Inhibition and Inactivation of Uropathogenic Escherichia coli Biofilms on Urinary Catheters by Sodium Selenite
Abstract
:1. Introduction
2. Results and Discussion
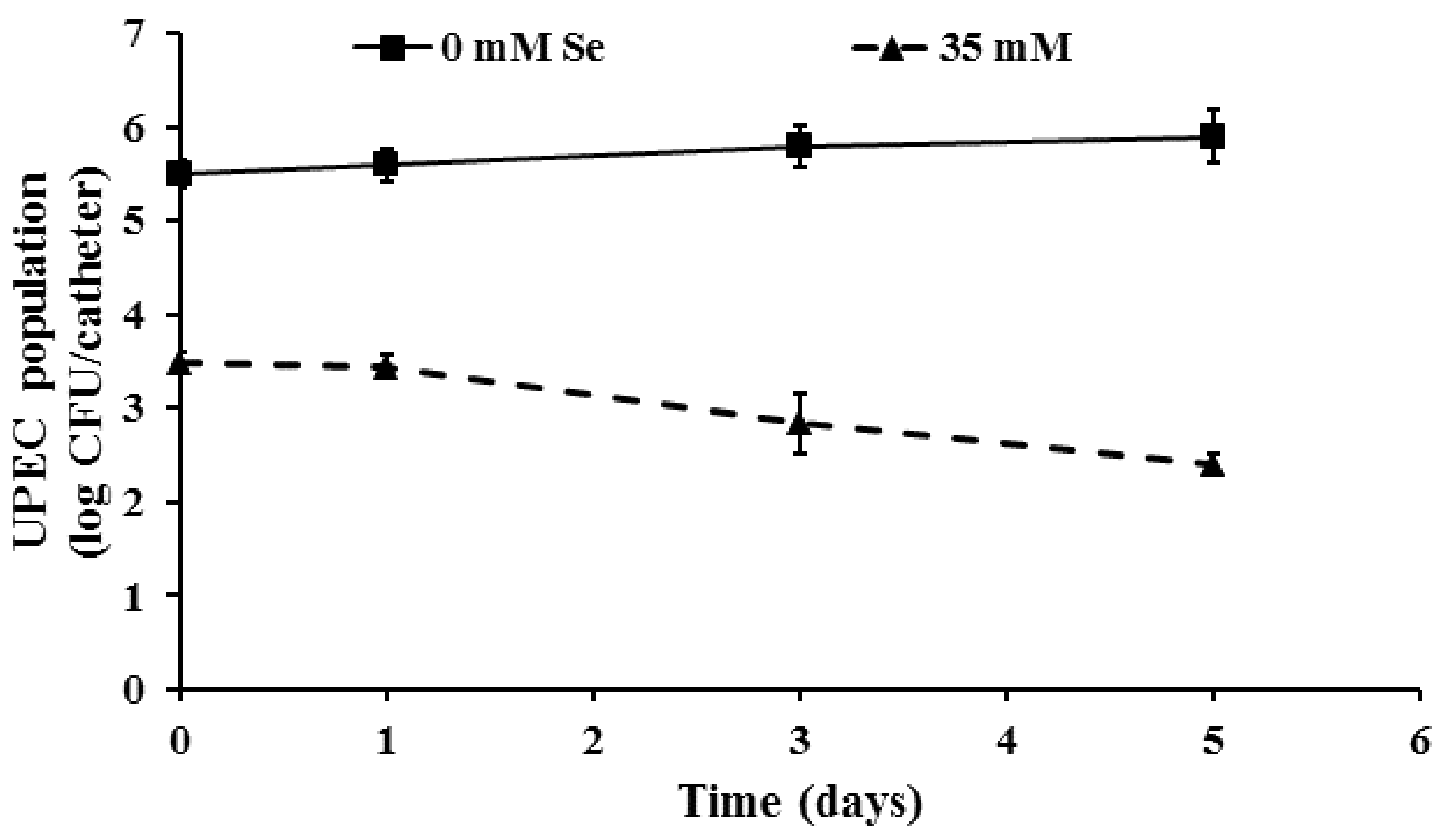
2.1. Inhibition of UPEC Biofilm Formation on Urinary Catheters by Selenium
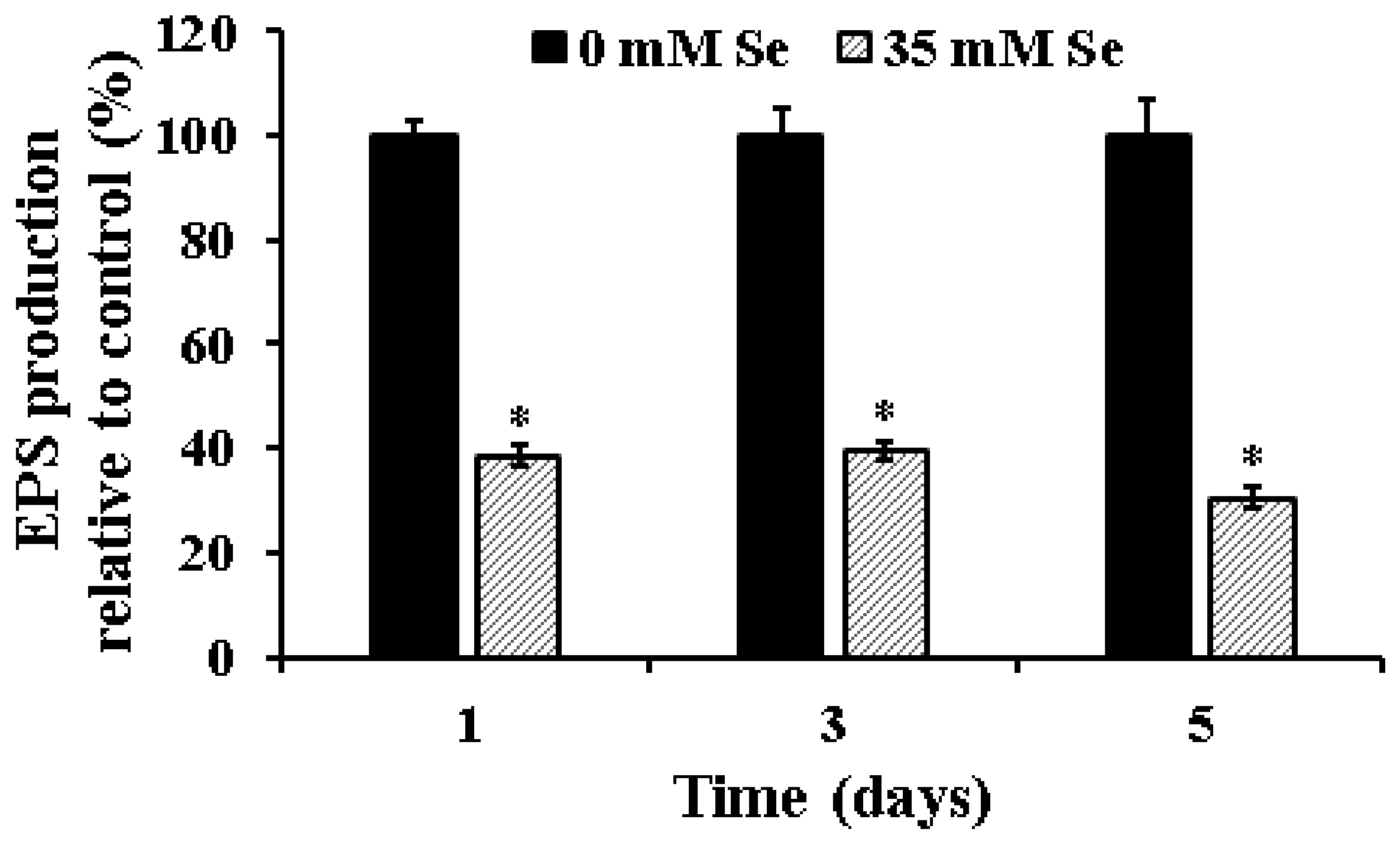
2.2. Effect of Selenium on EPS Production in UPEC Biofilms
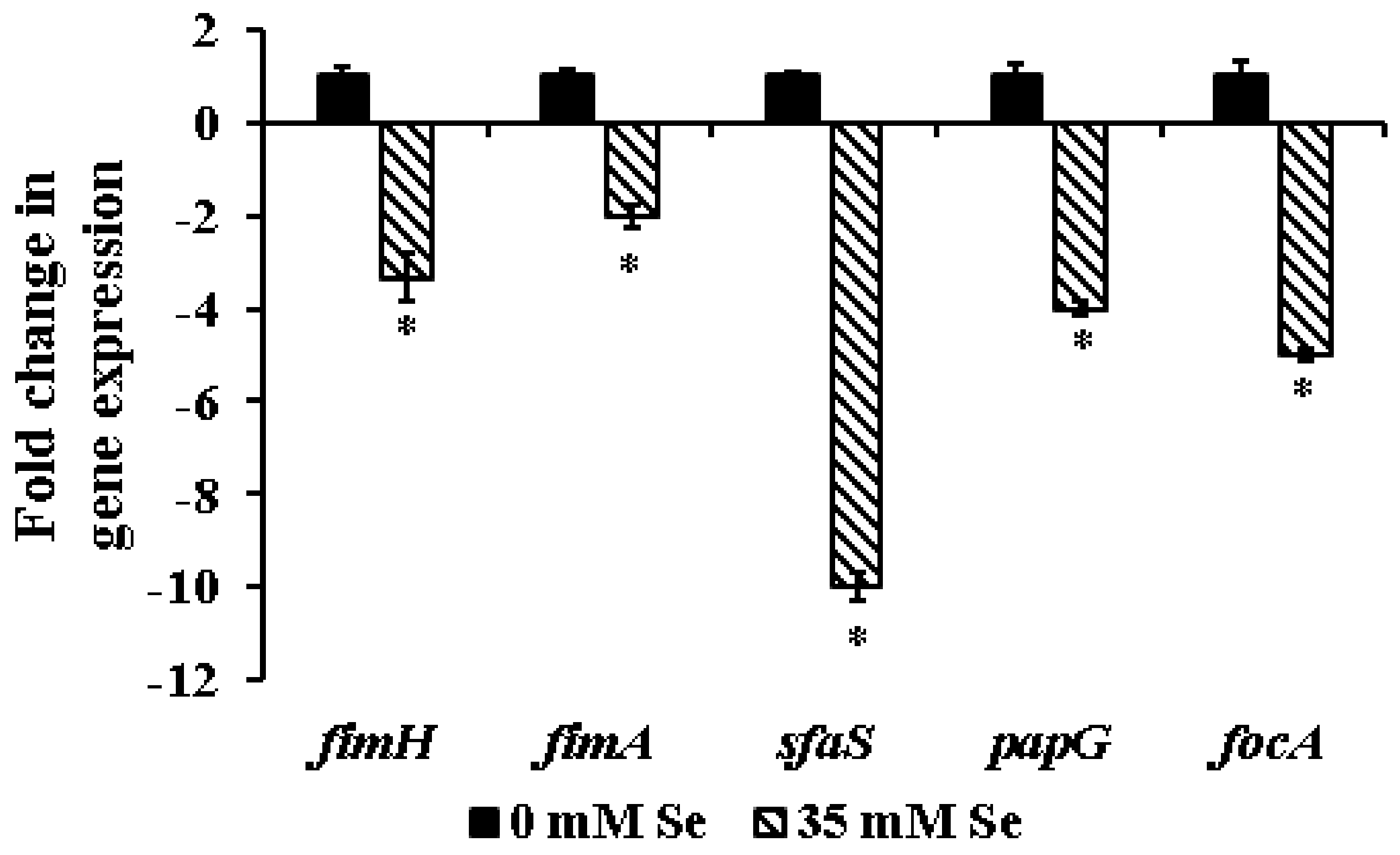
2.3. Effect of Selenium on the Expression of Biofilm-Associated Genes in UPEC
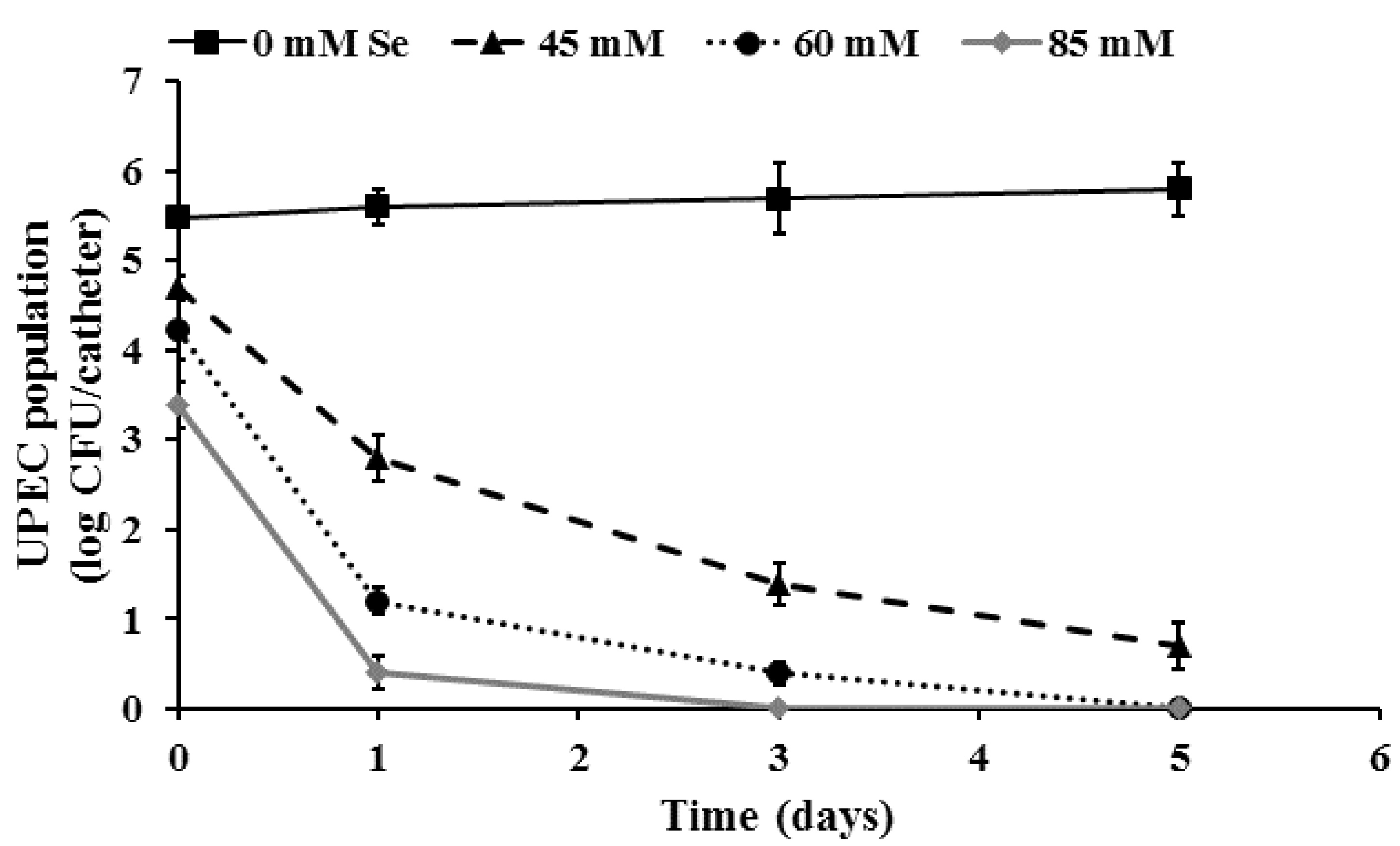
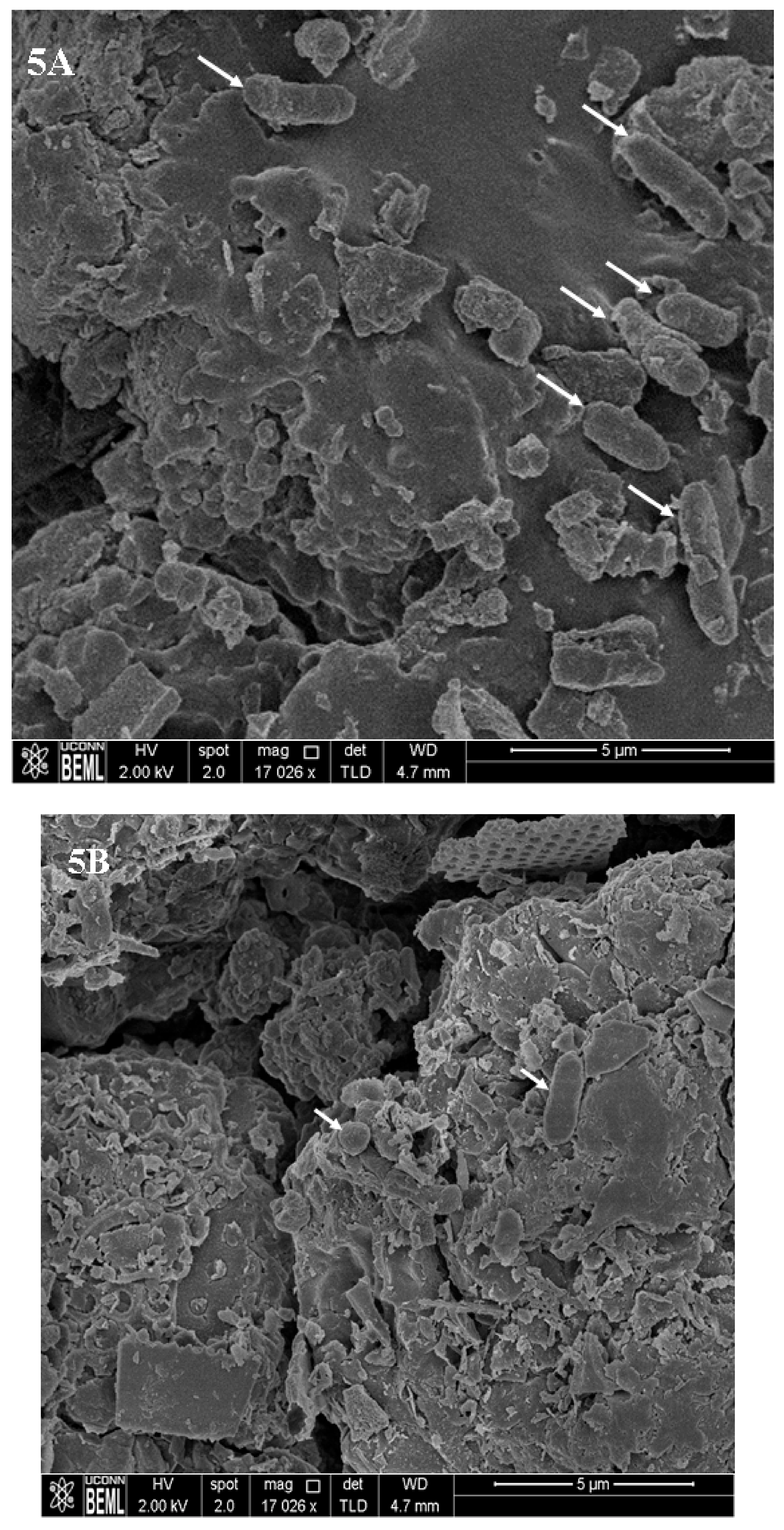
2.4. Inactivation of Pre-Formed UPEC Biofilms on Urinary Catheters by Selenium
3. Materials and Methods
3.1. Bacterial Isolates and Growth Conditions
3.2. Determining the SIC, MIC, and MBC of Se against EHEC
3.3. Inhibition of UPEC Biofilm Formation on Urinary Catheters by Selenium
3.4. Effect of Se on UPEC Exopolysaccharide (EPS) Production
3.5. Effect of Selenium on the Expression of UPEC Biofilm-Associated Genes
3.6. Inactivation of Preformed UPEC Biofilms on Urinary Catheters by Selenium
3.7. Scanning Electron Microscopy (SEM)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Parker, V.; Giles, M.; Graham, L.; Suthers, B.; Watts, W.; O’Brien, T.; Searles, A. Avoiding inappropriate urinary catheter use and catheter-associated urinary tract infection (CAUTI): A pre-post control intervention study. BMC Health Serv. Res. 2017, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.; Venkitanarayanan, K. Role of bacterial biofilms in catheter-associated urinary tract infections (CAUTI) and strategies for their control. In Recent Advances in the Field of Urinary Tract Infections; Nelius, T., Ed.; IntechOpen: London, UK, 2013; pp. 55–88. [Google Scholar]
- Meddings, J.; Reichert, H.; McMahon, L.F., Jr. Challenges and proposed improvements for reviewing symptoms and catheter use to identify National Healthcare Safety Network catheter-associated urinary tract infections. Am. J. Infect. Control 2014, 42, S236–S241. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Catheter-Associated Urinary Tract Infection (CAUTI). 2018. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf (accessed on 8 April 2018).
- Warren, J.W. Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 2001, 17, 299–303. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Chenoweth, C.E.; Gould, C.V.; Saint, S. Diagnosis, management, and prevention of catheter-associated urinary tract infections. Infect. Dis. Clin. N. Am. 2014, 28, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeak, C.S.; Schilling, A.L. The attributable cost of catheter-associated urinary tract infections in the United States: A systematic review. Am. J. Infect. Control 2018. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Laupland, K.B. Epidemiology of intensive care unit-acquired urinary tract infections. Curr. Opin. Infect. Dis. 2006, 19, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Guggenbichler, J.P.; Assadian, O.; Boeswald, M.; Kramer, A. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials—Catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg Interdiszip 2011, 6, Doc18. [Google Scholar] [CrossRef] [PubMed]
- Ronald, A. The etiology of urinary tract infection: Traditional and emerging pathogens. Dis. Mon. 2003, 49, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Eberly, A.R.; Floyd, K.A.; Beebout, C.J.; Colling, S.J.; Fitzgerald, M.J.; Stratton, C.W.; Schmitz, J.E.; Hadjifrangiskou, M. Biofilm formation by uropathogenic Escherichia coli is favored under oxygen conditions that mimic the bladder environment. Int. J. Mol. Sci. 2017, 18, 2077. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Catheter associated urinary tract infections. Antimicrob. Resist. Infect. Control 2014, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickel, J.C.; Gristina, A.G.; Costerton, J.W. Electron microscopic study of an infected Foley catheter. Can. J. Surg. 1985, 28, 50–51. [Google Scholar] [PubMed]
- Nickel, J.C.; Downey, J.A.; Costerton, J.W. Ultrastructural study of microbiologic colonization of urinary catheters. Urology 1989, 34, 284–291. [Google Scholar] [CrossRef]
- Ohkawa, M.; Sugata, T.; Sawaki, M.; Nakashima, T.; Fuse, H.; Hisazumi, H. Bacterial and crystal adherence to the surfaces of indwelling urethral catheters. J. Urol. 1990, 143, 717–721. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.; Narayanan, A.; Baskaran, S.A.; Venkitanarayanan, K. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J. Urol. 2010, 184, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Estevam, E.C.; Witek, K.; Faulstich, L.; Nasim, M.J.; Latacz, G.; Dominguez-Alvarez, E.; Kiec-Kononowicz, K.; Demasi, M.; Handzlik, J.; Jacob, C. Aspects of a distinct cytotoxicity of selenium salts and organic selenides in living cells with possible implications for drug design. Molecules 2015, 20, 13894–13912. [Google Scholar] [CrossRef] [PubMed]
- Sunde, R.A. Selenium. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 225–237. [Google Scholar]
- Wang, Q.; Larese-Casanova, P.; Webster, T.J. Inhibition of various Gram-positive and Gram-negative bacteria growth on selenium nanoparticle coated paper towels. Int. J. Nanomed 2015, 10, 2885–2894. [Google Scholar] [CrossRef]
- Vasić, S.; Radojević, I.; Pešić, N.; Čomić, L. Influence of sodium selenite on the growth of selected bacteria species and their sensitivity to antibiotics. Kragujev. J. Sci. 2011, 33, 55–61. [Google Scholar]
- Kumar, B.S.; Tiwari, S.K.; Manoj, G.; Kunwar, A.; Amrita, N.; Sivaram, G.; Abid, Z.; Ahmad, A.; Khan, A.A.; Priyadarsini, K.I. Anti-unlcer and antimicrobial activities of sodium selenite against Helicobacter pylori: In vitro and in vivo evaluation. Scand. J. Infect. Dis. 2010, 42, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.F.; Safhi, M.M.; Moni, S.S.; Jabeen, A. In Vitro antibacterial spectrum of sodium selenite against selected human pathogenic bacterial strains. Scientifica (Cairo) 2016, 2016, 9176273. [Google Scholar] [CrossRef]
- Khiralla, G.M.; El-Deeb, B.A. Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT-Food Sci. Technol. 2015, 63, 1001–1007. [Google Scholar] [CrossRef]
- Tran, P.A.; Webster, T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553–1558. [Google Scholar] [CrossRef]
- Denstedt, J.D.; Wollin, T.A.; Reid, G. Biomaterials used in urology: Current issues of biocompatibility, infection, and encrustation. J. Endourol. 1998, 12, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Trautner, B.W.; Darouiche, R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, H.D. Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS ONE 2013, 8, e76106. [Google Scholar] [CrossRef] [PubMed]
- Nair, M. Controlling Enterohemorrhagic E. coli O157:H7 Using Selenium and Rutin. Ph.D. Thesis, University of Connecticut, Storrs, CT, USA, 2017. 1552. Available online: https://opencommons.uconn.edu/dissertations/1552/ (accessed on 8 April 2018).
- Bhattaram, V. Investigating the Potential of Essential Minerals for Controlling Vibrio cholerae. Ph.D. Thesis, University of Connecticut, Storrs, CT, USA, 2017. 1498. Available online: https://opencommons.uconn.edu/cgi/viewcontent.cgi?article=7707&context=dissertations (accessed on 8 April 2018).
- Yang, X.; Sha, K.; Xu, G.; Tian, H.; Wang, X.; Chen, S.; Wang, Y.; Li, J.; Chen, J.; Huang, N. Subinhibitory concentrations of allicin decrease uropathogenic Escherichia coli (UPEC) biofilm formation, adhesion ability, and swimming motility. Int. J. Mol. Sci. 2016, 17, 979. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Devraj, A.; Justice, A.A.; Bakaletz, L.O.; Goodman, S.D. DNABII proteins play a central role in UPEC biofilm structure. Mol. Microbiol. 2015, 96, 1119–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Preston, J.F., 3rd; Romeo, T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 2004, 186, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Kocianova, S.; Voyich, J.M.; Yao, Y.; Fischer, E.R.; DeLeo, F.R.; Otto, M. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 2004, 279, 54881–54886. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, U.; Ulrich, M.; Steinhuber, A.; Doring, G.; Mack, D.; Landmann, R.; Goerke, C.; Wolz, C. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect. Immun. 2005, 73, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Sabri, A.N.; McHugh, T.D. Chloroform extract of turmeric inhibits biofilm formation, EPS production and motility in antibiotic resistant bacteria. J. Gen. Appl. Microbiol. 2018, 63, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Niba, E.T.; Naka, Y.; Nagase, M.; Mori, H.; Kitakawa, M. A genome-wide approach to identify the genes involved in biofilm formation in E. coli. DNA Res. 2007, 14, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Blumer, C.; Kleefeld, A.; Lehnen, D.; Heintz, M.; Dobrindt, U.; Nagy, G.; Michaelis, K.; Emody, L.; Polen, T.; Rachel, R.; et al. Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology 2005, 151, 3287–3298. [Google Scholar] [CrossRef] [PubMed]
- Gally, D.L.; Leathart, J.; Blomfield, I.C. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol. Microbiol. 1996, 21, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Hatt, J.K.; Rather, P.N. Role of bacterial biofilms in urinary tract infections. Curr. Top. Microbiol. Immunol. 2008, 322, 163–192. [Google Scholar] [PubMed]
- Hung, C.S.; Bouckaert, J.; Hung, D.; Pinkner, J.; Widberg, C.; DeFusco, A.; Auguste, C.G.; Strouse, R.; Langermann, S.; Waksman, G.; et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 2002, 44, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.J.; Mackenzie, R.; Asscher, A.W. A rapid bioluminescence method for quantifying bacterial adhesion to polystyrene. J. Gen. Microbiol. 1983, 129, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Hung, C.S.; Pinkner, J.S.; Walker, J.N.; Cusumano, C.K.; Li, Z.; Bouckaert, J.; Gordon, J.I.; Hultgren, S.J. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl. Acad. Sci. USA 2009, 106, 22439–22444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.; Zhou, Y.; Pinkner, J.S.; Dodson, K.W.; Crowley, J.R.; Heuser, J.; Chapman, M.R.; Hadjifrangiskou, M.; Henderson, J.P.; Hultgren, S.J. Escherichia coli biofilms have an organized and complex extracellular matrix structure. MBio 2013, 4, e00645-13. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins involved in attachment to abiotic surfaces by Gram-Negative bacteria. Microbiol. Spectr. 2015, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.O.; Soares, L.O.; Silva Junior, A.; Mantovani, H.C.; Chang, Y.F.; Moreira, M.A. Biofilm formation on biotic and abiotic surfaces in the presence of antimicrobials by Escherichia coli isolates from cases of bovine mastitis. Appl. Environ. Microbiol. 2014, 80, 6136–6145. [Google Scholar] [CrossRef] [PubMed]
- Riegman, N.; Kusters, R.; Van Veggel, H.; Bergmans, H.; Van Bergen en Henegouwen, P.; Hacker, J.; Van Die, I. F1C fimbriae of a uropathogenic Escherichia coli strain: Genetic and functional organization of the foc gene cluster and identification of minor subunits. J. Bacteriol. 1990, 172, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, G.; Wullta, B.; Svanborg, C. Escherichia coli, fimbriae, bacterial persistence and host response induction in the human urinary tract. Int. J. Med. Microbiol. 2005, 295, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Adamus-Bialek, W.; Kubiak, A.; Czerwonka, G. Analysis of uropathogenic Escherichia coli biofilm formation under different growth conditions. Acta Biochim. Pol. 2015, 62, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Jahandeh, N.; Ranjbar, R.; Behzadi, P.; Behzadi, E. Uropathogenic Escherichia coli virulence genes: Invaluable approaches for designing DNA microarray probes. Cent. Eur. J. Urol. 2015, 68, 452–458. [Google Scholar] [CrossRef]
- Zamani, H.; Salehzadeh, A. Biofilm formation in uropathogenic Escherichia coli: Association with adhesion factor genes. Turk. J. Med. Sci. 2018, 48, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Naves, P.; del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Dahbi, G.; Blanco, M.; Ponte Mdel, C.; Soriano, F. Correlation between virulence factors and in vitro biofilm formation by Escherichia coli strains. Microb. Pathog. 2008, 45, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.; Narayanan, A.; Venkitanarayanan, K. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 2011, 185, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M.; Smithson, A.; Horcajada, J.P.; Martinez, J.A.; Mensa, J.P.; Vila, J. Implication of biofilm formation in the persistence of urinary tract infection caused by uropathogenic Escherichia coli. Clin. Microbiol. Infect. 2006, 12, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Role of biofilms in antimicrobial resistance. ASAIO J. 2000, 46, 46–S52. [Google Scholar] [CrossRef]
- Hoyle, B.D.; Wong, C.K.; Costerton, J.W. Disparate efficacy of tobramycin on Ca2+-, Mg2+-, and HEPES-treated Pseudomonas aeruginosa biofilms. Can. J. Microbiol. 1992, 38, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Jin, J.; Abruna, H.D.; Houston, P.L.; Hay, A.G.; Ghiorse, W.C.; Shuler, M.L.; Hidalgo, G.; Lion, L.W. Spatial distributions of copper in microbial biofilms by scanning electrochemical microscopy. Environ. Sci. Technol. 2007, 41, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 2007, 5, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Trautner, B.W.; Hull, R.A.; Darouiche, R.O. Escherichia coli 83972 inhibits catheter adherence by a broad spectrum of uropathogens. Urology 2003, 61, 1059–1062. [Google Scholar] [CrossRef]
- Narayanan, A.; Nair, M.S.; Karumathil, D.P.; Baskaran, S.A.; Venkitanarayanan, K.; Amalaradjou, M.A. Inactivation of Acinetobacter baumannii biofilms on polystyrene, stainless steel, and urinary catheters by octenidine dihydrochloride. Front. Microbiol. 2016, 7, 847. [Google Scholar] [CrossRef] [PubMed]
- Borucki, M.K.; Peppin, J.D.; White, D.; Loge, F.; Call, D.R. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7336–7342. [Google Scholar] [CrossRef] [PubMed]
- Zameer, F.; Gopal, S.; Krohne, G.; Kreft, J. Development of a biofilm model for Listeria monocytogenes EGD-e. World J. Microbiol. Biotechnol. 2010, 26, 1143–1147. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.S.; Joo, S.W.; Chandra Regmi, S.; Kim, J.A.; Ryu, C.M.; Ryu, S.Y.; Cho, M.H.; Lee, J. Diverse plant extracts and trans-resveratrol inhibit biofilm formation and swarming of Escherichia coli O157:H7. Biofouling 2013, 29, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Djeribi, R.; Bouchloukh, W.; Jouenne, T.; Menaa, B. Characterization of bacterial biofilms formed on urinary catheters. Am. J. Infect. Control 2012, 40, 854–859. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, A.; Nair, M.S.; Muyyarikkandy, M.S.; Amalaradjou, M.A. Inhibition and Inactivation of Uropathogenic Escherichia coli Biofilms on Urinary Catheters by Sodium Selenite. Int. J. Mol. Sci. 2018, 19, 1703. https://doi.org/10.3390/ijms19061703
Narayanan A, Nair MS, Muyyarikkandy MS, Amalaradjou MA. Inhibition and Inactivation of Uropathogenic Escherichia coli Biofilms on Urinary Catheters by Sodium Selenite. International Journal of Molecular Sciences. 2018; 19(6):1703. https://doi.org/10.3390/ijms19061703
Chicago/Turabian StyleNarayanan, Amoolya, Meera S. Nair, Muhammed S. Muyyarikkandy, and Mary Anne Amalaradjou. 2018. "Inhibition and Inactivation of Uropathogenic Escherichia coli Biofilms on Urinary Catheters by Sodium Selenite" International Journal of Molecular Sciences 19, no. 6: 1703. https://doi.org/10.3390/ijms19061703




