Preparation of Biodegradable Oligo(lactide)s-Grafted Dextran Nanogels for Efficient Drug Delivery by Controlling Intracellular Traffic
Abstract
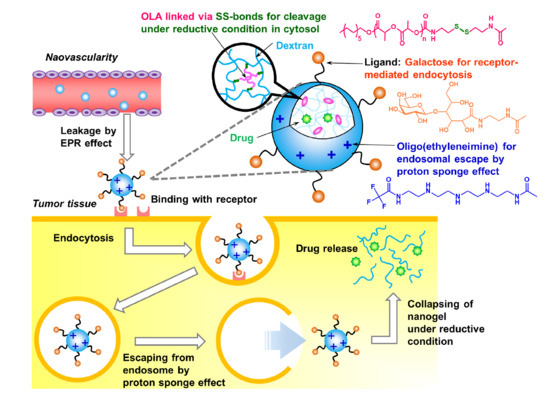
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Graft Copolymer
2.2. Characterization of Nanogels and Dissociation under Reductive Conditions
2.3. Cellular Uptake Behavior of EI4/Gal-Dex-g-SS-OLA Nanogels
3. Materials and Methods
3.1. Materials
3.2. Measurements
3.3. Synthesis of Aminoethyl-Disulfanyl-Ethyl-OLA (OLA-SS-NH2)
3.4. Synthesis of Tetraethylenepentamine/Galactose-Modified Dextran-Grafted-Disulfanyl-Ethyl-OLA (EI4/Gal-Dex-g-SS-OLA)
3.5. Preparation of Nanogel
3.6. Stability of Nanogels under Reductive Conditions
3.7. Cell Viability Test
3.8. Cellular Uptake of Nanogels into HepG2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuki, Y.; Nochi, T.; Kong, I.G.; Takahashi, H.; Sawada, S.; Akiyoshi, K.; Kiyono, H. Nanogel-based antigen-delivery system for nasal vaccines. Biotechnol. Genet. Eng. Rev. 2013, 29, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Hasegawa, U.; Sugawara, A.; Yamane, S.; Akiyoshi, K. Polysaccharide nanogel engineering: Design of nano-structured hydrogel materials and application to biotechnology and medicine. In Nanotechnololgy in Carbohydrate Chemistry; Yuasa, H., Ed.; Transworld Research Network: Keraka, India, 2006; pp. 67–87. [Google Scholar]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning Engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Ayame, H.; Morimoto, N.; Akiyoshi, K. Self-Assembled cationic nanogels for intracellular protein delivery. Bioconjug. Chem. 2008, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, D.M.; Composto, R.J.; Tsourkasc, A.; Muzykantov, V.R. Nanogel carrier design for targeted drug delivery. J. Mater. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, L.; Tomcin, S.; Miyata, K.; Mailänder, V.; Landfester, K.; Kataoka, K.; Zentel, R. Size-dependent knockdown potential of siRNA-loaded cationic nanohydrogel particles. Biomacromolecules 2014, 15, 4111–4121. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, D.; Harada, N.; Hayashi, T.; Tahara, Y.; Momose, F.; Sawada, S.; Mukai, S.; Akiyoshi, K.; Shiku, H. Nanogel-based immunologically stealth vaccine targets macrophages in the medulla of lymph node and induces potent antitumor immunity. ACS Nano 2014, 8, 9209–9218. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.H.; Huang, W.C.; Shen, M.Y.; Wang, C.H.; Huang, Y.F.; Lin, S.C.; Chern, C.S.; Chiu, H.C. Dual-layered nanogel-coated hollow lipid/polypeptide conjugate assemblies for potential pH-triggered intracellular drug release. PLoS ONE 2014, 9, e92268. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Hirano, S.; Takahashi, H.; Loethen, S.; Thompson, D.H.; Akiyoshi, K. Self-assembled pH-sensitive cholesteryl pullulan nanogel as a protein delivery vehicle. Biomacromolecules 2013, 14, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Averick, S.E.; Paredes, E.; Irastorza, A.; Shrivats, A.R.; Srinivasan, A.; Siegwart, D.J.; Magenau, A.J.; Cho, H.Y.; Hsu, E.; Averick, A.A.; et al. Preparation of cationic nanogels for nucleic acid delivery. Biomacromolecules 2012, 13, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.R.; Marcinko, T.; Prasad, P.; Deming, D.; Garman, S.C.; Thayumanavan, S. Unlocking a caged lysosomal protein from a polymeric nanogel with a pH trigger. Biomacromolecules 2014, 15, 4046–4053. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Senanayake, T.H.; Bohling, A.; Vinogradov, S.V. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: Synthesis, pharmacokinetics, and tumor growth inhibition. Mol. Pharmaceutics 2014, 11, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Mao, C.; Lai, B.; Yan, L. Synthesis of disulfide-cross-linked polypeptide nanogel conjugated with a near-infrared fluorescence probe for direct imaging of reduction-induced drug release. ACS Appl. Mater. Interfaces 2012, 4, 5662–5672. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Deguchi, S.; Tajima, H.; Nishikawa, T.; Sunamoto, J. Microscopic structure and thermoresponsiveness of a hydrogel nanoparticle by self-assembly of a hydrophobized polysaccharide. Macromolecules 1997, 30, 857–861. [Google Scholar] [CrossRef]
- Nagahama, K.; Mori, Y.; Ohya, Y.; Ouchi, T. Biodegradable nanogel formation of polylactide-grafted dextran copolymer in dilute aqueous solution and enhancement of its stability by stereocomplexation. Biomacromolecules 2007, 8, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, K.; Ouchi, T.; Ohya, Y. Biodegradable nanogels prepared by self-assembly of poly(l-lactide)-grafted dextran: Entrapment and release of protein. Macromol. Biosci. 2008, 8, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Kataoka, K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 2009, 61, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit. Rev. Ther. Drugs Carr. Syst. 1989, 6, 193–210. [Google Scholar]
- Kusumoto, K.; Akita, H.; Ishitsuka, T.; Matsumoto, Y.; Nomoto, T.; Furukawa, R.; El-Sayed, A.; Hatakeyama, H.; Kajimoto, K.; Yamada, Y.; et al. Lipid envelope-type nanoparticle incorporating a multifunctional peptide for systemic siRNA delivery to the pulmonary endothelium. ACS Nano 2013, 7, 7534–7541. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Kumar, P.V.; Jain, N.K. Dendrimers in oncology: An expanding horizon. Chem. Rev. 2009, 109, 49–87. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Zern, B.J.; Anselmo, A.C.; Shuvaev, V.V.; Mitragotri, S.; Muzykantov, V. Vascular targeting of nanocarriers: Perplexing aspects of the seemingly straightforward paradigm. ACS Nano 2014, 8, 4100–4132. [Google Scholar] [CrossRef] [PubMed]
- Trail, P.A.; Willner, D.; Lasch, S.J.; Henderson, A.J.; Hofstead, S.; Casazza, A.M.; Firestone, R.A.; Hellstrom, I.; Hellstrom, K.E. Cure of xenografted human carcinomas by BR96-doxorubicin immunoconjugates. Science 1993, 261, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Jaracz, S.; Chen, J.; Kuznetsova, L.V.; Ojima, I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg. Med. Chem. 2005, 13, 5043–5054. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualch, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Schubert, S.; Ochrimenko, S.; Fischer, D.; Schubert, U.S. Branched and linear poly(ethylene imine)-based conjugates: Synthetic modification, characterization, and application. Chem. Soc. Rev. 2012, 41, 4755–4767. [Google Scholar] [CrossRef] [PubMed]
- Midoux, P.; Pichon, C.; Yaouanc, J.J.; Jaffrès, P.A. Chemical vectors for gene delivery: A current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br. J. Pharmacol. 2009, 157, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Ko, N.R.; Oh, J.K. Glutathione-triggered disassembly of dual disulfide located degradable nanocarriers of polylactide-based block copolymers for rapid drug release. Biomacromolecules 2014, 15, 3180–3189. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, X.; Dong, H.; Ren, T. Sheddable, degradable, cationic micelles enabling drug and gene delivery. RSC Adv. 2014, 4, 8165–8176. [Google Scholar] [CrossRef]
- Tang, L.Y.; Wang, Y.C.; Li, Y.; Du, J.Z.; Wang, J. Shell-detachable micelles based on disulfide-linked block copolymer as potential carrier for intracellular drug delivery. Bioconjug. Chem. 2009, 20, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, R.K.; Gupta, G.S. Targeting cells for drug and gene delivery: Emerging application of mannans and mannan binding lectines. J. Sci. Ind. Res. 2009, 68, 465–483. [Google Scholar]
- Tsuji, H.; Sato, S.; Masaki, N.; Arakawa, Y.; Kuzuya, A.; Ohya, Y. Stereocomplex crystallization and homo-crystallization of enantiomeric poly(lactic acid-co-alanine)s with ester and amide linkages. Polym. Chem. 2018, 9, 565–575. [Google Scholar] [CrossRef]
- Murata, J.; Ohya, Y.; Ouchi, T. Possibility of application of quaternary chitosan having pendant galactose residues as gene delivery tool. Carbohydr. Polym. 1996, 29, 69–74. [Google Scholar] [CrossRef]
- Niu, J.; Liu, Z.; Fu, L.; Shi, F.; Ma, H.; Ozaki, Y.; Zhang, X. Surface-imprinted nanostructured layer-by-layer film for molecular recognition of theophylline derivatives. Langmuir 2008, 24, 11988–11994. [Google Scholar] [CrossRef] [PubMed]
- Konig, S.G.; Kramer, R. Polyamine-modified near-infrared cyanine dyes for targeting the nuclei and nucleoli of cells. Dyes Pigments 2017, 145, 80–94. [Google Scholar] [CrossRef]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohya, Y.; Takahashi, A.; Kuzuya, A. Preparation of Biodegradable Oligo(lactide)s-Grafted Dextran Nanogels for Efficient Drug Delivery by Controlling Intracellular Traffic. Int. J. Mol. Sci. 2018, 19, 1606. https://doi.org/10.3390/ijms19061606
Ohya Y, Takahashi A, Kuzuya A. Preparation of Biodegradable Oligo(lactide)s-Grafted Dextran Nanogels for Efficient Drug Delivery by Controlling Intracellular Traffic. International Journal of Molecular Sciences. 2018; 19(6):1606. https://doi.org/10.3390/ijms19061606
Chicago/Turabian StyleOhya, Yuichi, Akihiro Takahashi, and Akinori Kuzuya. 2018. "Preparation of Biodegradable Oligo(lactide)s-Grafted Dextran Nanogels for Efficient Drug Delivery by Controlling Intracellular Traffic" International Journal of Molecular Sciences 19, no. 6: 1606. https://doi.org/10.3390/ijms19061606
APA StyleOhya, Y., Takahashi, A., & Kuzuya, A. (2018). Preparation of Biodegradable Oligo(lactide)s-Grafted Dextran Nanogels for Efficient Drug Delivery by Controlling Intracellular Traffic. International Journal of Molecular Sciences, 19(6), 1606. https://doi.org/10.3390/ijms19061606