Physiological Hypoxia (Physioxia) Impairs the Early Adhesion of Single Lymphoma Cell to Marrow Stromal Cell and Extracellular Matrix. Optical Tweezers Study
Abstract
:1. Introduction
2. Results
2.1. DLBCL Cell Lines Exhibit Differential Proliferative Response to Experimental Physioxia and Hypoxia
2.2. Hypoxia-Inducible Factor 1-Alpha Expression is Altered in Physioxia
2.3. The Influence of Laser Beam on Living Cells
2.4. Single Cell Adhesion in Optical Tweezers
2.4.1. Varying Single Cell Adhesion to MSC and Matrigel among DLBCL Cell Lines
2.4.2. Physioxia Decreased Single Lymphoma Cell Adhesion to Stromal Cell and Matrigel
2.4.3. Physioxia Decreased Primary B-Cell Adhesion to Stromal Cell
2.5. Cell Adhesion for Entire Lymphoma Population Does Not Reflect Results from Single Cell Assay
2.6. Adhesion Changes under Physioxia Are Fully Reversible
2.7. Anti-ITGB1 and Anti-CDH2 Treatment Caused Significant Decrease in Single Lymphoma Adhesion
2.8. Physioxia Impaired Integrin β1, β2 and Cadherin-2 Expression
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Primary Cells
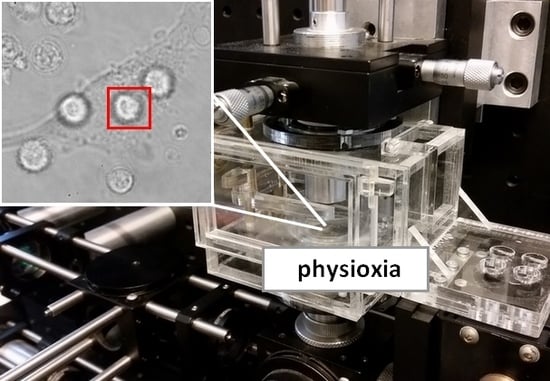
4.2. Optical Tweezers
4.3. Physioxic Conditioning and Reoxidation
4.4. Cell Viability Assay
4.5. The Influence of Laser Beam on Living Cells
4.6. Evaluation of Single Cell Adhesion in Optical Tweezers
4.7. Adhesion to Matrigel
4.8. Cell Attachment Bulky Assays
4.8.1. Lymphoma Adherence to Matrigel
4.8.2. Lymphoma Adherence to Mesenchymal Stromal Cells
4.9. Inhibition of Cell Adhesion by Antibodies Targeting Cellular Adhesion Molelecules
4.10. Integrin Profile of Lymphoma Cells. Immunofluorescence using Confocal Microscopy
4.11. Immunocytochemical Analysis of Hypoxia Inducible Factor Alpha
4.12. Western Blot Analysis of HIF1α
4.13. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| BS | Beamsplitter |
| BSA | Bovine serum albumin |
| CAMs | Cellular adhesion molecules |
| CDH2 | Cadherin-2 |
| DLBCL | Diffuse large B-cell lymphoma |
| DM | Dichroic mirror |
| DSMZ | German Collection of Microorganisms and Cell Cultures |
| EMC | Extracellular matrix |
| FBS | Fetal bovine serum |
| GMXY | Galvano-mirror XY scanning system |
| HIF1α | Hypoxia-inducible factor 1-alpha |
| ITGA1 | Integrin alpha-1 |
| ITGB1 | Integrin beta-1 |
| ITGB2 | Integrin beta-2 |
| ITGB7 | Integrin beta-7 |
| L | Lens |
| M | Mirror |
| MSC | Mesenchymal stromal cell |
| NA | Numerical aperture |
| OA | Optical absorbance |
| OCC | Oxygen controlling chamber |
| OT | Optical tweezers |
| PBS | Phosphate buffered saline |
| S | Spectrophotometer |
| SLM | Spatial light modulator |
References
- Shankland, K.R.; Armitage, J.O.; Hancock, B.W. Non-Hodgkin lymphoma. Lancet 2012, 380, 848–857. [Google Scholar] [CrossRef]
- Yao, Z.; Deng, L.; Xu-Monette, Z.Y.; Manyam, G.C.; Jain, P.; Tzankov, A.; Visco, C.; Bhagat, G.; Wang, J.; Dybkaer, K. Concordant bone marrow involvement of diffuse large B-cell lymphoma represents a distinct clinical and biological entity in the era of immunotherapy. Leukemia 2018, 32, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.J.; Goodell, L.; Glod, J.; Gélinas, C.; Rabson, A.B.; Strair, R.K. Mesenchymal stromal cells protect mantle cell lymphoma cells from spontaneous and drug-induced apoptosis through secretion of B-cell activating factor and activation of the canonical and non-canonical nulear factor κB pathways. Hematologica 2012, 97, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Lwin, T.; Hazlehurst, L.A.; Li, Z.; Dessureault, S.; Sotomayor, E.; Moscinski, L.C.; Dalton, W.S.; Tao, J. Bone marrow stromal cells prevent apoptosis of lymphoma cells by upregulation of anti-apoptotic proteins associated with activation of NF-kappaB (RelB/p52) in non-Hodgkin’s lymphoma cells. Leukemia 2007, 21, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Rudelius, M.; Rosenfeldt, M.Y.; Leich, E.; Rauert-Wunderlich, H.; Solimando, A.G.; Beilhack, A.; Ott, G.; Rosenwald, A. Inhibition of focal adhesion kinase overcomes resistance of mantle cell lymphoma to ibrutinib in the bone marrow microenvironment. Haematologica 2018, 103, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Kurtova, A.V.; Tamayo, A.T.; Ford, R.J.; Burger, J.A. Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): Importance for interactions with the stromal microenvironment and specific targeting. Blood 2009, 113, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, N.S.; Apollonio, B.; Ramsay, A.G. Tumor microenvironment (TME)-driven immune suppression in B cell malignancy. Biochim. Biophys. Acta 2016, 1863, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Blonska, M.; Zhu, Y.; Chuang, H.H.; You, M.J.; Kunkalla, K.; Vega, F.; Lin, X. Jun-regulated genes promote interaction of diffuse large B-cell lymphoma with the microenvironment. Blood 2015, 125, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Brahimi-Horn, M.C.; Pouyssegur, J. Oxygen, a source of life and stress. FEBS Lett. 2007, 581, 3582–3591. [Google Scholar] [CrossRef] [PubMed]
- Giaccia, A.J.; Simon, M.C.; Johnson, R. The biology of hypoxia: The role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004, 18, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress in cell culture: An under-appreciated problem? FEBS Lett. 2003, 540, 3–6. [Google Scholar] [CrossRef]
- Evens, A.M.; Schumacker, P.T.; Helenowski, I.B.; Singh, A.T.; Dokic, D.; Keswani, A.; Kordeluk, E.; Raji, A.; Winter, J.N.; Jovanovic, B.D. Hypoxia inducible factor-alpha activation in lymphoma and relationship to the thioredoxin family. Br. J. Haematol. 2008, 141, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Cosme, J.; Guo, H.; Hadipour-Lakmehsari, S.; Emili, A.; Gramolini, A.O. Hypoxia-induced changes in the fibroblast secretome, nexosome, and whole-cell proteome using cultured, cardiac-derived cells isolated from neonatal mice. J. Proteome Res. 2017, 16, 2836–2847. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, K.; Jaber, S.; Nahid, M.N.; Underwood, K.; Beheshti, A.; Landon, A.; Bhandary, B.; Bastain, P.; Evens, A.M.; Haley, J. Role of hypoxia in Diffuse Large B-cell Lymphoma: Metabolic repression and selective translation of HK2 facilitates development of DLBCL. Sci. Rep. 2018, 8, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, L.H.; Withers, H.G.; Garbens, A.; Love, H.D.; Magnoni, L.; Hayward, S.W.; Moyes, C.D. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochim. Biophys. Acta 2009, 1787, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Ali, M.; McCallum, G.P.; Kumareswaran, R.; Koritzinsky, M.; Wouters, B.G.; Wells, P.G.; Gallinger, S.; Bristow, R.G. Hypoxia provokes base excision repair changes and a repair-deficient, mutator phenotype in colorectal cancer cells. Mol. Cancer Res. 2014, 12, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, E.R.; Udartseva, O.O.; Zhidkova, O.V.; Buravkov, S.V.; Ezdakova, M.I.; Buravkova, L.B. IFN-gamma priming of adipose-derived stromal cells at “physiological” hypoxia. J. Cell. Physiol. 2018, 233, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.K. Optical tweezers for single cells. J. R. Soc. Interface 2008, 5, 671–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, X.; Yang, H.; Fahmy, T.; Wang, Y.; Sun, D. Direct measurement of cell protrusion force utilizing a robot-aided cell manipulation system with optical tweezers for cell migration control. Int. J. Robot. Res. 2014, 33, 1782–1792. [Google Scholar] [CrossRef]
- Yang, H.; Gou, X.; Wang, Y.; Fahmy, T.M.; Leung, A.Y.H.; Lu, J.; Sun, D. Dynamic Model of Chemoattractant-Induced Cell Migration. Biophys. J. 2015, 108, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Schwingel, M.; Bastmeyer, M. Force Mapping during the Formation and Maturation of Cell Adhesion Sites with Multiple Optical Tweezers. PLoS ONE 2013, 8, e54850. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Madgavkar, A.; Stjerndahl, M.; Wu, Y.; Tan, W.; Duran, R.; Niehren, S.; Mustafa, K.; Arvidson, K.; Wennerberg, A. Using optical tweezers for measuring the interaction forces between human bone cells and implant surfaces: System design and force calibration. Rev. Sci. Instrum. 2007, 78, 074302. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Gou, X.; Han, H.; Leung, A.Y.; Sun, D. Manipulating cell adhesions with optical tweezers for study of cell-to-cell interactions. J. Biomed. Nanotechnol. 2013, 9, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Bambardekar, K.; Clément, R.; Blanc, O.; Chardès, C.; Lenne, P.F. Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 1416–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkham, G.R.; Britchford, E.; Upton, T.; Ware, J.; Gibson, G.M.; Devaud, Y.; Ehrbar, M.; Padgett, M.; Allen, S.; Buttery, L.D.; et al. Precision assembly of complex cellular microenvironments using holographic optical tweezers. Sci. Rep. 2015, 5, 8577. [Google Scholar] [CrossRef] [PubMed]
- Drobczyński, S.; Prorok, K.; Tamarov, K.; Duś-Szachniewicz, K.; Lehto, V.-P.; Bednarkiewicz, A. Towards controlled photothermal treatment of single cell: Optically induced heating and remote temperature monitoring in-vitro through double wavelength optical tweezers. ACS Photonics 2017, 4, 1993–2002. [Google Scholar] [CrossRef]
- Drobczyński, S.; Duś-Szachniewicz, K. Real-time force measurement in double wavelength optical tweezers. J. Opt. Soc. Am. B 2017, 34, 38–43. [Google Scholar] [CrossRef]
- Chan, B.P.; Reichert, W.M.; Truskey, G.A. Effect of streptavidin–biotin on endothelial vasoregulation and leukocyte adhesion. Biomaterials 2004, 25, 3951–3961. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.Q.; Zhang, J.; Feng, C. Biotin-Avidin Based Universal Cell-Matrix Interaction for Promoting Three-Dimensional Cell Adhesion. ACS Appl. Mater. Interfaces 2015, 7, 20786–20792. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Reuss-Borst, M.A.; Klein, G.; Waller, H.D.; Müller, C.A. Differential expression of adhesion molecules in acute leukemia. Leukemia 1995, 9, 869–874. [Google Scholar] [PubMed]
- Shishido, S.; Bönig, H.; Kim, Y.M. Role of integrin alpha4 in drug resistance of leukemia. Front. Oncol. 2014, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Prosper, F.; Stroncek, D.; McCarthy, J.B.; Verfaillie, C.M. Mobilization and homing of peripheral blood progenitors is related to reversible downregulation of α4 β1 integrin expression and function. J. Clin. Investig. 1998, 101, 2456–2467. [Google Scholar] [CrossRef] [PubMed]
- Neri, P.; Ren, L.; Azab, A.K.; Brentnall, M.; Gratton, K.; Klimowicz, A.C.; Lin, C.; Duggan, P.; Tassone, P.; Mansoor, A. Integrin β7-mediated regulation of multiple myeloma cell adhesion, migration, and invasion. Blood 2011, 117, 6202–6213. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.A.; Godet, I.; Ye, I.C.; Byun, J.; Jayatilaka, H.; Lee, S.J.; Xiang, L.; Samanta, D.; Lee, M.H.; Wu, P.H. Hypoxia Selectively Enhances Integrin α5β1 Receptor Expression in Breast Cancer to Promote Metastasis. Mol. Cancer Res. 2017, 15, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.L.; Schwab, L.P.; Krutilina, R.; Parke, D.N.; Sethuraman, A.; Hoogewijs, D.; Schörg, A.; Gotwald, L.; Fan, M.; Wenger, R.H.; et al. ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol. Cancer 2016, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Carrera, S.; Senra, J.; Acosta, M.I.; Althubiti, M.; Hammond, E.M.; de Verdier, P.J.; Macip, S. The role of the HIF-1α transcription factor in increased cell division at physiological oxygen tensions. PLoS ONE 2014, 9, e97938. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kiedaa, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattei, G.; Giusti, S.; Ahluwalia, A. Design Criteria for Generating Physiologically Relevant In Vitro Models in Bioreactors. Processes 2014, 2, 548–569. [Google Scholar] [CrossRef] [Green Version]
- Packer, L.; Fuehr, K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature 1977, 267, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Guillaume, L.; Grimes, D.R.; Fehrenbach, J.; Lobjois, V.; Ducommun, B. Oxygen Partial Pressure Is a Rate-Limiting Parameter for Cell Proliferation. PLoS ONE 2016, 11, e0161239. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.D.; Schirmer, K.; Münst, B.; Heinz, S.; Ghafoory, S.; Wölfl, S.; Simon-Keller, K.; Marx, A.; Øie, C.; Ebert, M.P.; et al. In Vitro Generation of Functional Liver Organoid-Like Structures Using Adult Human Cells. PLoS ONE 2015, 10, e0139345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mignon, C.; Uzunbajakava, N.E.; Raafs, B.; Botchkareva, N.V.; Tobin, D.J. Photobiomodulation of human dermal fibroblasts in vitro: Decisive role of cell culture conditions and treatment protocols on experimental outcome. Sci. Rep. 2017, 7, 2797. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Markway, B.D.; Bond, D.; McCarthy, H.E.; Johnstone, B. Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity. Stem. Cell Res. Ther. 2016, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Timpano, S.; Uniacke, J. Human Cells Cultured under Physiological Oxygen Utilize Two Cap-binding Proteins to recruit Distinct mRNAs for Translation. J. Biol. Chem. 2016, 291, 10772–10782. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, Z.; Loughna, P.T. Oxygen concentration modulates the differentiation of muscle stem cells toward myogenic and adipogenic fates. Differentiation 2012, 84, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Han, H.C.; Hu, S.; Leung, A.Y.; Sun, D. Applying combined optical tweezers and fluorescence microscopy technologies to manipulate cell adhesions for cell-to-cell interaction study. IEEE Trans. Biomed. Eng. 2013, 60, 2308–2315. [Google Scholar] [PubMed]
- Zhou, Z.L.; Ma, J.; Tong, M.H.; Chan, B.P.; Wong, A.S.; Ngan, A.H. Nanomechanical measurement of adhesion and migration of leukemia cells with phorbol 12-myristate 13-acetate treatment. Int. J. Nanomed. 2016, 11, 6533–6545. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.G.; McAlinden, N.; Millington, O.R.; Wright, A.J. A minimally invasive optical trapping system to understand cellular interactions at onset of an immune response. PLoS ONE 2017, 12, e0188581. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Ghobrial, I.M.; Azab, A.K. Hypoxia promotes dissemination and colonization in new bone marrow niches in Waldenström macroglobulinemia. Mol. Cancer Res. 2015, 13, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Fitzpatrick, T.E.; Graham, C.H. Effect of hypoxia on cellular adhesion to vitronectin and fibronectin. Biochem. Biophys. Res. Commun. 2001, 287, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.M.; Adams, G.E.; Joiner, M.C.; Marshall, J.F.; Hart, I.R. Hypoxia facilitates tumour cell detachment by reducing expression of surface adhesion molecules and adhesion to extracellular matrices without loss of cell viability. Br. J. Cancer 1998, 77, 1799–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, R.; Friedrich, D.; Chavakis, E.; Böhm, M.; Friedrich, E.B. Effect of hypoxia on integrin-mediated adhesion of endothelial progenitor cells. J. Cell. Mol. Med. 2012, 16, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.W.; Lin, S.C.; Lai, Y.Y.; Lin, B.W.; Lin, S.C.; Lee, J.C.; Tsai, S.J. Regulation of CD151 by hypoxia controls cell adhesion and metastasis in colorectal cancer. Clin. Cancer Res. 2008, 14, 8043–8051. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.; Alabiso, O.; Galetto, A.S.; Baldanzi, G. Integrins in T Cell Physiology. Int. J. Mol. Sci. 2018, 19, 485. [Google Scholar] [CrossRef] [PubMed]
- Huveneers, S.; Truong, H.; Fässler, R.; Sonnenberg, A.; Danen, E.H.J. Binding of soluble fibronectin to integrin α5β1- link to focal adhesion redistribution and contractile shape. J. Cell Sci. 2008, 121, 2452–2462. [Google Scholar] [CrossRef] [PubMed]
- Cowden Dahl, K.D.; Robertson, S.E.; Weaver, V.M.; Simon, M.C. Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Mol. Biol. Cell 2005, 16, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Caaveiro, J.M.; Nagatoishi, S.; Miyafusa, T.; Matsuura, T.; Sudou, Y.; Tsumoto, K. Disruption of cell adhesion by an antibody targeting the cell-adhesive intermediate (X-dimer) of human P-cadherin. Sci. Rep. 2017, 7, 39518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallo, S.; Rutz, J.; Kautsch, M.; Winkelmann, R.; Michaelis, M.; Wezel, F.; Bartsch, G.; Haferkamp, A.; Rothweiler, F.; Blaheta, R.A. Blocking integrin β1 decreases adhesion in chemoresistant urothelial cancer cell lines. Oncol. Lett. 2017, 14, 5513–5518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, Y.; Ni, H.; Wilkins, J.A. The selective inhibition of beta 1 and beta 7 integrin-mediated lymphocyte adhesion by bacitracin. J. Immunol. 1998, 161, 6323–6329. [Google Scholar] [PubMed]
- Fraczkowska, K.; Bacia, M.; Przybyło, M.; Drabik, D.; Kaczorowska, A.; Rybka, J.; Stefanko, E.; Drobczynski, S.; Masajada, J.; Podbielska, H. Alterations of biomechanics in cancer and normal cells induced by doxorubicin. Biomed. Pharmacother. 2018, 97, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Cell Line (Supplier) | Type | Time-Dependent Adhesion to MSCs [s] ± SD | N | Time-Dependent Adhesion to Matrigel [s] ± SD | N | Adhesion to Matrigel vs. MSC Ratio | Adhesion Properties in OT |
|---|---|---|---|---|---|---|---|
| Ri-1 (DSMZ) | ABC | 15.5 ± 8.4 | 81 | 67.5 ± 25.5 | 75 | 4.4 | high/medium |
| U-2904 (DSMZ) | ABC | 103.5 ± 34.2 | 77 | 291.2 ± 41.8 | 75 | 2.8 | low |
| Pfeiffer (ATCC) | GCB | 55.9 ± 19.4 | 80 | 89.9 ± 26.9 | 73 | 1.6 | medium |
| Toledo (ATCC) | GCB | 132.9 ± 48.8 | 81 | 223.6 ± 58.7 | 73 | 1.7 | low |
| U-2932 (DSMZ) | GCB | 34.6 ± 11.6 | 79 | 50.7 ± 16.8 | 74 | 1.5 | high |
| SUDHL-10 (DSMZ) | GCB | 19.7 ± 7.5 | 81 | 43.8 ± 14.1 | 79 | 2.2 | high |
| Fold Change | ||
|---|---|---|
| Cell Line | Physioxia vs. Normoxia to MSCs | Physioxia vs. Normoxia to Matrigel |
| Ri-1 | 5.1 | 3.2 |
| U2904 | 2.1 | 1.1 |
| Pfeiffer | 1.7 | 2.5 |
| Toledo | 2.1 | 1.6 |
| U2932 | 3.3 | 2.8 |
| SUDHL-10 | 4.0 | 2.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duś-Szachniewicz, K.; Drobczyński, S.; Ziółkowski, P.; Kołodziej, P.; Walaszek, K.M.; Korzeniewska, A.K.; Agrawal, A.; Kupczyk, P.; Woźniak, M. Physiological Hypoxia (Physioxia) Impairs the Early Adhesion of Single Lymphoma Cell to Marrow Stromal Cell and Extracellular Matrix. Optical Tweezers Study. Int. J. Mol. Sci. 2018, 19, 1880. https://doi.org/10.3390/ijms19071880
Duś-Szachniewicz K, Drobczyński S, Ziółkowski P, Kołodziej P, Walaszek KM, Korzeniewska AK, Agrawal A, Kupczyk P, Woźniak M. Physiological Hypoxia (Physioxia) Impairs the Early Adhesion of Single Lymphoma Cell to Marrow Stromal Cell and Extracellular Matrix. Optical Tweezers Study. International Journal of Molecular Sciences. 2018; 19(7):1880. https://doi.org/10.3390/ijms19071880
Chicago/Turabian StyleDuś-Szachniewicz, Kamila, Sławomir Drobczyński, Piotr Ziółkowski, Paweł Kołodziej, Kinga M. Walaszek, Aleksandra K. Korzeniewska, Anil Agrawal, Piotr Kupczyk, and Marta Woźniak. 2018. "Physiological Hypoxia (Physioxia) Impairs the Early Adhesion of Single Lymphoma Cell to Marrow Stromal Cell and Extracellular Matrix. Optical Tweezers Study" International Journal of Molecular Sciences 19, no. 7: 1880. https://doi.org/10.3390/ijms19071880
APA StyleDuś-Szachniewicz, K., Drobczyński, S., Ziółkowski, P., Kołodziej, P., Walaszek, K. M., Korzeniewska, A. K., Agrawal, A., Kupczyk, P., & Woźniak, M. (2018). Physiological Hypoxia (Physioxia) Impairs the Early Adhesion of Single Lymphoma Cell to Marrow Stromal Cell and Extracellular Matrix. Optical Tweezers Study. International Journal of Molecular Sciences, 19(7), 1880. https://doi.org/10.3390/ijms19071880