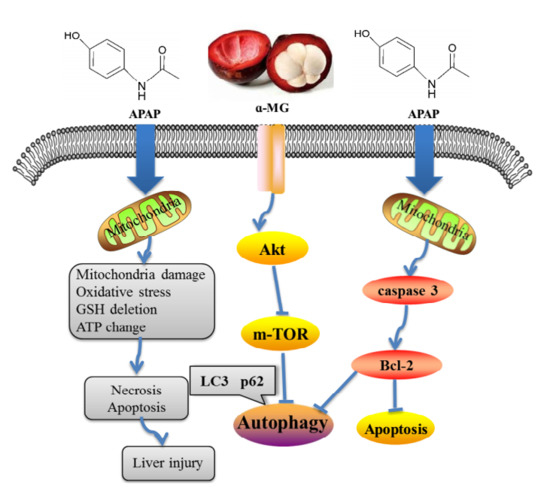
Dietary α-Mangostin Provides Protective Effects against Acetaminophen-Induced Hepatotoxicity in Mice via Akt/mTOR-Mediated Inhibition of Autophagy and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Preparation of α-MG from Mangosteen Pericarp
2.3. Animals and Experimental Design
2.4. Determination of α-MG by HPLC
2.5. Analysis of Histology Changes and TUNEL Assay
2.6. Analysis of Immunofluorescence Staining
2.7. Estimation of Lipid Peroxidation and Biochemical Parameters
2.8. Measurement of Serum Inflammatory Markers TNF-α and IL-1β
2.9. Western Blotting Analysis
2.10. Statistical Analysis and Data Presentation
3. Results
3.1. α-MG Restores APAP-Induced Liver Histopathological Changes
3.2. Ameliorating Effects of α-MG against APAP-Induced Aberrant Transaminase
3.3. Inhibitory Effects of α-MG on Oxidative Stress
3.4. α-MG Inhibits APAP-Inducible Inflammation
3.5. Inhibitory Effects of α-MG on Apoptotic Molecular Expression
3.6. Suppression of Molecular Expression in Autophagic Pathway in Mice by α-MG
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Larson, A.M.; Polson, J.; Fontana, R.J.; Davern, T.J.; Lalani, E.; Hynan, L.S.; Reisch, J.S.; Schiodt, F.V.; Ostapowicz, G.; Shakil, A.O.; et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005, 42, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- De Achaval, S.; Suarez-Almazor, M. Acetaminophen overdose: A little recognized public health threat. Pharmacoepidemiol. Drug Saf. 2011, 20, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Gandillet, A.; Vidal, I.; Alexandre, E.; Audet, M.; Chenard-Neu, M.P.; Stutzmann, J.; Heyd, B.; Jaeck, D.; Richert, L. Experimental models of acute and chronic liver failure in nude mice to study hepatocyte transplantation. Cell Transplant. 2005, 14, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Tittarelli, R.; Pellegrini, M.; Scarpellini, M.G.; Marinelli, E.; Bruti, V.; di Luca, N.M.; Busardo, F.P.; Zaami, S. Hepatotoxicity of paracetamol and related fatalities. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 95–101. [Google Scholar] [PubMed]
- McGill, M.R.; Sharpe, M.R.; Williams, C.D.; Taha, M.; Curry, S.C.; Jaeschke, H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Investig. 2012, 122, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ponce, H.A.; Martinez-Saldana, M.C.; Rincon-Sanchez, A.R.; Sumaya-Martinez, M.T.; Buist-Homan, M.; Faber, K.N.; Moshage, H.; Jaramillo-Juarez, F. Hepatoprotective Effect of Opuntia robusta and Opuntia streptacantha Fruits against Acetaminophen-Induced Acute Liver Damage. Nutrients 2016, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Song, H.; Yang, Y.; Zhou, H.; Liu, Y.; Liu, Z. Smashing Tissue Extraction of Five Lignans From the Fruit of Schisandra chinensis. J. Chromatogr. Sci. 2016, 54, 246–256. [Google Scholar] [PubMed]
- Lavallard, V.J.; Meijer, A.J.; Codogno, P.; Gual, P. Autophagy, signaling and obesity. Pharmacol. Res. 2012, 66, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Kanaseki, T.; Mizushima, N.; Mizuta, T.; Arakawa-Kobayashi, S.; Thompson, C.B.; Tsujimoto, Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 2004, 6, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Baulies, A.; Ribas, V.; Nunez, S.; Torres, S.; Alarcon-Vila, C.; Martinez, L.; Suda, J.; Ybanez, M.D.; Kaplowitz, N.; Garcia-Ruiz, C.; et al. Lysosomal Cholesterol Accumulation Sensitizes To Acetaminophen Hepatotoxicity by Impairing Mitophagy. Sci. Rep. 2015, 5, 18017. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverri, J.; Cardenas-Rodriguez, N.; Orozco-Ibarra, M.; Perez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food. Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Hu, W.; Cai, Z.; Liu, Y.; Li, S.; Tao, W.; Xiang, H. New medicinal properties of mangostins: Analgesic activity and pharmacological characterization of active ingredients from the fruit hull of Garcinia mangostana L. Pharmacol. Biochem. Behav. 2010, 95, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Patruno, A.; Pasqualone, L.; Carlucci, G.; Ferrone, V.; Carlucci, M.; de Lutiis, M.A.; Grilli, A.; et al. A Novel Biological Role of alpha-Mangostin in Modulating Inflammatory Response Through the Activation of SIRT-1 Signaling Pathway. J. Cell Physiol. 2016, 231, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Mahabusarakam, W.; Proudfoot, J.; Taylor, W.; Croft, K. Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin. Free Radic Res. 2000, 33, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Arunrattiyakorn, P.; Suksamrarn, S.; Suwannasai, N.; Kanzaki, H. Microbial metabolism of alpha-mangostin isolated from Garcinia mangostana L. Phytochemistry 2011, 72, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Zhang, K.J.; Gu, Q.L.; Bi, X.L.; Wang, J.X. Pharmacology of mangostins and their derivatives: A comprehensive review. Chin. J. Nat. Med. 2017, 15, 81–93. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, Y.M.; Huh, J.H.; Lee, E.S.; Kwon, M.H.; Lee, B.R.; Ko, H.J.; Chung, C.H. alpha-Mangostin ameliorates hepatic steatosis and insulin resistance by inhibition C-C chemokine receptor 2. PLoS ONE 2017, 12, e0179204. [Google Scholar]
- Tsai, S.Y.; Chung, P.C.; Owaga, E.E.; Tsai, I.J.; Wang, P.Y.; Tsai, J.I.; Yeh, T.S.; Hsieh, R.H. Alpha-mangostin from mangosteen (Garcinia mangostana Linn.) pericarp extract reduces high fat-diet induced hepatic steatosis in rats by regulating mitochondria function and apoptosis. Nutr. Metab. 2016, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Supawadee, S.; Thanet, S.; Wisut, P.; Somneuk, N.; Sirinun, N.; Ramida, W. Investigation of Therapeutic Effects of alpha-Mangostin on Thioacetamide-Induced Cirrhosis in Rats. J. Med. Assoc. Thai 2015, 98 (Suppl. 9), S91–S97. [Google Scholar] [PubMed]
- Phitaktim, S.; Chomnawang, M.; Sirichaiwetchakoon, K.; Dunkhunthod, B.; Hobbs, G.; Eumkeb, G. Synergism and the mechanism of action of the combination of alpha-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol. 2016, 16, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, J.N.; Yan, M.H.; Xing, J.J.; Liu, W.C.; Li, W. Caspase-Mediated Anti-Apoptotic Effect of Ginsenoside Rg5, a Main Rare Ginsenoside, on Acetaminophen-Induced Hepatotoxicity in Mice. J. Agric. Food Chem. 2017, 65, 9226–9236. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.N.; Liu, Z.; Wang, Z.; Li, X.D.; Zhang, L.X.; Li, W.; Wang, Y.P. Ameliorative Effects and Possible Molecular Mechanism of Action of Black Ginseng (Panax ginseng) on Acetaminophen-Mediated Liver Injury. Molecules 2017, 22, 664. [Google Scholar] [CrossRef]
- Ray, R.; Chen, G.; Vande Velde, C.; Cizeau, J.; Park, J.H.; Reed, J.C.; Gietz, R.D.; Greenberg, A.H. BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J. Biol. Chem. 2000, 275, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, A.; Chung, R.T. Acute liver failure: Mechanisms of hepatocyte injury and regeneration. Semin. Liver Dis. 2008, 28, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Hu, J.N.; Liu, Z.; Zhang, R.; He, Y.F.; Hou, W.; Wang, Z.Q.; Yang, G.; Li, W. Saponins (Ginsenosides) from the Leaves of Panax quinquefolius Ameliorated Acetaminophen-Induced Hepatotoxicity in Mice. J. Agric. Food Chem. 2017, 65, 3684–3692. [Google Scholar] [CrossRef] [PubMed]
- Michael Brown, J.; Ball, J.G.; Wright, M.S.; Van Meter, S.; Valentovic, M.A. Novel protective mechanisms for S-adenosyl-l-methionine against acetaminophen hepatotoxicity: improvement of key antioxidant enzymatic function. Toxicol. Lett. 2012, 212, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen-related hepatotoxicity. Clin. Liver Dis. 2013, 17, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Ghassam, B.J.; Prakash, H.S. Hepatoprotective and cytoprotective properties of Hyptis suaveolens against oxidative stress-induced damage by CCl(4) and H(2)O(2). Asian Pac. J. Trop. Med. 2012, 5, 868–874. [Google Scholar] [CrossRef]
- Sidahmed, H.M.; Abdelwahab, S.I.; Mohan, S.; Abdulla, M.A.; Mohamed Elhassan Taha, M.; Hashim, N.M.; Hadi, A.H.; Vadivelu, J.; Loke Fai, M.; Rahmani, M.; Yahayu, M. α-Mangostin from Cratoxylum arborescens (Vahl) Blume Demonstrates Anti-Ulcerogenic Property: A Mechanistic Study. Evid. Based Complement. Altern. Med. 2013, 2013, 450840. [Google Scholar] [CrossRef] [PubMed]
- Blazka, M.E.; Wilmer, J.L.; Holladay, S.D.; Wilson, R.E.; Luster, M.I. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 1995, 133, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, A.; Eipel, C.; Abshagen, K.; Siebert, N.; Menger, M.D.; Vollmar, B. Role of the perforin/granzyme cell death pathway in d-Gal/LPS-induced inflammatory liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1069–G1076. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, W.; Ling, J.; Jiang, C. alpha-Mangostin Inhibits alpha-Synuclein-Induced Microglial Neuroinflammation and Neurotoxicity. Cell Mol. Neurobiol. 2016, 36, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.S.; Jaques-Robinson, K.M.; Hadzimichalis, N.M.; Golfetti, R.; Merrill, G.F. Acetaminophen reduces mitochondrial dysfunction during early cerebral postischemic reperfusion in rats. Brain Res. 2010, 1319, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.L.; Pierce, R.H.; Vail, M.E.; White, C.C.; Tonge, R.P.; Kavanagh, T.J.; Fausto, N.; Nelson, S.D.; Bruschi, S.A. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol. Pharmacol. 2001, 60, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Boulares, A.H.; Zoltoski, A.J.; Stoica, B.A.; Cuvillier, O.; Smulson, M.E. Acetaminophen induces a caspase-dependent and Bcl-XL sensitive apoptosis in human hepatoma cells and lymphocytes. Pharmacol. Toxicol. 2002, 90, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Massen, S.; Terenzio, M.; Lang, V.; Chen-Lindner, S.; Eils, R.; Novak, I.; Dikic, I.; Hamacher-Brady, A.; Brady, N.R. Modulation of serines 17 and 24 in the LC3-interacting region of Bnip3 determines pro-survival mitophagy versus apoptosis. J. Biol. Chem. 2013, 288, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lei, M. alpha-Mangostin protects against high-glucose induced apoptosis of human umbilical vein endothelial cells. Biosci. Rep. 2017, 37, 6. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; Boggess, N.; McGill, M.R.; Lebofsky, M.; Borude, P.; Apte, U.; Jaeschke, H.; Ding, W.X. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol. Sci. 2012, 127, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yu, S.; Wang, Z.; Yang, K.; Liu, Z.; Li, C.; Liang, Y. Nicotinamide Adenine Dinucleotide Protects against Spinal Cord Ischemia Reperfusion Injury-Induced Apoptosis by Blocking Autophagy. Oxid. Med. Cell Longev. 2017, 2017, 7063874. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Autophagy and apoptosis in liver injury. Cell Cycle 2015, 14, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Reimers, K.; Choi, C.Y.; Bucan, V.; Vogt, P.M. The Bax Inhibitor-1 (BI-1) family in apoptosis and tumorigenesis. Curr. Mol. Med. 2008, 8, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.C.; Hsu, Y.L.; Liu, C.K.; Kuo, P.L. alpha-Mangostin, a dietary xanthone, induces autophagic cell death by activating the AMP-activated protein kinase pathway in glioblastoma cells. J. Agric. Food Chem. 2011, 59, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Limaye, P.B.; Bowen, W.C.; Orr, A.V.; Luo, J.; Tseng, G.C.; Michalopoulos, G.K. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology 2008, 47, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Nam, E.J.; Hayashida, K.; Aquino, R.S.; Couchman, J.R.; Kozar, R.A.; Liu, J.; Park, P.W. Syndecan-1 limits the progression of liver injury and promotes liver repair in acetaminophen-induced liver injury in mice. Hepatology 2017, 66, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Ma, L.; Sun, J.E.; Zhu, L.J.; Green, M.R. BCR-ABL suppresses autophagy through ATF5-mediated regulation of mTOR transcription. Blood 2011, 118, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Wiedenhoft, H.; Hayashi, L.; Coffin, A.B. PI3K and Inhibitor of Apoptosis Proteins Modulate Gentamicin-Induced Hair Cell Death in the Zebrafish Lateral Line. Front. Cell Neurosci. 2017, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kageyama, S.; Ichimura, Y. p62/SQSTM1/A170: physiology and pathology. Pharmacol. Res. 2012, 66, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Dinic, M.; Lukic, J.; Djokic, J.; Milenkovic, M.; Strahinic, I.; Golic, N.; Begovic, J. Lactobacillus fermentum Postbiotic-induced Autophagy as Potential Approach for Treatment of Acetaminophen Hepatotoxicity. Front. Microbiol. 2017, 8, 594. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.-t.; Sun, Y.-s.; Ren, S.; Zhao, L.-c.; Liu, W.-c.; Chen, C.; Wang, Z.; Li, W. Dietary α-Mangostin Provides Protective Effects against Acetaminophen-Induced Hepatotoxicity in Mice via Akt/mTOR-Mediated Inhibition of Autophagy and Apoptosis. Int. J. Mol. Sci. 2018, 19, 1335. https://doi.org/10.3390/ijms19051335
Yan X-t, Sun Y-s, Ren S, Zhao L-c, Liu W-c, Chen C, Wang Z, Li W. Dietary α-Mangostin Provides Protective Effects against Acetaminophen-Induced Hepatotoxicity in Mice via Akt/mTOR-Mediated Inhibition of Autophagy and Apoptosis. International Journal of Molecular Sciences. 2018; 19(5):1335. https://doi.org/10.3390/ijms19051335
Chicago/Turabian StyleYan, Xiao-tong, Yin-shi Sun, Shen Ren, Li-chun Zhao, Wen-cong Liu, Chen Chen, Zi Wang, and Wei Li. 2018. "Dietary α-Mangostin Provides Protective Effects against Acetaminophen-Induced Hepatotoxicity in Mice via Akt/mTOR-Mediated Inhibition of Autophagy and Apoptosis" International Journal of Molecular Sciences 19, no. 5: 1335. https://doi.org/10.3390/ijms19051335