The Effect of Bornyl cis-4-Hydroxycinnamate on Melanoma Cell Apoptosis Is Associated with Mitochondrial Dysfunction and Endoplasmic Reticulum Stress
Abstract
:1. Introduction
2. Results
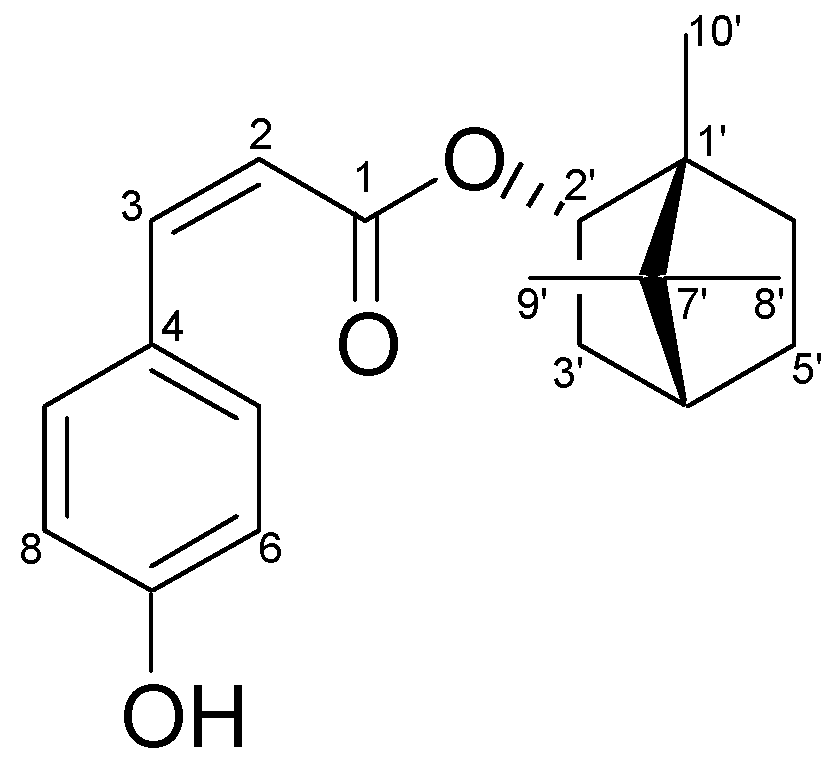
2.1. Characterization of the Constituents of the Ethyl Acetate (EA) Fraction of P. betle Stems
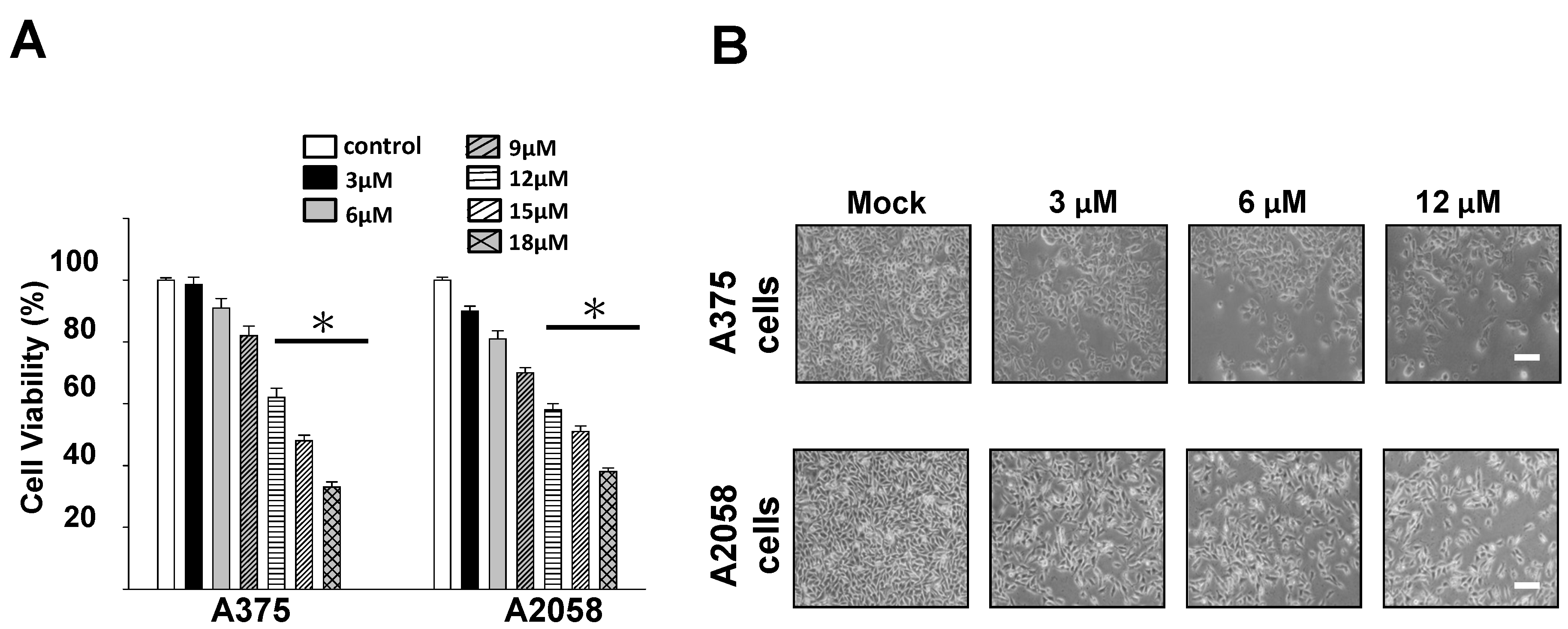
2.2. Cytotoxic and Antiproliferative Effects of Bornyl cis-4-Hydroxycinnamate on Melanoma Cells
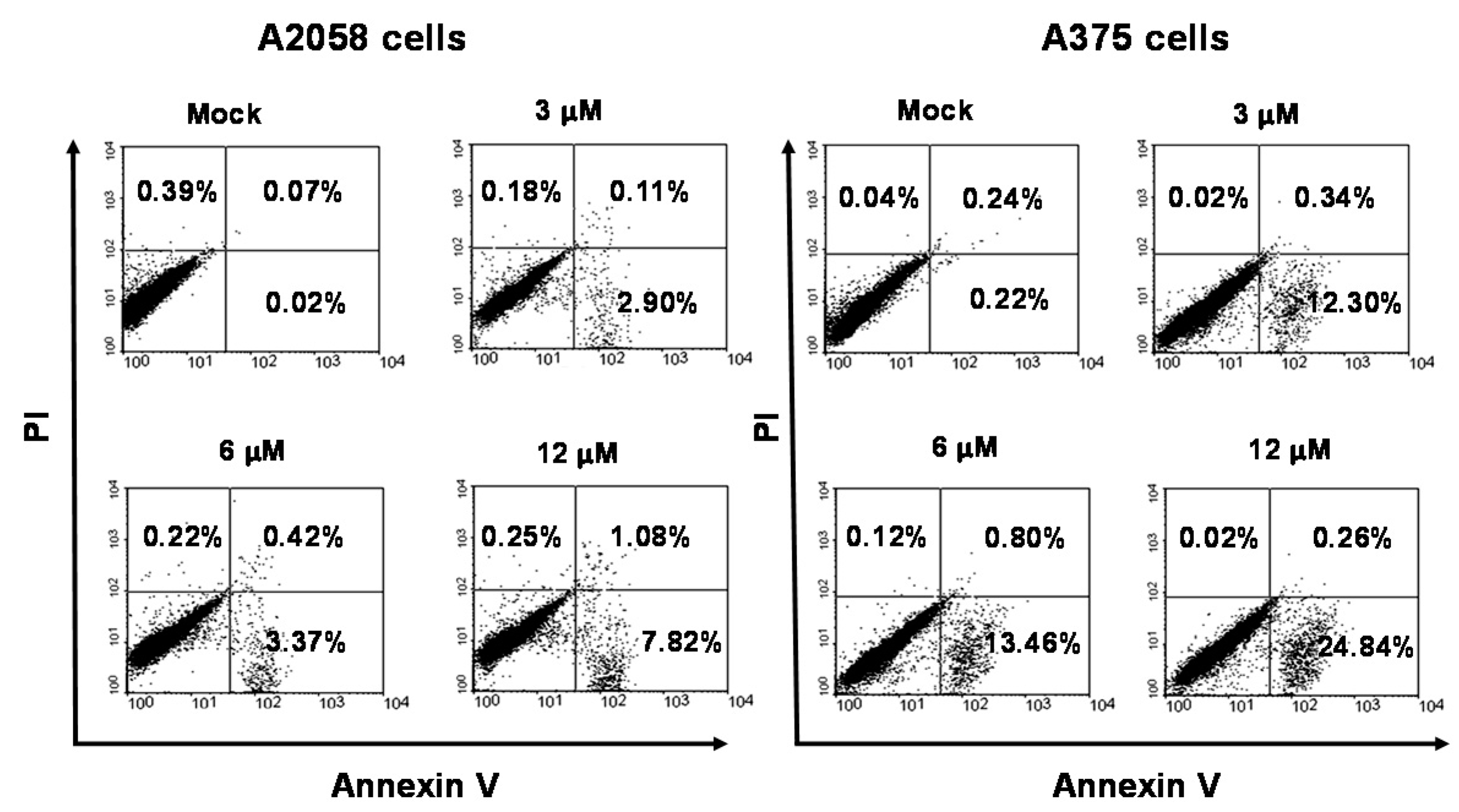
2.3. Apoptosis Is Triggered by Bornyl cis-4-Hydroxycinnamate in Melanoma Cells
2.4. Proteomic Analysis of Bornyl cis-4-Hydroxycinnamate-Treated A375 Cells
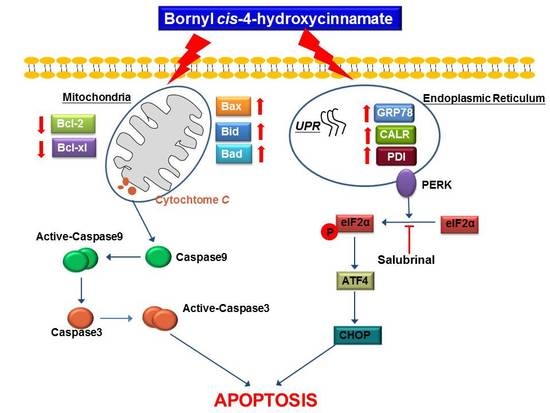
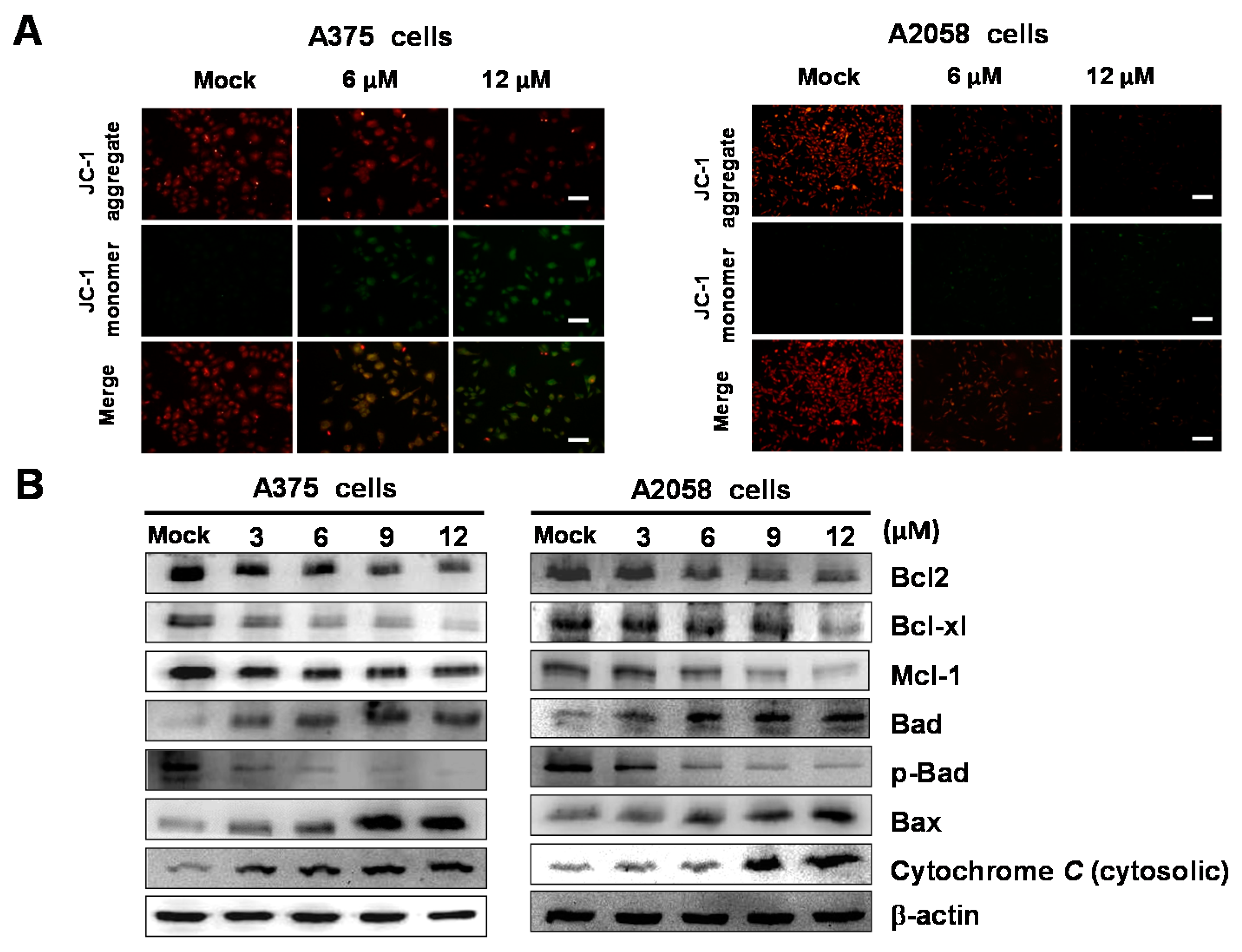
2.5. Bornyl cis-4-Hydroxycinnamate Activates the Apoptosis Pathway through the Induction of Mitochondrial Depolarization
2.6. Bornyl cis-4-Hydroxycinnamate Activates the Caspase-Dependent Pathway Leading to Cell Apoptosis
2.7. Bornyl cis-4-Hydroxycinnamate Treatment Induces the Endoplasmic Reticulum (ER) Stress Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. General Instrumental Operation for the Isolation and Identification of Compounds
4.3. Source, Extraction, Fractionation, and Purification of P. betle Stem Compounds
4.4. Cell Lines and Cell Culture Conditions
4.5. Cytotoxicity Assessment
4.6. Flow Cytometric Analysis
4.7. Protein Lysate Preparation
4.8. Two-Dimensional Gel Electrophoresis and Differential Proteomic Analyses
4.9. Assessment of the Mitochondrial Membrane Potential (Δψm)
4.10. Antibody and Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA A Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- American Cancer Society. Melanoma Skin Cancer. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about.html (accessed on 19 November 2017).
- Fuglede, N.; Brinck-Claussen, U.; Deltour, I.; Boesen, E.; Dalton, S.; Johansen, C. Incidence of cutaneous malignant melanoma in denmark, 1978–2007. Br. J. Dermatol. 2011, 165, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Sasse, A.D.; Sasse, E.C.; Clark, L.; Ulloa, L.; Clark, O. Chemoimmunotherapy versus chemotherapy for metastatic malignant melanoma. Cochrane Database Syst. Rev. 2007, 24, CD005413. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Buch, S.C. Chemotherapy resistance abrogation in metastatic melanoma. Clin. Adv. Hematol. Oncol. 2010, 8, 259–266. [Google Scholar] [PubMed]
- Chang, J. Cutaneous melanoma: Taiwan experience and literature review. Chang Gung Med. J. 2010, 33, 602–612. [Google Scholar] [PubMed]
- Essner, R. Surgical treatment of malignant melanoma. Surg. Clin. 2003, 83, 109–156. [Google Scholar] [CrossRef]
- Bleehen, N.; Newlands, E.; Lee, S.M.; Thatcher, N.; Selby, P.; Calvert, A.; Rustin, G.; Brampton, M.; Stevens, M. Cancer research campaign phase II trial of temozolomide in metastatic melanoma. J. Clin. Oncol. 1995, 13, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Treisman, J.; Garlie, N. Systemic therapy for cutaneous melanoma. Clin. Plast. Surg. 2010, 37, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Acquavella, N.; Kluger, H.; Rhee, J.; Farber, L.; Tara, H.; Ariyan, S.; Narayan, D.; Kelly, W.; Sznol, M. Toxicity and activity of a twice daily high-dose bolus interleukin 2 regimen in patients with metastatic melanoma and metastatic renal cell cancer. J. Immunother. 2008, 31, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Haluska, F.G. Molecular pathogenesis of cutaneous melanocytic neoplasms. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 551–579. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.L.; Rajab, N.F.; Then, S.M.; Yusof, Y.A.M.; Ngah, W.Z.W.; Pin, K.Y.; Looi, M.L. Piper betle leaf extract enhances the cytotoxicity effect of 5-fluorouracil in inhibiting the growth of HT29 and HCT116 colon cancer cells. J. Zhejiang Univ. Sci. B 2014, 15, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.P.; Thilakchand, K.R.; Palatty, P.L.; Rao, P.; Rao, S.; Bhat, H.P.; Baliga, M.S. Piper betel Linn (betel vine), the maligned southeast asian medicinal plant possesses cancer preventive effects: Time to reconsider the wronged opinion. Asian Pac. J. Cancer Prev. 2011, 12, 2149–2156. [Google Scholar] [PubMed]
- Venkadeswaran, K.; Muralidharan, A.R.; Annadurai, T.; Ruban, V.V.; Sundararajan, M.; Anandhi, R.; Thomas, P.A.; Geraldine, P. Antihypercholesterolemic and antioxidative potential of an extract of the plant, Piper betle, and its active constituent, eugenol, in triton WR-1339-induced hypercholesterolemia in experimental rats. Evid.-Based Complement. Altern. Med. 2014, 2014, 478973. [Google Scholar] [CrossRef] [PubMed]
- Al-Adhroey, A.H.; Nor, Z.M.; Al-Mekhlafi, H.M.; Amran, A.A.; Mahmud, R. Antimalarial activity of methanolic leaf extract of Piper betle L. Molecules 2011, 16, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Saito, T.; Matsunami, J.; Asakawa, Y. A comparative study on three chmo-type of the liverwort conocephalum conicum using volatile constituents. Phytochemistry 1997, 44, 1265–1270. [Google Scholar] [CrossRef]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, G.; Dasgupta, P.; Rastogi, S.; Joshi, B.; Chellappan, S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J. Biol. Chem. 2003, 278, 47853–47861. [Google Scholar] [CrossRef] [PubMed]
- Nijtmans, L.G.; de Jong, L.; Sanz, M.A.; Coates, P.J.; Berden, J.A.; Back, J.W.; Muijsers, A.O.; van der Spek, H.; Grivell, L.A. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 2000, 19, 2444–2451. [Google Scholar] [CrossRef] [PubMed]
- Rajalingam, K.; Wunder, C.; Brinkmann, V.; Churin, Y.; Hekman, M.; Sievers, C.; Rapp, U.R.; Rudel, T. Prohibitin is required for ras-induced Raf–MEK–ERK activation and epithelial cell migration. Nat. Cell Biol. 2005, 7, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Kämäräinen, O.; Hofmann, A. Molecular modeling of prohibitin domains. Proteins Struct. Funct. Bioinform. 2007, 68, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.-F.; Cheong, W.-F.; Shu, M.-H.; Teh, C.-H.; Chan, K.-L.; AbuBakar, S. Eurycomanone suppresses expression of lung cancer cell tumor markers, prohibitin, annexin 1 and endoplasmic reticulum protein 28. Phytomedicine 2012, 19, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-B.; Song, W.; Chen, L.-Y.; Li, Q.-F.; Shi, S.-L.; Kong, H.-Y.; Chen, P. Differential expression and regulation of prohibitin during curcumin-induced apoptosis of immortalized human epidermal hacat cells. Int. J. Mol. Med. 2014, 33, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-I.; Wang, R.Y.-L.; Lin, J.-J.; Su, J.-H.; Chiu, C.-C.; Chen, J.-C.; Chen, J.Y.-F.; Wu, Y.-J. Proteomic profiling of the 11-dehydrosinulariolide-treated oral carcinoma cells Ca9–22: Effects on the cell apoptosis through mitochondrial-related and ER stress pathway. J. Proteom. 2012, 75, 5578–5589. [Google Scholar] [CrossRef] [PubMed]
- Denicourt, C.; Dowdy, S.F. Targeting apoptotic pathways in cancer cells. Science 2004, 305, 1411–1413. [Google Scholar] [CrossRef] [PubMed]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-T.; Chang, J.T.-C.; Wang, H.-M.; Ng, S.-H.; Hsueh, C.; Lee, L.-Y.; Lin, C.-H.; Chen, I.-H.; Huang, S.-F.; Cheng, A.-J. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann. Surg. Oncol. 2008, 15, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, D.W.; Thornberry, N.A. Life and death decisions. Science 2003, 299, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Amarante-Mendes, G.P.; Kim, C.N.; Liu, L.; Huang, Y.; Perkins, C.L.; Green, D.R.; Bhalla, K. Bcr-abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome c and activation of caspase-3. Blood 1998, 91, 1700–1705. [Google Scholar] [PubMed]
- Rao, R.V.; Poksay, K.S.; Castro-Obregon, S.; Schilling, B.; Row, R.H.; del Rio, G.; Gibson, B.W.; Ellerby, H.M.; Bredesen, D.E. Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Yuan, J. Cellular response to endoplasmic reticulum stress: A matter of life or death. Cell Death Differ. 2006, 13, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Kouroku, Y.; Fujita, E.; Tanida, I.; Ueno, T.; Isoai, A.; Kumagai, H.; Ogawa, S.; Kaufman, R.; Kominami, E.; Momoi, T. ER stress (PERK/eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007, 14, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Bollo, M.; Paredes, R.M.; Holstein, D.; Zheleznova, N.; Camacho, P.; Lechleiter, J.D. Calcineurin interacts with perk and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS ONE 2010, 5, e11925. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Bandemer, J.; Vindis, C.; Camaré, C.; Mucher, E.; Guéraud, F.; Larroque-Cardoso, P.; Bernis, C.; Auge, N.; Salvayre, R. Protein disulfide isomerase modification and inhibition contribute to ER stress and apoptosis induced by oxidized low density lipoproteins. Antioxid. Redox Signal. 2013, 18, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Teske, B.F.; Wek, S.A.; Bunpo, P.; Cundiff, J.K.; McClintick, J.N.; Anthony, T.G.; Wek, R.C. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol. Biol. Cell 2011, 22, 4390–4405. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Chiu, J.-H. Use of chinese medicine among patients with liver cancer in taiwan. J. Altern. Complement. Med. 2010, 16, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-J.; Wang, R.Y.; Chen, J.-C.; Chiu, C.-C.; Liao, M.-H.; Wu, Y.-J. Cytotoxicity of 11-epi-sinulariolide acetate isolated from cultured soft corals on HA22T cells through the endoplasmic reticulum stress pathway and mitochondrial dysfunction. Int. J. Mol. Sci. 2016, 17, 1787. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-J.; Wong, B.-S.; Yea, S.-H.; Lu, C.-I.; Weng, S.-H. Sinularin induces apoptosis through mitochondria dysfunction and inactivation of the pi3k/akt/mtor pathway in gastric carcinoma cells. Mar. Drugs 2016, 14, 142. [Google Scholar] [CrossRef] [PubMed]
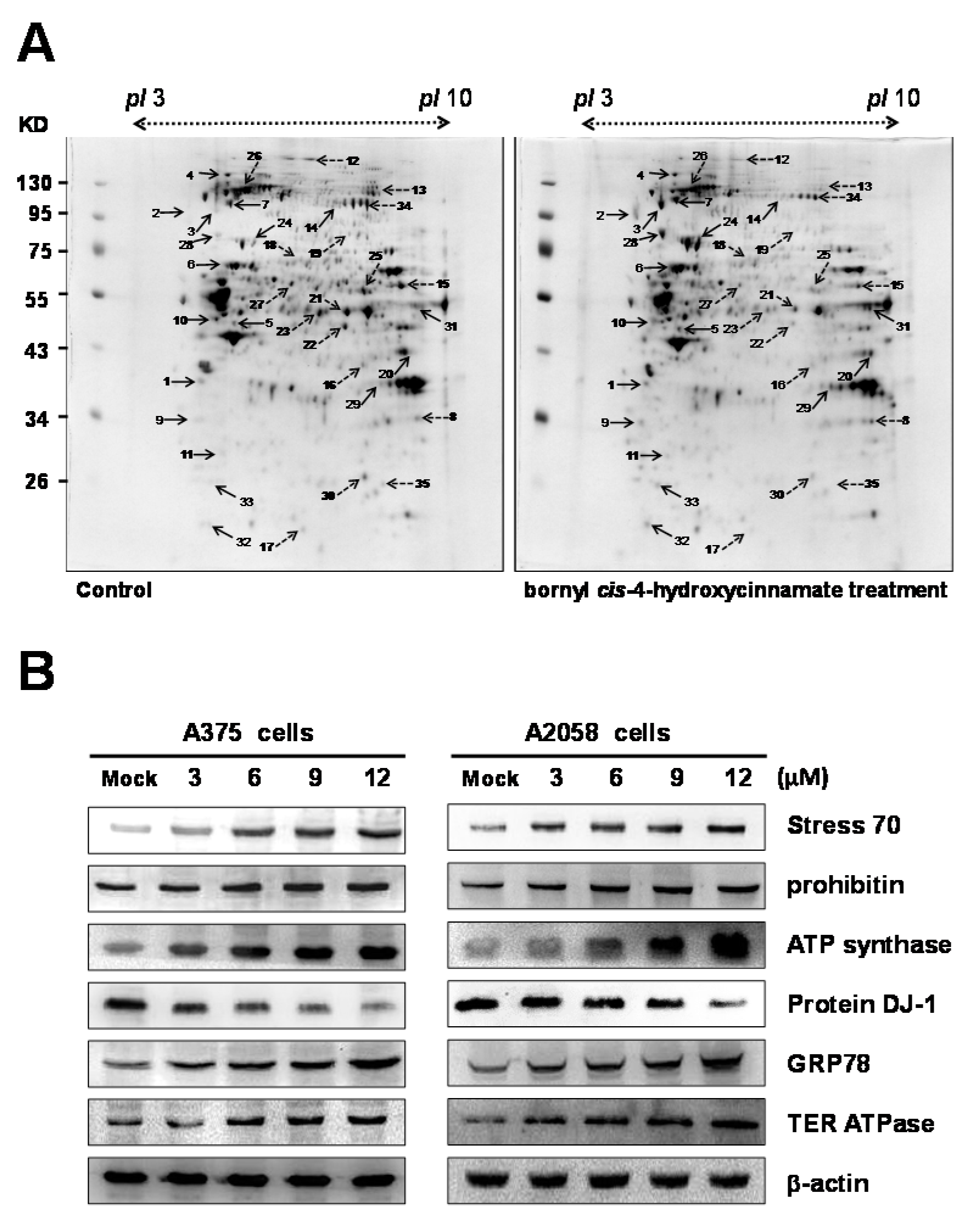




| Spot No. | Protein Name | Accession. No. | Mw/pI | Peptide Matched | Sequence Covered (%) | MASCOT Score | Regulation (Fold-Change) | Cellular Component | Protein Function |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Nucleophosmin | P06748 | 32.72/4.64 | 40 | 30 | 1737 | +1.53 | Nucleus | Regulation of ARF–p53 tumor suppressor pathway |
| 2 | Sodium/potassium-transporting ATPase subunit α-1 | P05023 | 114.15/5.33 | 47 | 28 | 2125 | +2.76 | Mitochondrion | Sodium/potassium-exchanging ATPase activity |
| 3 | Hsp90 | P08238 | 83.56/4.97 | 113 | 49 | 3086 | +2.74 | Cytoplasm | Protein folding |
| 4 | Hypoxia upregulated protein 1 (heat shock protein 70 family) | Q9Y4L1 | 111.49/5.16 | 6 | 6 | 52 | +2.21 | Endoplasmic reticulum | Stress response |
| 5 | ATP synthase subunit β | P06576 | 56.52/5.26 | 51 | 57 | 1865 | +2.89 | Mitochondrion | Mitochondrial membrane ATP synthase |
| 6 | Vimentin | P08670 | 53.67/5.06 | 5 | 9 | 58 | +2.72 | Cytoplasm | Cytoskeletal protein |
| 7 | Transitional endoplasmic reticulum ATPase | P55072 | 89.96/5.14 | 29 | 20 | 432 | +3.12 | Endoplasmic reticulum | Involved in the formation of the transitional endoplasmic reticulum (tER) |
| 8 | Voltage-dependent anion-selective channel protein | P21796 | 30.87/8.62 | 42 | 68 | 1261 | −1.55 | Cytoplasm | Involved in cell volume regulation and apoptosis |
| 9 | Proliferating cell nuclear antigen | P12004 | 29.09/4.57 | 61 | 73 | 1246 | +1.36 | Nucleus | DNA repair and damage |
| 10 | Tubulin β-4B | P68371 | 50.26/4.79 | 27 | 40 | 601 | +3.4 | Cytoplasm | Structural constituent of cytoskeleton |
| 11 | Prohibitin | P35232 | 29.84/5.57 | 73 | 78 | 2345 | +1.51 | Mitochondrion | Inhibits DNA synthesis |
| 12 | Clathrin heavy chain 1 | Q00610 | 193.29/5.48 | 41 | 29 | 1104 | −1.51 | Cytoplasm | Intracellular trafficking |
| 13 | C-1-tetrahydrofolate synthase | P11586 | 102.19/6.89 | 6 | 6 | 54 | −1.56 | Cytoplasm | Hydrolase |
| 14 | Glycogen phosphorylase | P11216 | 97.33/6.40 | 43 | 44 | 1331 | +1.59 | Mitochondrion | Glycosyltransferase |
| 15 | Serine hydroxymethyltransferase | P34897 | 56.42/8.76 | 22 | 18 | 198 | −2.11 | Mitochondrion | Associates with mitochondrial DNA |
| 16 | Poly(rC)-binding protein 2 | P40555 | 37.99/6.66 | 30 | 28 | 491 | −1.52 | Cytoplasm | Chaperone |
| 17 | Protein DJ-1 | Q99497 | 20.05/6.33 | 56 | 68 | 2434 | −3.21 | Endoplasmic Reticulum | Protects cells against oxidative stress and cell death |
| 18 | Prelamin-A/C | P02545 | 74.38/6.57 | 67 | 48 | 2452 | −3.13 | Nucleus | Structural molecule activity |
| 19 | Bifunctional UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase | Q9Y223 | 80.21/6.32 | 3 | 2 | 102 | −1.52 | Cytoplasm | Regulates and initiates biosynthesis of N-acetylneuraminic acid |
| 20 | Fructose-bisphosphate aldolase A | P04075 | 39.85/8.30 | 6 | 10 | 121 | +3.25 | Cytoplasm | Plays a key role in glycolysis and gluconeogenesis |
| 21 | α-enolase | P06733 | 47.48/7.01 | 355 | 75 | 8369 | −1.81 | Cytoplasm | Multifunctional enzyme (Transcription regulation) |
| 22 | Elongation factor Tu, | P49411 | 49.85/7.26 | 201 | 69 | 5544 | −2.36 | Mitochondrion | Elongation factor |
| 23 | α-enolase | P06733 | 47.48/7.01 | 143 | 76 | 3720 | −2.94 | Cytoplasm | Lyase |
| 24 | Stress-70 protein | P38646 | 73.92/5.87 | 432 | 72 | 11,267 | +3.15 | Mitochondrion | Chaperone |
| 25 | Dihydrolipoyl dehydrogenase, mitochondrial | P09622 | 54.72/7.95 | 28 | 27 | 630 | −2.73 | Mitochondrion | Oxidoreductase |
| 26 | Heat-shock protein 105 kDa | Q92598 | 97.73/5.28 | 120 | 60 | 2474 | −1.52 | Cytoplasm | Response to unfolded protein |
| 27 | Tryptophan-tRNA ligase | P23381 | 53.48/5.83 | 57 | 52 | 1802 | −1.53 | Cytoplasm | ATP binding |
| 28 | GRP78 | P11021 | 72.40/2.07 | 49 | 53 | 1322 | +3.23 | Endoplasmic reticulum | Stress response |
| 29 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | P04406 | 36.20/8.57 | 88 | 84 | 1998 | +1.54 | Cytoplasm | Assembly of the cytoskeleton |
| 30 | Triosephosphate isomerase | P60174 | 31.06/5.65 | 54 | 70 | 1494 | −2.78 | Cytoplasm | Triose-phosphate isomerase activity |
| 31 | ATP synthase subunit β | P06576 | 56.52/5.26 | 23 | 37 | 524 | +2.29 | Mitochondrion | Mitochondrial membrane ATP synthase |
| 32 | RNA-binding protein 8A | Q9Y5S9 | 19.93/5.50 | 15 | 33 | 348 | +1.57 | Nucleus | mRNA binding |
| 33 | Ran-specific GTPase-activating protein | P43487 | 23.47/5.19 | 24 | 54 | 484 | +1.52 | Cytoplasm | GTPase activation |
| 34 | Elongation factor 2 | P13639 | 96.26/6.41 | 175 | 58 | 2493 | −1.51 | Cytoplasm | GTPase activity |
| 35 | 3-hydroxyacyl-CoA dehydrogenase type-2 | Q99714 | 27.13/7.66 | 59 | 86 | 1770 | −1.55 | Mitochondrion | Mitochondrial tRNA maturation |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-Y.; Wu, Y.-J.; Chang, C.-I.; Chiu, C.-C.; Wu, M.-L. The Effect of Bornyl cis-4-Hydroxycinnamate on Melanoma Cell Apoptosis Is Associated with Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2018, 19, 1370. https://doi.org/10.3390/ijms19051370
Yang T-Y, Wu Y-J, Chang C-I, Chiu C-C, Wu M-L. The Effect of Bornyl cis-4-Hydroxycinnamate on Melanoma Cell Apoptosis Is Associated with Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. International Journal of Molecular Sciences. 2018; 19(5):1370. https://doi.org/10.3390/ijms19051370
Chicago/Turabian StyleYang, Tzu-Yen, Yu-Jen Wu, Chi-I Chang, Chien-Chih Chiu, and Mei-Li Wu. 2018. "The Effect of Bornyl cis-4-Hydroxycinnamate on Melanoma Cell Apoptosis Is Associated with Mitochondrial Dysfunction and Endoplasmic Reticulum Stress" International Journal of Molecular Sciences 19, no. 5: 1370. https://doi.org/10.3390/ijms19051370
APA StyleYang, T.-Y., Wu, Y.-J., Chang, C.-I., Chiu, C.-C., & Wu, M.-L. (2018). The Effect of Bornyl cis-4-Hydroxycinnamate on Melanoma Cell Apoptosis Is Associated with Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. International Journal of Molecular Sciences, 19(5), 1370. https://doi.org/10.3390/ijms19051370