Kudzu Leaf Extract Suppresses the Production of Inducible Nitric Oxide Synthase, Cyclooxygenase-2, Tumor Necrosis Factor-Alpha, and Interleukin-6 via Inhibition of JNK, TBK1 and STAT1 in Inflammatory Macrophages
Abstract
:1. Introduction
2. Results
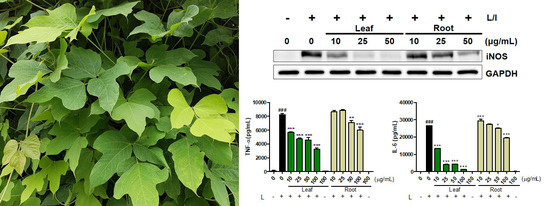
2.1. Effects of Kudzu Leaf Extract on Cell Viability and the Production of Inducible Nitric Oxide Synthase (iNOS) and Nitric Oxide in Mouse Peritoneal Macrophages
2.2. Effects of Kudzu Leaf Extract on Cyclooxygeanse-2, Tumor Necrosis Factor-α, and Interleukin-6 in Mouse Peritoneal Macrophages
2.3. Effects of Kudzu Leaf Extract on Nuclear Factor-κB, Mitogen-Activated Protein Kinase, and TABK-Binding Kinase 1 Activation in Mouse Peritoneal Macrophages
2.4. Effects of Kudzu Leaf Extract on Signal Transducer and Activator of Transcription 1 Activation in Mouse Peritoneal Macrophages
2.5. High-performance Liquid Chromatography (HPLC) Analysis of Robinin and Puerarin in Kudzu Leaves and Roots
2.6. Effects of Robinin on iNOS Production and Signaling Molecules in Activated Macrophages
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Extraction Procedure
4.3. Isolation of Robinin
4.4. HPLC Analysis
4.5. Isolation of Mouse Peritoneal Macrophages
4.6. Cell Viability Assay
4.7. Stimulation of Cells with LPS or LPS Plus IFN-γ
4.8. Determination of Nitrite Accumulation
4.9. Cytokine Analysis
4.10. Western Blot Analysis
4.11. Luciferase Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Adachi, O.; Ogawa, T.; Takeda, K.; Akira, S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 1999, 11, 115–122. [Google Scholar] [CrossRef]
- Kolb, J.P.; Casella, C.R.; SenGupta, S.; Chilton, P.M.; Mitchell, T.C. Type I interferon signaling contributes to the bias that toll-like receptor 4 exhibits for signaling mediated by the adaptor protein TRIF. Sci. Signal. 2014, 7, ra108. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κb. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995, 270, 16483–16486. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. Mapk signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Filla, M.B.; Fultz, M.J.; Vogel, S.N.; Russell, S.W.; Murphy, W.J. Autocrine/paracrine IFN-αβ mediates the lipopolysaccharide-induced activation of transcription factor STAT1α in mouse macrophages: Pivotal role of STAT1α in induction of the inducible nitric oxide synthase gene. J. Immunol. 1998, 161, 4803–4810. [Google Scholar] [PubMed]
- Ohmori, Y.; Hamilton, T.A. Requirement for STAT1 in LPS-induced gene expression in macrophages. J. Leukoc. Biol. 2001, 69, 598–604. [Google Scholar] [PubMed]
- Blanchette, J.; Jaramillo, M.; Olivier, M. Signalling events involved in interferon-γ-inducible macrophage nitric oxide generation. Immunology 2003, 108, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Sweet, M.J.; Hume, D.A. Signal integration between IFNγ and TLR signalling pathways in macrophages. Immunobiology 2006, 211, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Carrier, D.J.; Beitle, R.R.; Howard, L.R.; Lay, J.O.; Liyanage, R.; Clausen, E.C. A glycoside flavonoid in kudzu (Pueraria lobata). Appl. Biochem. Biotechnol. 2005, 121, 783–794. [Google Scholar] [CrossRef]
- Sharma, G.K.; Chandler, C.; Salemi, L. Environmental pollution and leaf cuticular variation in kudzu (Pueraria lobata Willd.). Ann. Bot. 1980, 45, 77–80. [Google Scholar] [CrossRef]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Kudzu root: Traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J. Ethnopharmacol. 2011, 134, 584–607. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, J.; Takeshida, T.; Abe, Y.; Terada, N.; Yamashita, H.; Yamasaki, M.; Takeuchi, K.; Murakami, K.; Tomimatsu, T.; Nohara, T. Studies on the constituents of pueraria lobta. IV. Chemical constituents in the flowers and the leaves. Chem. Pharm. Bull. 1988, 36, 1174–1179. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, N.; Ben-Neriah, Y. Regulation of NF-κb by ubiquitination and degradation of the Iκbs. Immunol. Rev. 2012, 246, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Kaufman, P.B.; Warber, S.; Bolling, S.; Chang, S.C.; Duke, J.A. Quantification of major isoflavonoids and l-canavanine in several organs of kudzu vine (Pueraria montana) and in starch samples derived from kudzu roots. Plant Sci. 2003, 164, 883–888. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Trebicka, E.; Fu, Y.; Waggoner, L.; Akira, S.; Fitzgerald, K.A.; Kagan, J.C.; Cherayil, B.J. Regulation of lipopolysaccharide-induced translation of tumor necrosis factor-alpha by the toll-like receptor 4 adaptor protein TRAM. J. Innate Immun. 2011, 3, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Hoebe, K.; Janssen, E.M.; Kim, S.O.; Alexopoulou, L.; Flavell, R.A.; Han, J.; Beutler, B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by TRIF-dependent and TRIF-independent pathways. Nat. Immunol. 2003, 4, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Sakai, J.; Cammarota, E.; Wright, J.A.; Cicuta, P.; Gottschalk, R.A.; Li, N.; Fraser, I.D.C.; Bryant, C.E. Lipopolysaccharide-induced NF-κb nuclear translocation is primarily dependent on MyD88, but TNFalpha expression requires TRIF and MyD88. Sci. Rep. 2017, 7, 1428. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P. Post-transcriptional control of cytokine production. Nat. Immunol. 2008, 9, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Swantek, J.L.; Cobb, M.H.; Geppert, T.D. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: Glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol. Cell. Biol. 1997, 17, 6274–6282. [Google Scholar] [CrossRef] [PubMed]
- Lahti, A.; Jalonen, U.; Kankaanranta, H.; Moilanen, E. c-Jun NH2-terminal kinase inhibitor anthra(1,9-cd)pyrazol-6(2H)-one reduces inducible nitric-oxide synthase expression by destabilizing mRNA in activated macrophages. Mol. Pharmacol. 2003, 64, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Zhao, Y.; Kay, T.W.; Muglia, L.J. Glucocorticoids target suppressor of cytokine signaling 1 (SOCS1) and type 1 interferons to regulate toll-like receptor-induced STAT1 activation. Proc. Natl. Acad. Sci. USA 2011, 108, 9554–9559. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.E.; Galligan, C.L.; Newman, R.D.; Fish, E.N.; Vogel, S.N. Contribution of interferon-β to the murine macrophage response to the toll-like receptor 4 agonist, lipopolysaccharide. J. Biol. Chem. 2006, 281, 31119–31130. [Google Scholar] [CrossRef] [PubMed]
- Fultz, M.J.; Barber, S.A.; Dieffenbach, C.W.; Vogel, S.N. Induction of IFN-γ in macrophages by lipopolysaccharide. Int. Immunol. 1993, 5, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.D.; Kalvakolanu, D.V.; Chen, W.; Zhang, L.; Kang, T.J.; Thomas, K.E.; Vogel, S.N.; Cross, A.S. A role for STAT1 in the regulation of lipopolysaccharide-induced interleukin-1β expression. J. Interferon Cytokine Res. 2006, 26, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κb activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κb activation along with their inhibitory effect on inos expression and no production in activated macrophages. Mediators Inflamm. 2007, 2007, 45673. [Google Scholar] [PubMed]
- Hu, W.; Yang, X.; Zhe, C.; Zhang, Q.; Sun, L.; Cao, K. Puerarin inhibits INOS, COX-2 and CRP expression via suppression of NF-κb activation in LPS-induced RAW264.7 macrophage cells. Pharmacol. Rep. 2011, 63, 781–789. [Google Scholar] [CrossRef]
- Wang, J.; Mazza, G. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor alpha in LPS/IFN-γ-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 4183–4189. [Google Scholar] [CrossRef] [PubMed]
- Bratkov, V.M.; Shkondrov, A.M.; Zdraveva, P.K.; Krasteva, I.N. Flavonoids from the genus astragalus: Phytochemistry and biological activity. Pharmacogn. Rev. 2016, 10, 11–32. [Google Scholar] [PubMed]
- Tsiklauri, L.; An, G.; Ruszaj, D.M.; Alaniya, M.; Kemertelidze, E.; Morris, M.E. Simultaneous determination of the flavonoids robinin and kaempferol in human breast cancer cells by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 55, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, J.G.; Lee, J.; Yang, W.S.; Park, G.W.; Kim, H.G.; Yi, Y.S.; Baek, K.S.; Sung, N.Y.; Hossen, M.J.; et al. The dietary flavonoid kaempferol mediates anti-inflammatory responses via the Src, Syk, IRAK1, and IRAK4 molecular targets. Mediators Inflamm. 2015, 2015, 904142. [Google Scholar] [CrossRef] [PubMed]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.W.; Cho, Y.R.; Kim, W.; Eom, S.H. Phytochemical linarin enriched in the flower of chrysanthemum indicum inhibits proliferation of A549 human alveolar basal epithelial cells through suppression of the Akt-dependent signaling pathway. J. Med. Food 2013, 16, 1086–1094. [Google Scholar] [CrossRef] [PubMed]








| Robinin | Puerarin | Yield of Crude Extract | |
|---|---|---|---|
| Leaf | 4.61 ± 0.18 | N.D. | 110.2 |
| Root | N.D. | 43.54 ± 0.83 | 253.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, S.H.; Jin, S.-J.; Jeong, H.-Y.; Song, Y.; Lim, Y.J.; Kim, J.-I.; Lee, Y.-H.; Kang, H. Kudzu Leaf Extract Suppresses the Production of Inducible Nitric Oxide Synthase, Cyclooxygenase-2, Tumor Necrosis Factor-Alpha, and Interleukin-6 via Inhibition of JNK, TBK1 and STAT1 in Inflammatory Macrophages. Int. J. Mol. Sci. 2018, 19, 1536. https://doi.org/10.3390/ijms19051536
Eom SH, Jin S-J, Jeong H-Y, Song Y, Lim YJ, Kim J-I, Lee Y-H, Kang H. Kudzu Leaf Extract Suppresses the Production of Inducible Nitric Oxide Synthase, Cyclooxygenase-2, Tumor Necrosis Factor-Alpha, and Interleukin-6 via Inhibition of JNK, TBK1 and STAT1 in Inflammatory Macrophages. International Journal of Molecular Sciences. 2018; 19(5):1536. https://doi.org/10.3390/ijms19051536
Chicago/Turabian StyleEom, Seok Hyun, So-Jung Jin, Hee-Yeong Jeong, Youngju Song, You Jin Lim, Jong-In Kim, Youn-Hyung Lee, and Hee Kang. 2018. "Kudzu Leaf Extract Suppresses the Production of Inducible Nitric Oxide Synthase, Cyclooxygenase-2, Tumor Necrosis Factor-Alpha, and Interleukin-6 via Inhibition of JNK, TBK1 and STAT1 in Inflammatory Macrophages" International Journal of Molecular Sciences 19, no. 5: 1536. https://doi.org/10.3390/ijms19051536