Generation of Human Breg-Like Phenotype with Regulatory Function In Vitro with Bacteria-Derived Oligodeoxynucleotides
Abstract
:1. Introduction
2. Results
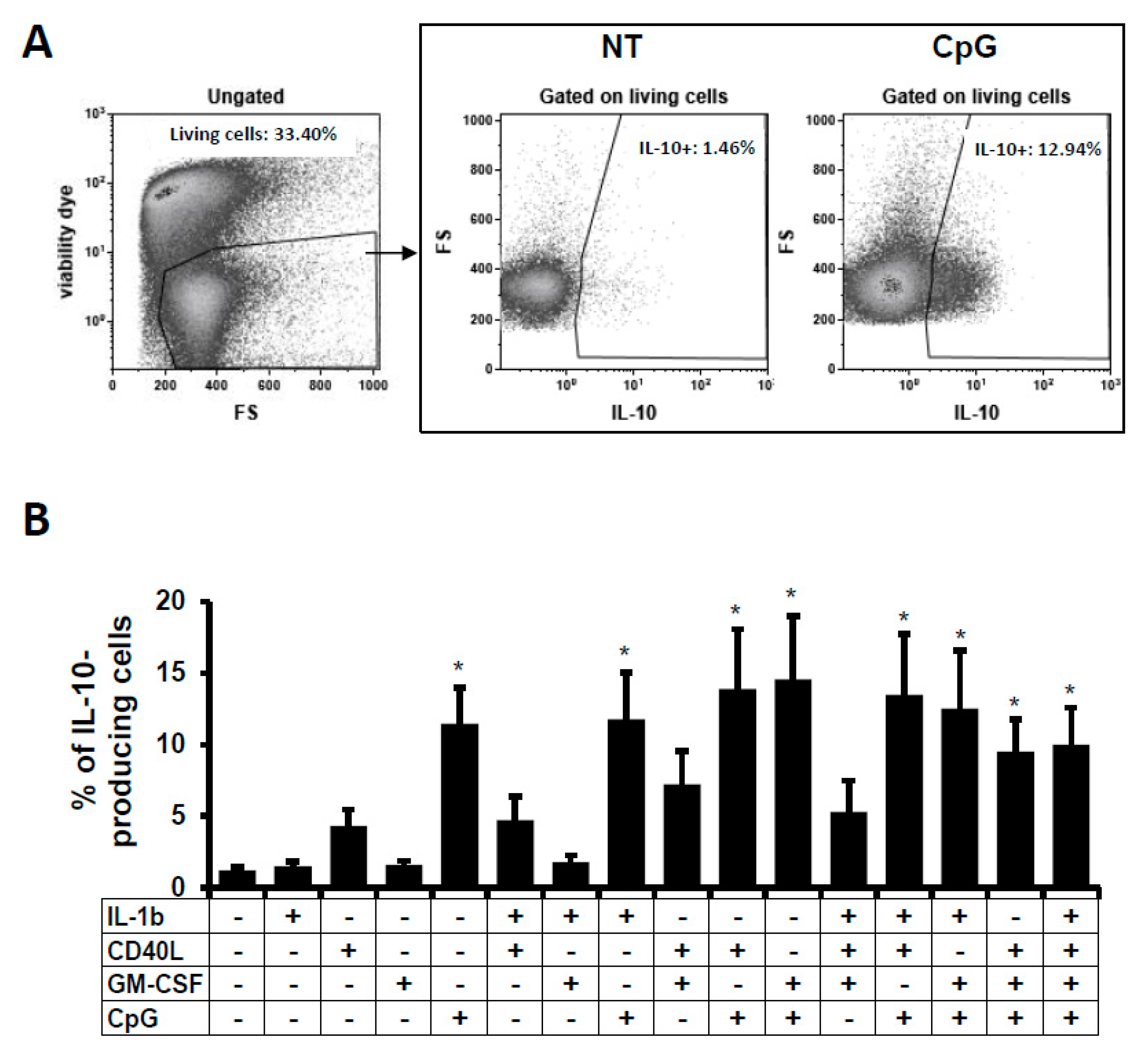
2.1. IL-10-Producing B Cells In Vitro
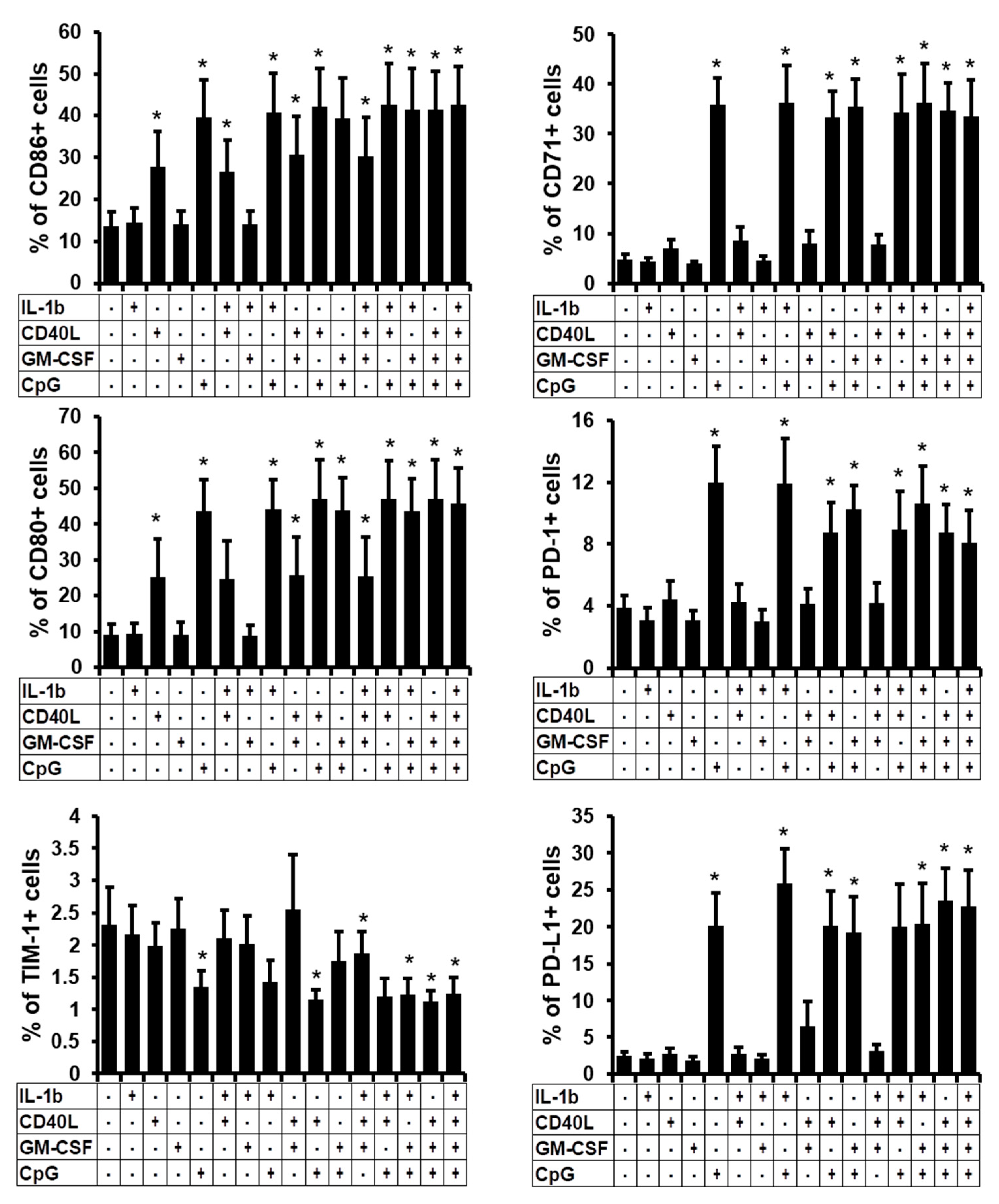
2.2. Phenotype of the B Cells Stimulated In Vitro
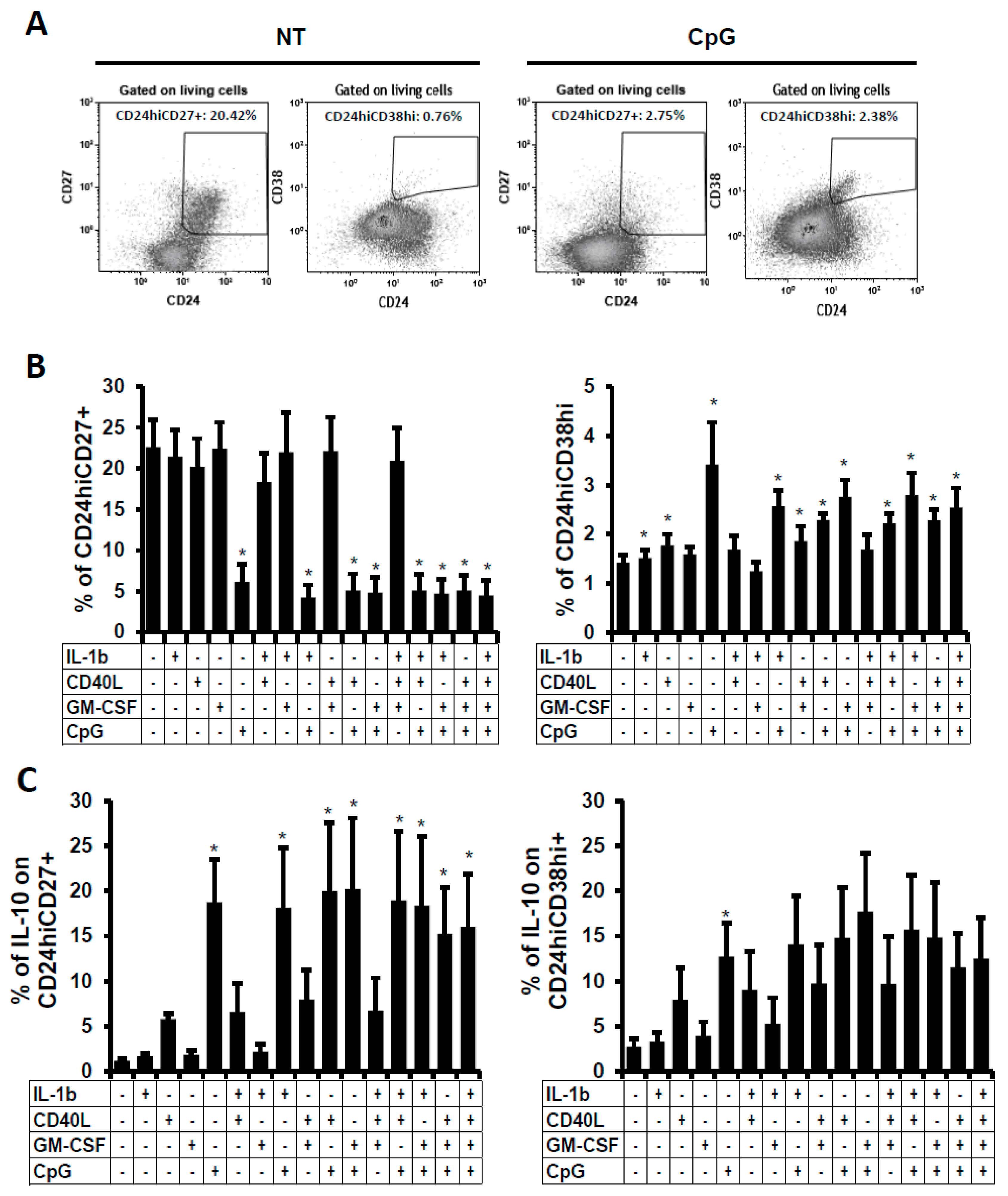
2.3. Phenotype Characterisation of the B Cells Stimulated In Vitro
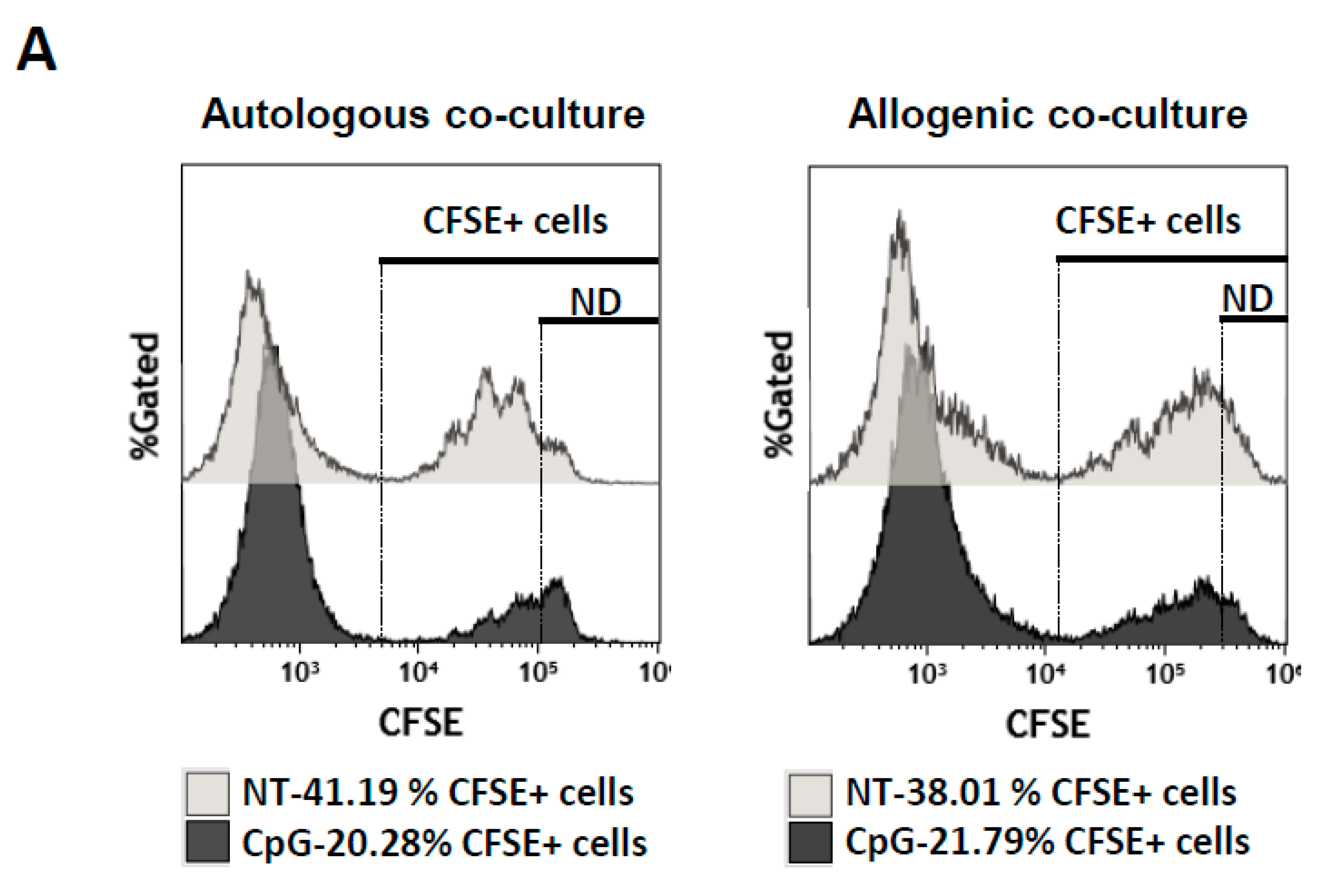
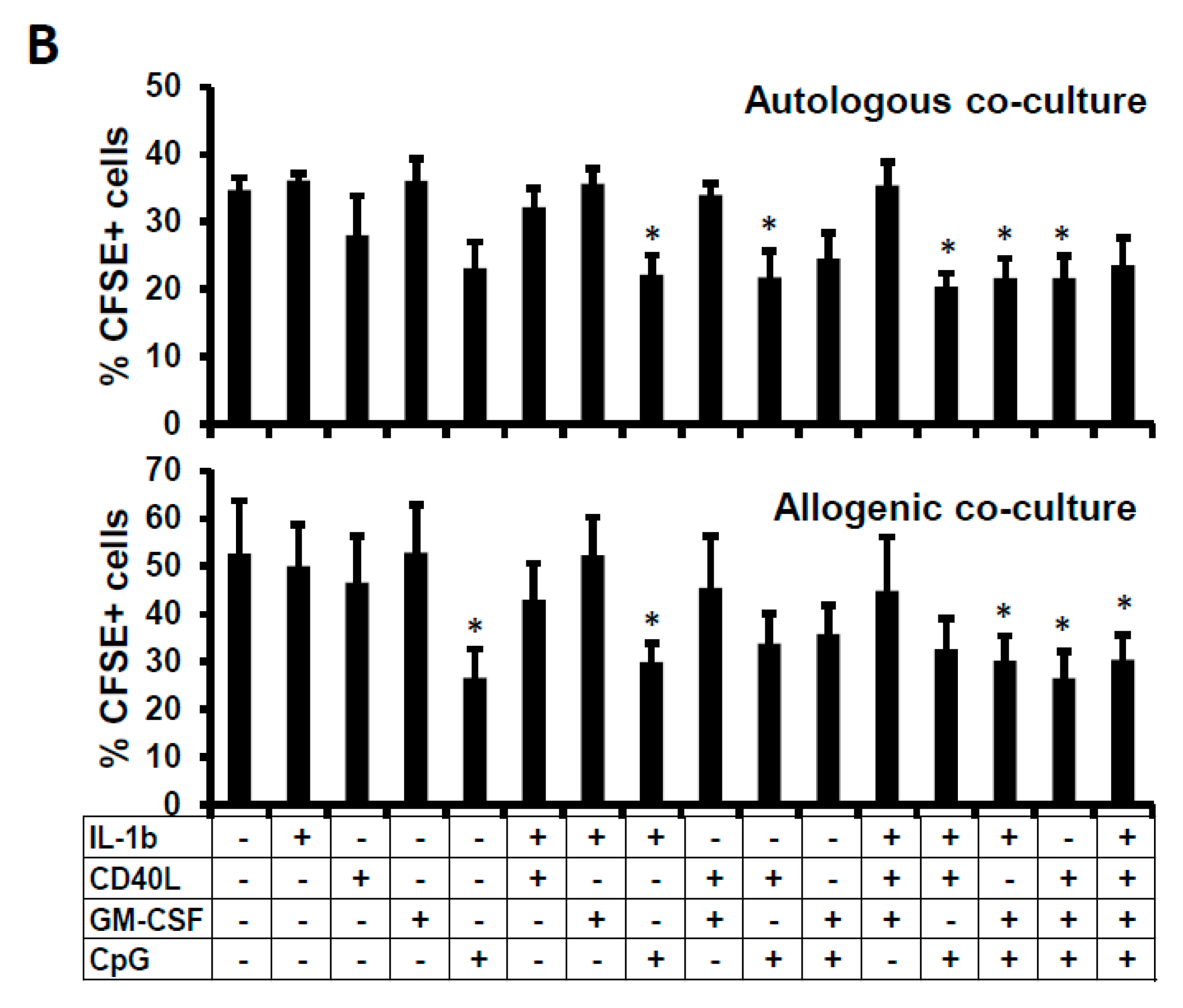
2.4. Suppressive Function of the B Cells Stimulated In Vitro
3. Discussion
4. Materials and Methods
4.1. Isolation of B Cells from PBMCs
4.2. Culture and Treatment of B Cells
4.3. Flow Cytometry for Determination of B-Cell Phenotype
4.4. Proliferation Assay
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.; Rudensky, A.Y. Control of inflammation by integration of environmental cues by regulatory T cells. J. Clin. Investig. 2013, 123, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkura, N.; Kitagawa, Y.; Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 2013, 38, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Montfort, A.; Capasso, M. B regulatory cells in cancer. Trends Immunol. 2013, 34, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, J.M.; Jamin, C.; Amrouche, K.; le Goff, B.; Maugars, Y.; Youinou, P. Regulatory B cells play a key role in immune system balance. Joint Bone Spine 2013, 80, 18–22. [Google Scholar] [CrossRef]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Sarvaria, A.; Basar, R.; Mehta, R.S.; Shaim, H.; Muftuoglu, M.; Khoder, A.; Sekine, T.; Gokdemir, E.; Kondo, K.; Marin, D.; et al. IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood 2016, 128, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Moreau, A.; Blair, P.A.; Chai, J.G.; Ratnasothy, K.; Stolarczyk, E.; Alhabbab, R.; Rackham, C.L.; Jones, P.M.; Smyth, L.; Elgueta, R.; et al. Transitional-2 B cells acquire regulatory function during tolerance induction and contribute to allograft survival. Eur. J. Immunol. 2015, 45, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Matsushita, T.; Horikawa, M.; Dilillo, D.J.; Yanaba, K.; Venturi, G.M.; Szabolcs, P.M.; Bernstein, S.H.; Magro, C.M.; Williams, A.D.; et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011, 117, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zheng, X.; Zhang, J.; Zhu, Y.; Zhu, X.; Liu, H.; Zeng, M.; Graner, M.W.; Zhou, B.; Chen, X. CD19+CD1d+CD5+ B cell frequencies are increased in patients with tuberculosis and suppress Th17 responses. Cell. Immunol. 2012, 274, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Van de Veen, W.; Stanic, B.; Yaman, G.; Wawrzyniak, M.; Sollner, S.; Akdis, D.G.; Ruckert, B.; Akdis, C.A.; Akdis, M. IgG4 production is confined to human IL-10–producing regulatory B cells that suppress antigen-specific immune responses. J. Allergy Clin. Immunol. 2013, 131, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Floudas, A.; Amu, S.; Fallon, P.G. New insights into IL-10 dependent and IL-10 independent mechanisms of regulatory B cell immune suppression. J Clin. Immunol. 2016, 36 (Suppl. 1), 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Wang, L.; Dittel, B.N. IL-10-independent regulatory B-cell subsets and mechanisms of action. Int. Immunol. 2015, 27, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Menon, M. The expanding family of regulatory B cells. Int. Immunol. 2015, 27, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosser, E.C.; Oleinika, K.; Tonon, S.; Doyle, R.; Bosma, A.; Carter, N.A.; Harris, K.A.; Jones, S.A.; Klein, N.; Mauri, C. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat. Med. 2014, 20, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Yu, C.R.; Dambuza, I.M.; Mahdi, R.M.; Dolinska, M.B.; Sergeev, Y.V.; Wingfield, P.T.; Kim, S.H.; Egwuagu, C.E. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 2014, 20, 633–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauri, C.; Menon, M. Human regulatory B cells in health and disease: Therapeutic potential. J. Clin. Investig. 2017, 127, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.R.; Quan, S.; Soliven, B. CD1dhiCD5+ B cells expanded by GM-CSF in vivo suppress experimental autoimmune myasthenia gravis. J. Immunol. 2014, 193, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Aravena, O.; Ferrier, A.; Menon, M.; Mauri, C.; Aguillon, J.C.; Soto, L.; Catalan, D. Tim-1 defines a human regulatory B cell population that is altered in frequency and function in systemic sclerosis patients. Arthritis Res. Ther. 2017, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M. Interpretation of cellular proliferation data: Avoid the panglossian. Cytometry A 2011, 79, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Abente, J.; Prieto-Sanchez, A.; Munoz-Fernandez, M.A.; Correa-Rocha, R.; Pion, M. Human immunodeficiency virus type-1 induces a regulatory B cell-like phenotype in vitro. Cell. Mol. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Hams, E.; Floudas, A.; Sparwasser, T.; Weaver, C.T.; Fallon, P.G. PD-L1hi B cells are critical regulators of humoral immunity. Nat. Commun. 2015, 6, 5997. [Google Scholar] [CrossRef] [PubMed]
- Banko, Z.; Pozsgay, J.; Szili, D.; Toth, M.; Gati, T.; Nagy, G.; Rojkovich, B.; Sarmay, G. Induction and differentiation of IL-10-producing regulatory b cells from healthy blood donors and rheumatoid arthritis patients. J. Immunol. 2017, 198, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Poe, J.C.; Smith, S.H.; Haas, K.M.; Yanaba, K.; Tsubata, T.; Matsushita, T.; Tedder, T.F. Amplified B lymphocyte CD40 signaling drives regulatory B10 cell expansion in mice. PLoS ONE 2011, 6, e22464. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego-Valle, J.; Pérez-Fernández, V.A.; Correa-Rocha, R.; Pion, M. Generation of Human Breg-Like Phenotype with Regulatory Function In Vitro with Bacteria-Derived Oligodeoxynucleotides. Int. J. Mol. Sci. 2018, 19, 1737. https://doi.org/10.3390/ijms19061737
Gallego-Valle J, Pérez-Fernández VA, Correa-Rocha R, Pion M. Generation of Human Breg-Like Phenotype with Regulatory Function In Vitro with Bacteria-Derived Oligodeoxynucleotides. International Journal of Molecular Sciences. 2018; 19(6):1737. https://doi.org/10.3390/ijms19061737
Chicago/Turabian StyleGallego-Valle, Jorge, Verónica Astrid Pérez-Fernández, Rafael Correa-Rocha, and Marjorie Pion. 2018. "Generation of Human Breg-Like Phenotype with Regulatory Function In Vitro with Bacteria-Derived Oligodeoxynucleotides" International Journal of Molecular Sciences 19, no. 6: 1737. https://doi.org/10.3390/ijms19061737



