits Vinyl Carbamate-Induced Lung Carcinogenesis via Activation of the Nrf2 Protective Response. Antioxid. Redox Signal. 2015, 23, 651–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.; Kim, G.T.; Kim, E.J.; La, J.H.; Lee, J.S.; Lee, E.S.; Park, J.Y.; Hong, S.G.; Han, J. Lamotrigine inhibits TRESK regulated by G-protein coupled receptor agonists. Biochem. Biophys. Res. Commun. 2008, 367, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Manteniotis, S.; Lehmann, R.; Flegel, C.; Vogel, F.; Hofreuter, A.; Schreiner, B.S.; Altmuller, J.; Becker, C.; Schobel, N.; Hatt, H.; et al. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in Trigeminal ganglia. PLoS ONE 2013, 8, e79523. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiao, Z.; Ren, F.; Guo, Z.; Chen, Z.; Zhao, H.; Cao, Y.Q. Functional analysis of a migraine-associated TRESK K+ channel mutation. J. Neurosci. 2013, 33, 12810–12824. [Google Scholar] [CrossRef] [PubMed]
- Callejo, G.; Giblin, J.P.; Gasull, X. Modulation of TRESK background K+ channel by membrane stretch. PLoS ONE 2013, 8, e64471. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Mariash, E.; Kim, D. Functional expression of TRESK-2, a new member of the tandem-pore K+ channel family. J. Biol. Chem. 2004, 279, 28063–28070. [Google Scholar] [CrossRef] [PubMed]
- Thut, P.D.; Wrigley, D.; Gold, M.S. Cold transduction in rat trigeminal ganglia neurons in vitro. Neuroscience 2003, 119, 1071–1083. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hatakeyama, T.; Taniguchi, K. Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells. Neurosci. Lett. 2009, 454, 129–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadaveira-Mosquera, A.; Perez, M.; Reboreda, A.; Rivas-Ramirez, P.; Fernandez-Fernandez, D.; Lamas, J.A. Expression of K2P channels in sensory and motor neurons of the autonomic nervous system. J. Mol. Neurosci. 2012, 48, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kim, D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 2006, 291, C138–C146. [Google Scholar] [CrossRef] [PubMed]
- Dobler, T.; Springauf, A.; Tovornik, S.; Weber, M.; Schmitt, A.; Sedlmeier, R.; Wischmeyer, E.; Doring, F. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J. Physiol. 2007, 585, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Sigal, Y.M.; Milstein, A.D.; Garrison, J.L.; Zorn, J.A.; Tsuruda, P.R.; Nicoll, R.A.; Julius, D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat. Neurosci. 2008, 11, 772–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulleuda, A.; Cokic, B.; Callejo, G.; Saiani, B.; Serra, J.; Gasull, X. TRESK channel contribution to nociceptive sensory neurons excitability: Modulation by nerve injury. Mol. Pain 2011, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Yoon, S.Y.; Hur, C.G.; Yang, H.Y.; Choe, C.; Kim, E.J.; Joo, J.S.; Kang, K.R.; Park, J.Y.; Hong, S.G.; et al. Acetylcholine rescues two-cell block through activation of IP3 receptors and Ca2+/calmodulin-dependent kinase II in an ICR mouse strain. Pflugers Arch. 2009, 458, 1125–1136. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Kim, E.-J.; Ryu, J.H.; Lee, D.K.; Hong, S.-G.; Han, J.; Han, J.; Kang, D. Verapamil Inhibits TRESK (K2P18.1) Current in Trigeminal Ganglion Neurons Independently of the Blockade of Ca2+ Influx. Int. J. Mol. Sci. 2018, 19, 1961. https://doi.org/10.3390/ijms19071961
Park H, Kim E-J, Ryu JH, Lee DK, Hong S-G, Han J, Han J, Kang D. Verapamil Inhibits TRESK (K2P18.1) Current in Trigeminal Ganglion Neurons Independently of the Blockade of Ca2+ Influx. International Journal of Molecular Sciences. 2018; 19(7):1961. https://doi.org/10.3390/ijms19071961
Chicago/Turabian StylePark, Hyun, Eun-Jin Kim, Ji Hyeon Ryu, Dong Kun Lee, Seong-Geun Hong, Jaehee Han, Jongwoo Han, and Dawon Kang. 2018. "Verapamil Inhibits TRESK (K2P18.1) Current in Trigeminal Ganglion Neurons Independently of the Blockade of Ca2+ Influx" International Journal of Molecular Sciences 19, no. 7: 1961. https://doi.org/10.3390/ijms19071961





