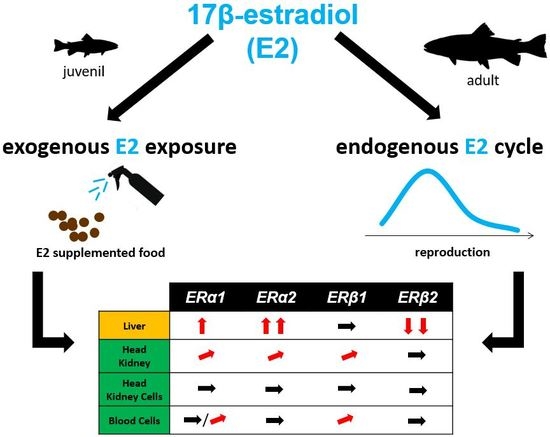
Immune-Specific Expression and Estrogenic Regulation of the Four Estrogen Receptor Isoforms in Female Rainbow Trout (Oncorhynchus mykiss)
Abstract
:1. Introduction
2. Results
2.1. Absolute Gene Transcript Levels of Erα1, α2, β1, and β2 in Immune Organs and Cells of Juvenile Rainbow Trout in Comparison to Liver ER Gene Transcript Levels
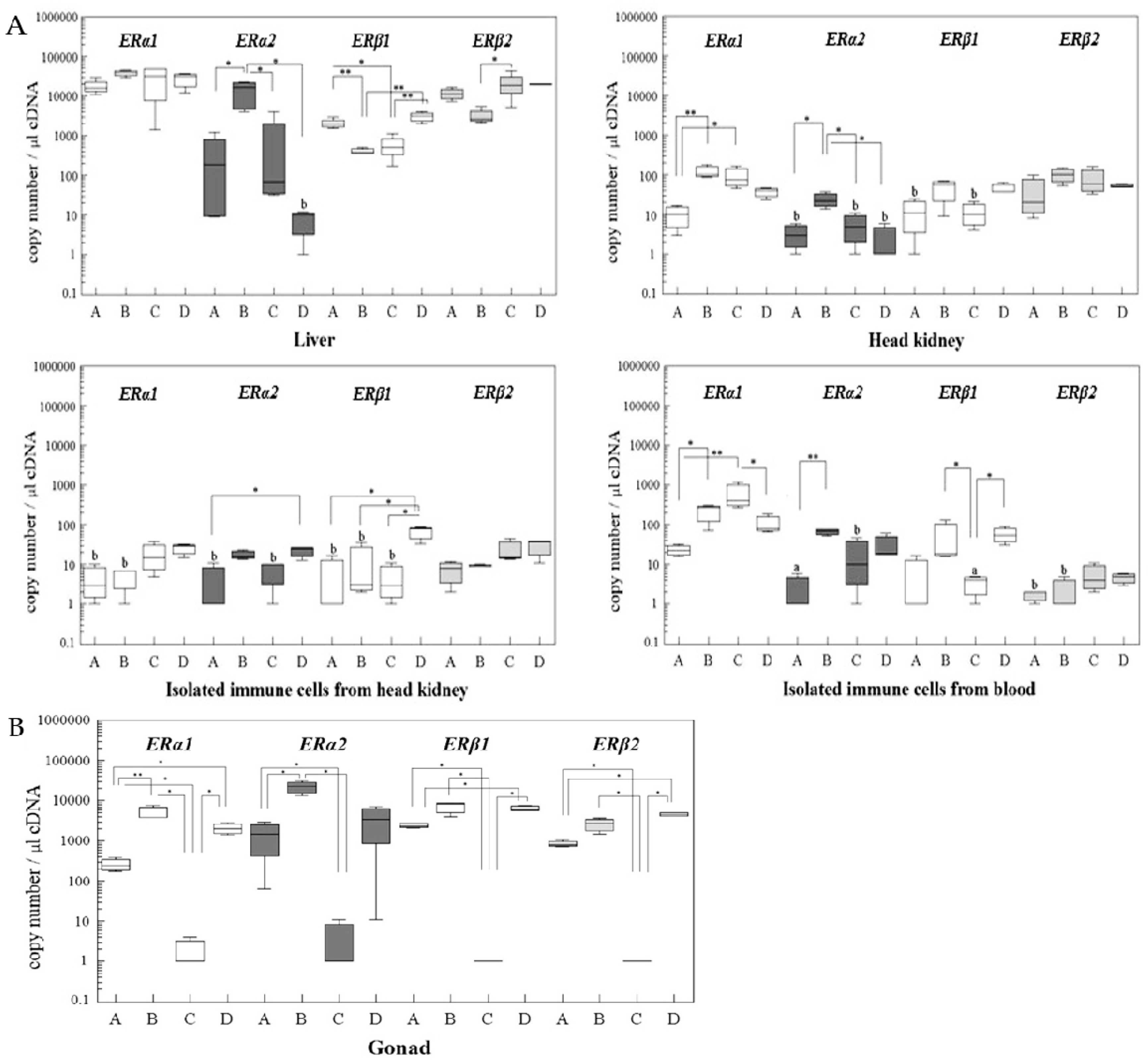
2.2. Changes of ER Gene Transcript Levels in Sexually Immature Juvenile Rainbow Trout Exposed to Exogenous E2
2.3. Changes of ER Gene Transcript Levels in Sexually Mature Adult Rainbow Trout Females during the Reproductive Cycle
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.1.1. Juvenile Rainbow Trout
4.1.2. Adult rainbow trout
4.2. Preparation of Samples and Immune Cell Isolation
4.3. RNA Extraction and Gene Expression Analysis
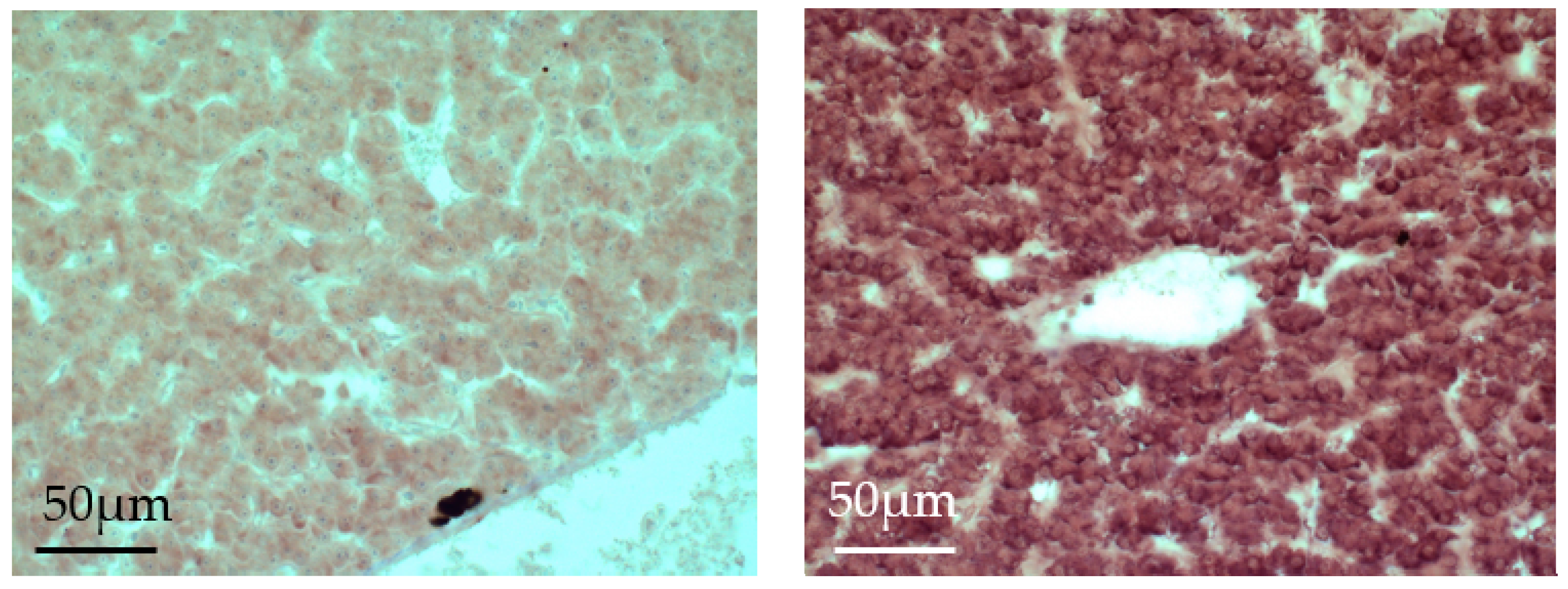
4.4. In Situ Hybridization
4.5. Competitive Enzyme-Linked Immunosorbent Assay (Celisa) to Determine 17β-Estradiol Concentrations in Serum
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hall, J.M.; Couse, J.F.; Korach, K.S. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J. Biol. Chem. 2001, 276, 36869–36872. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, S.; McArthur, S. Oestrogen and immunomodulation: New mechanisms that impact on peripheral and central immunity. Curr. Opin. Pharmacol. 2013, 13, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Ansar Ahmed, S. The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 2016, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Klein, S. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004, 26, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Nunn, C.L.; Lindenfors, P.; Pursall, E.R.; Rolff, J. On sexual dimorphism in immune function. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, M.; Gilkeson, G. Estrogen receptors in immunity and autoimmunity. Clin. Rev. Allergy Immunol. 2011, 40, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Kouro, T.; Yokota, T.; Kincade, P.W. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc. Natl. Acad. Sci. USA 2001, 98, 15131–15136. [Google Scholar] [CrossRef] [PubMed]
- Phiel, K.L.; Henderson, R.A.; Adelman, S.J.; Elloso, M.M. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 2005, 97, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Stygar, D.; Masironi, B.; Eriksson, H.; Sahlin, L. Studies on estrogen receptor (ER) α and β responses on gene regulation in peripheral blood leukocytes in vivo using selective ER agonists. J. Endocrinol. 2007, 194, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.C.; Curran, E.M.; Judy, B.M.; Milligan, G.N.; Lubahn, D.B.; Estes, D.M. Estrogen receptor α (ERα) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17β-estradiol acts through ERα to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J. Immunol. 2005, 175, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Tiwari-Woodruff, S.; Morales, L.B.J.; Lee, R.; Voskuhl, R.R. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER) α and ERβ ligand treatment. Proc. Natl. Acad. Sci. USA 2007, 104, 14813–14818. [Google Scholar] [CrossRef] [PubMed]
- Cvoro, A.; Tatomer, D.; Tee, M.-K.; Zogovic, T.; Harris, H.A.; Leitman, D.C. Selective estrogen receptor-β agonists repress transcription of proinflammatory genes. J. Immunol. 2008, 180, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.J.; Kovacs, W.J. Gonadal steroids and immunity. Endocr. Rev. 1996, 17, 369–384. [Google Scholar] [PubMed]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Gilliver, S.C.; Emmerson, E.; Campbell, L.; Chambon, P.; Hardman, M.J.; Ashcroft, G.S. 17β-Estradiol inhibits wound healing in male mice via estrogen receptor-α. Am. J. Pathol. 2010, 176, 2707–2721. [Google Scholar] [CrossRef] [PubMed]
- Beagley, K.W.; Gockel, C.M. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. Pathog. Dis. 2003, 38, 13–22. [Google Scholar] [CrossRef]
- Abrams, E.T.; Miller, E.M. The roles of the immune system in women’s reproduction: Evolutionary constraints and life history trade-offs. Am. J. Phys. Anthropol. 2011, 146, 134–154. [Google Scholar] [CrossRef] [PubMed]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Milla, S.; Depiereux, S.; Kestemont, P. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: A review. Ecotoxicology 2011, 20, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Cabas, I.; García-Ayala, A. Sex steroids modulate fish immune response. In Sex Steroids; InTech: London, UK, 2012. [Google Scholar]
- Segner, H.; Casanova-Nakayama, A.; Kase, R.; Tyler, C.R. Impact of environmental estrogens on fish considering the diversity of estrogen signaling. Gen. Comp. Endocrinol. 2013, 191, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Nakayama, A.; Wenger, M.; Burki, R.; Eppler, E.; Krasnov, A.; Segner, H. Endocrine disrupting compounds: Can they target the immune system of fish? Mar. Pollut. Bull. 2011, 63, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Iwanowicz, L.R.; Ottinger, C.A. Estrogens, estrogen receptors and their role as immunoregulators in fish. Fish Def. 2009, 1, 277–322. [Google Scholar]
- Shved, N.; Berishvili, G.; Häusermann, E.; D’cotta, H.; Baroiller, J.-F.; Eppler, E. Challenge with 17α-ethinylestradiol (EE2) during early development persistently impairs growth, differentiation, and local expression of IGF-I and IGF-II in immune organs of tilapia. Fish Shellfish Immunol. 2009, 26, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Chaves-Pozo, E.; Abellán, E.; Meseguer, J.; Mulero, V.; García-Ayala, A. 17β-Estradiol regulates gilthead seabream professional phagocyte responses through macrophage activation. Dev. Comp. Immunol. 2011, 35, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cabas, I.; Rodenas, M.C.; Abellán, E.; Meseguer, J.; Mulero, V.; García-Ayala, A. Estrogen Signaling through the G Protein–Coupled Estrogen Receptor Regulates Granulocyte Activation in Fish. J. Immunol. 2013, 191, 4628–4639. [Google Scholar] [CrossRef] [PubMed]
- Massart, S.; Milla, S.; Kestemont, P. Expression of gene, protein and immunohistochemical localization of the estrogen receptor isoform ERα1 in male rainbow trout lymphoid organs; indication of the role of estrogens in the regulation of immune mechanisms. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 174, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Shelley, L.K.; Osachoff, H.L.; van Aggelen, G.C.; Ross, P.S.; Kennedy, C.J. Alteration of immune function endpoints and differential expression of estrogen receptor isoforms in leukocytes from 17β-estradiol exposed rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2013, 180, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Szwejser, E.; Maciuszek, M.; Casanova-Nakayama, A.; Segner, H.; Verburg-van Kemenade, B.L.; Chadzinska, M. A role for multiple estrogen receptors in immune regulation of common carp. Dev. Comp. Immunol. 2017, 66, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Moggs, J.G.; Orphanides, G. Estrogen receptors: Orchestrators of pleiotropic cellular responses. EMBO Rep. 2001, 2, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Guyre, P.M.; Wira, C.R.; Pioli, P.A. Estradiol regulates expression of estrogen receptor ERα46 in human macrophages. PLoS ONE 2009, 4, e5539. [Google Scholar] [CrossRef] [PubMed]
- Bagamasbad, P.; Denver, R.J. Mechanisms and significance of nuclear receptor auto-and cross-regulation. Gen. Comp. Endocrinol. 2011, 170, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Habibi, H.R. Estrogen receptor function and regulation in fish and other vertebrates. Gen. Comp. Endocrinol. 2013, 192, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Tecalco-Cruz, A.C.; Ramírez-Jarquín, J.O. Mechanisms that increase stability of estrogen receptor alpha in breast cancer. Clin. Breast Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Slingerland, J.M. Links between oestrogen receptor activation and proteolysis: Relevance to hormone-regulated cancer therapy. Nat. Rev. Cancer 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; Lacroix, M.; Laïos, I.; Laurent, G. Estrogen receptor alpha: Impact of ligands on intracellular shuttling and turnover rate in breast cancer cells. Curr. Cancer Drug Targets 2006, 6, 39–64. [Google Scholar] [CrossRef] [PubMed]
- Iwanowicz, L.R.; Stafford, J.L.; Patiño, R.; Bengten, E.; Miller, N.W.; Blazer, V.S. Channel catfish (Ictalurus punctatus) leukocytes express estrogen receptor isoforms ERα and ERβ2 and are functionally modulated by estrogens. Fish Shellfish Immunol. 2014, 40, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Nagler, J.J.; Cavileer, T.; Sullivan, J.; Cyr, D.G.; Rexroad, C. The complete nuclear estrogen receptor family in the rainbow trout: Discovery of the novel ERα2 and both ERβ isoforms. Gene 2007, 392, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Szwejser, E.; Pijanowski, L.; Maciuszek, M.; Ptak, A.; Wartalski, K.; Duda, M.; Segner, H.; Verburg-van Kemenade, B.L.; Chadzinska, M. Stress differentially affects the systemic and leukocyte estrogen network in common carp. Fish Shellfish Immunol. 2017, 68, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Stygar, D.; Westlund, P.; Eriksson, H.; Sahlin, L. Identification of wild type and variants of oestrogen receptors in polymorphonuclear and mononuclear leucocytes. Clin. Endocrinol. 2006, 64, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Yakimchuk, K.; Jondal, M.; Okret, S. Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. Mol. Cell. Endocrinol. 2013, 375, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McMurray, R.W. Effects of estrogen receptor subtype-selective agonists on immune functions in ovariectomized mice. Int. Immunopharmacol. 2006, 6, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Gerhold, L.M.; Böttner, M.; Rau, S.W.; Dela Cruz, C.; Yang, E.; Zhu, H.; Yu, J.; Cashion, A.B.; Kindy, M.S. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors α and β. J. Comp. Neurol. 2007, 500, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.I.S.; Estevao, M.D.; Redruello, B.; Socorro, S.M.; Canario, A.V.M.; Power, D.M. Immunohistochemical detection of estrogen receptors in fish scales. Gen. Comp. Endocrinol. 2009, 160, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Stellato, C.; Porreca, I.; Cuomo, D.; Tarallo, R.; Nassa, G.; Ambrosino, C. The “busy life” of unliganded estrogen receptors. Proteomics 2016, 16, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Van den Hurk, R.; Lambert, J. Temperature and steroid effects on gonadal sex differentiation in rainbow trout. In Proceedings of the International Symposium on Reproductive Physiology of Fish, Wageningen, The Netherlands, 2–6 August 1982; pp. 69–72. [Google Scholar]
- Feist, G.; Schreck, C.B.; Fitzpatrick, M.S.; Redding, J.M. Sex steroid profiles of coho salmon (Oncorhynchus kisutch) during early development and sexual differentiation. Gen. Comp. Endocrinol. 1990, 80, 299–313. [Google Scholar] [CrossRef]
- Marlatt, V.L.; Lakoff, J.; Crump, K.; Martyniuk, C.J.; Watt, J.; Jewell, L.; Atkinson, S.; Blais, J.M.; Sherry, J.; Moon, T.W. Sex-and tissue-specific effects of waterborne estrogen on estrogen receptor subtypes and E2-mediated gene expression in the reproductive axis of goldfish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Szwejser, E.; Verburg-van Kemenade, B.L.; Maciuszek, M.; Chadzinska, M. Estrogen-dependent seasonal adaptations in the immune response of fish. Horm. Behav. 2017, 88, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, F.; Le Guellec, C.; Vaillant, C.; Le Roux, M.G.; Valotaire, Y. Identification and estrogen induction of two estrogen receptors (ER) messenger ribonucleic acids in the rainbow trout liver: Sequence homology with other ERs. Mol. Endocrinol. 1989, 3, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Molero, L.; García-Durán, M.; Diaz-Recasens, J.; Rico, L.; Casado, S.; López-Farré, A. Expression of estrogen receptor subtypes and neuronal nitric oxide synthase in neutrophils from women and men: Regulation by estrogen. Cardiovasc. Res. 2002, 56, 43–51. [Google Scholar] [CrossRef]
- Menuet, A.; Le Page, Y.; Torres, O.; Kern, L.; Kah, O.; Pakdel, F. Analysis of the estrogen regulation of the zebrafish estrogen receptor (ER) reveals distinct effects of ERα, ERβ1 and ERβ2. J. Mol. Endocrinol. 2004, 32, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Sabo-Attwood, T.; Kroll, K.J.; Denslow, N.D. Differential expression of largemouth bass (Micropterus salmoides) estrogen receptor isotypes alpha, beta, and gamma by estradiol. Mol. Cell. Endocrinol. 2004, 218, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Filby, A.; Tyler, C. Molecular characterization of estrogen receptors 1, 2a, and 2b and their tissue and ontogenic expression profiles in fathead minnow (Pimephales promelas). Biol. Reprod. 2005, 73, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Boyce-Derricott, J.; Nagler, J.J.; Cloud, J.G. Variation among Rainbow Trout (Oncorhynchus mykiss) Estrogen Receptor Isoform 3′ Untranslated Regions and the Effect of 17β-Estradiol on mRNA Stability in Hepatocyte Culture. DNA Cell Biol. 2010, 29, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, V.; Martyniuk, C.; Zhang, D.; Xiong, H.; Watt, J.; Xia, X.; Moon, T.; Trudeau, V. Auto-regulation of estrogen receptor subtypes and gene expression profiling of 17β-estradiol action in the neuroendocrine axis of male goldfish. Mol. Cell. Endocrinol. 2008, 283, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Grčević, M.; Kralik, Z.; Kralik, G.; Galović, D.; Pavić, M. The effect of lutein additives on biochemical parameters in blood of laying hens. Poljoprivreda 2016, 22, 34–38. [Google Scholar] [CrossRef]
- Chandrasekar, G.; Archer, A.; Gustafsson, J.-Å.; Lendahl, M.A. Levels of 17β-estradiol receptors expressed in embryonic and adult zebrafish following in vivo treatment of natural or synthetic ligands. PLoS ONE 2010, 5, e9678. [Google Scholar] [CrossRef] [PubMed]
- Shelley, L.K.; Ross, P.S.; Kennedy, C.J. The effects of an in vitro exposure to 17β-estradiol and nonylphenol on rainbow trout (Oncorhynchus mykiss) peripheral blood leukocytes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Dulos, J.; Vijn, P.; van Doorn, C.; Hofstra, C.L.; Veening-Griffioen, D.; de Graaf, J.; Dijcks, F.A.; Boots, A.M. Suppression of the inflammatory response in experimental arthritis is mediated via estrogen receptor α but not estrogen receptor β. Arthritis Res. Ther. 2010, 12, R101. [Google Scholar] [CrossRef] [PubMed]
- Segner, H.; Verburg-van Kemenade, B.L.; Chadzinska, M. The immunomodulatory role of the hypothalamus-pituitary-gonad axis: Proximate mechanism for reproduction-immune trade offs? Dev. Comp. Immunol. 2017, 66, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.J.; Butler, L.D.; Petersen, B.H. Estradiol-induced alteration in the immune system. II. Suppression of cellular immunity in the rat is not the result of direct estrogenic action. Immunopharmacology 1986, 11, 47–55. [Google Scholar] [CrossRef]
- McMurray, R.W. Estrogen, prolactin, and autoimmunity: Actions and interactions. Int. Immunopharmacol. 2001, 1, 995–1008. [Google Scholar] [CrossRef]
- Lang, T.J. Estrogen as an immunomodulator. Clin. Immunol. 2004, 113, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Giefing-Kröll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Burki, R.; Krasnov, A.; Bettge, K.; Rexroad, C.E.; Afanasyev, S.; Antikainen, M.; Burkhardt-Holm, P.; Wahli, T.; Segner, H. Pathogenic infection confounds induction of the estrogenic biomarker vitellogenin in rainbow trout. Environ. Toxicol. Chem. 2012, 31, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
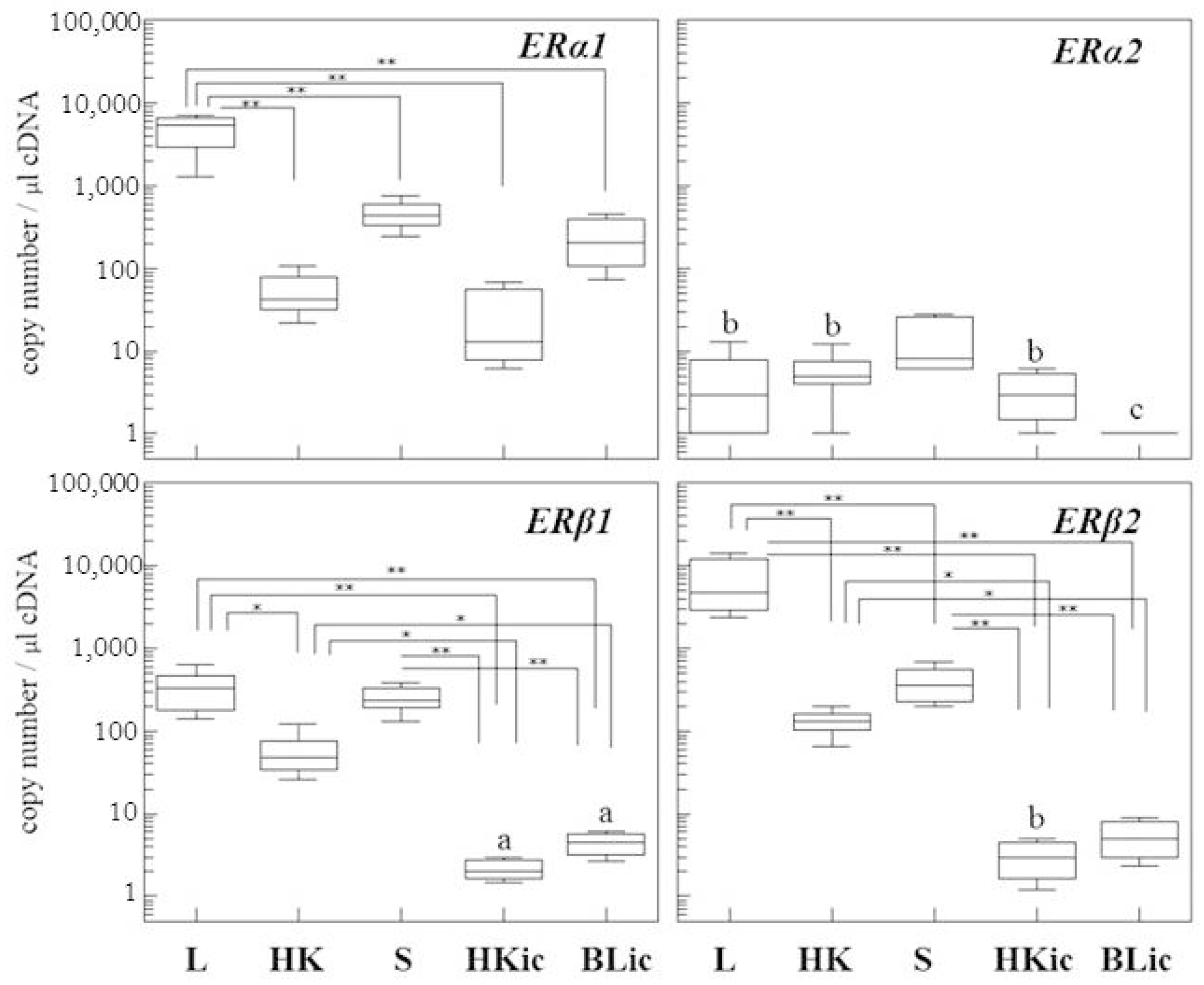


| Organ | Liver | Head Kidney | Spleen | HK Leukocytes | Blood Leukocytes |
|---|---|---|---|---|---|
| Ratio | Ratio ERα1 mRNA to Other Isoforms | Ratio ERα1 mRNA to Other Isoforms | Ratio ERα1 mRNA to Other Isoforms | Ratio ERα1 mRNA to Other Isoforms | Ratio ERα1 mRNA to Other Isoforms |
| ERα1 mRNA | 1 | 1 | 1 | 1 | 1 |
| ERα2 mRNA | 994 | 10 | 30 | 10 | 450 |
| ERβ1 mRNA | 14 | 1 | 2 | 10 | 110 |
| ERβ2 mRNA | 0.7 | 0.5 | 1.1 | 10 | 110 |
| ER Isoforms | Liver | Head Kidney Leukocytes | Blood Leukocytes |
|---|---|---|---|
| ERα1 | 0.138 ↑ | 0.039 ↑ | 0.044 ↑ |
| ERα2 | 0.241 ↑ | 0.203 ↑ | 0.014 ↑ |
| ERβ1 | 0.282 ↓ | 0.282 ↑ | 0.009 ↓ |
| ERβ2 | 0.019 ↓ | 0.054 ↓ | 0.041 ↓ |
| Gene | Sequence (5′-3′) | Accession No. | |
|---|---|---|---|
| ERα1 | Forward | CCCCCCAAGCCACCAT | AJ242741 |
| Reverse | TGATTGGTTACCACACTCGACCTATAT | ||
| Probe | CATACTACCTGGAGACCTCGTCCACACCC | ||
| ERα2 | Forward | TCCTGGAGCACAGCAAAGC | DQ177438 |
| Reverse | TGATCTTGAGACGCCCTTCTC | ||
| Probe | CCTCAGGACAGTAGCAAGAACAGCAGCTTC | ||
| ERβ1 | Forward | GGAGCGAGCCAATCAAGGA | DQ177439 |
| Reverse | GCCATGATCCGGCCAAT | ||
| Probe | TCTGCCCCACAGTATTAACCCCGGA | ||
| ERβ2 | Forward | CAGCTCCTGCTGTAGACACTCAGT | DQ248229 |
| Reverse | GGATGTACTAATGCTCTCGAGTGTTT | ||
| Probe | TGCTAACATTCCAAAACCCAGAGGAGAGC | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova-Nakayama, A.; Wernicke von Siebenthal, E.; Kropf, C.; Oldenberg, E.; Segner, H. Immune-Specific Expression and Estrogenic Regulation of the Four Estrogen Receptor Isoforms in Female Rainbow Trout (Oncorhynchus mykiss). Int. J. Mol. Sci. 2018, 19, 932. https://doi.org/10.3390/ijms19040932
Casanova-Nakayama A, Wernicke von Siebenthal E, Kropf C, Oldenberg E, Segner H. Immune-Specific Expression and Estrogenic Regulation of the Four Estrogen Receptor Isoforms in Female Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences. 2018; 19(4):932. https://doi.org/10.3390/ijms19040932
Chicago/Turabian StyleCasanova-Nakayama, Ayako, Elena Wernicke von Siebenthal, Christian Kropf, Elisabeth Oldenberg, and Helmut Segner. 2018. "Immune-Specific Expression and Estrogenic Regulation of the Four Estrogen Receptor Isoforms in Female Rainbow Trout (Oncorhynchus mykiss)" International Journal of Molecular Sciences 19, no. 4: 932. https://doi.org/10.3390/ijms19040932
APA StyleCasanova-Nakayama, A., Wernicke von Siebenthal, E., Kropf, C., Oldenberg, E., & Segner, H. (2018). Immune-Specific Expression and Estrogenic Regulation of the Four Estrogen Receptor Isoforms in Female Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences, 19(4), 932. https://doi.org/10.3390/ijms19040932