Identification, Expression Analysis of the Hsf Family, and Characterization of Class A4 in Sedum Alfredii Hance under Cadmium Stress
Abstract
:1. Introduction
2. Results
2.1. Twenty Two SaHsf Members were Identified and Classified into Three Classes
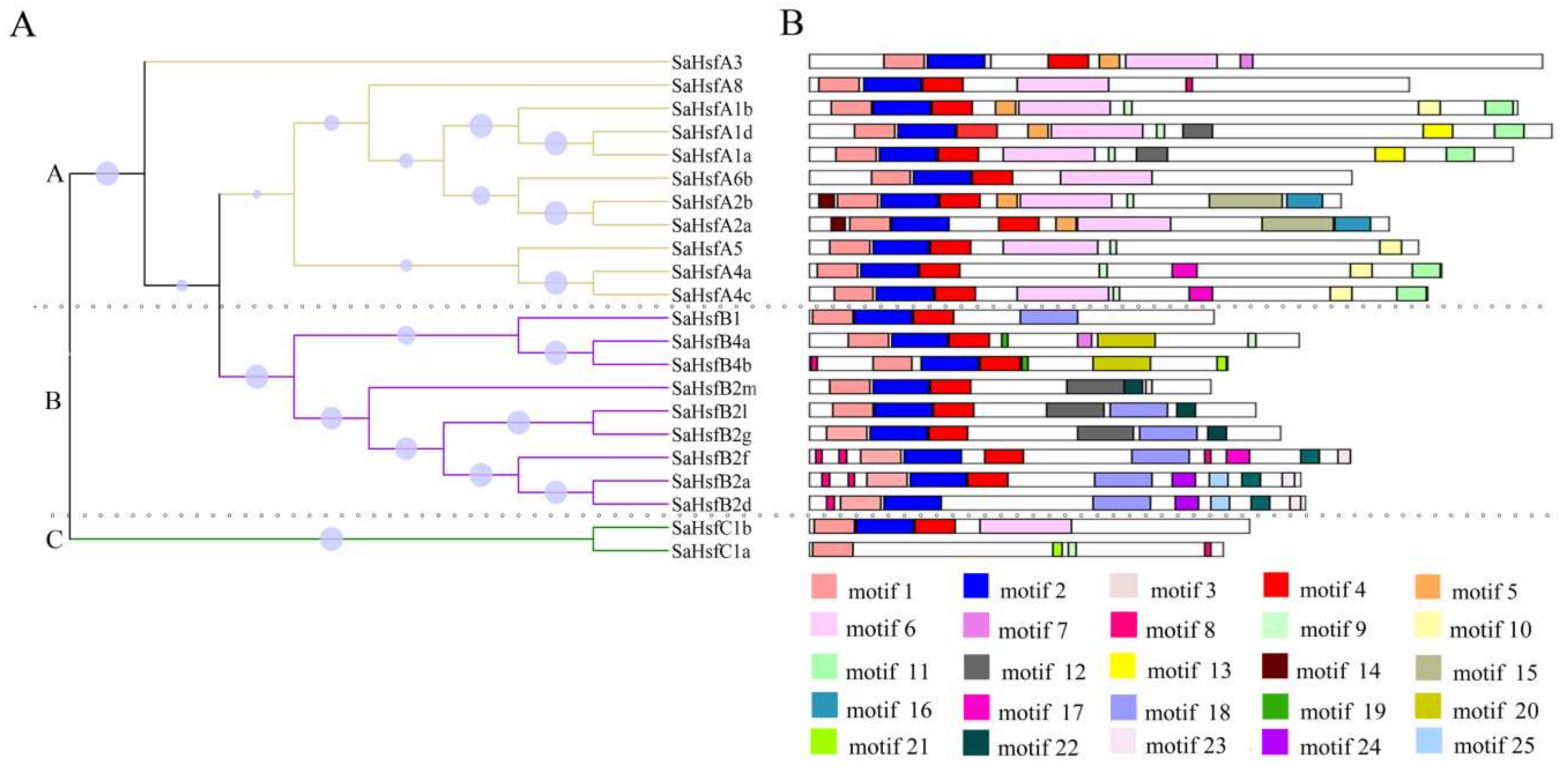
2.2. Phylogenetic Analysis of Hsfs in S. alfredii
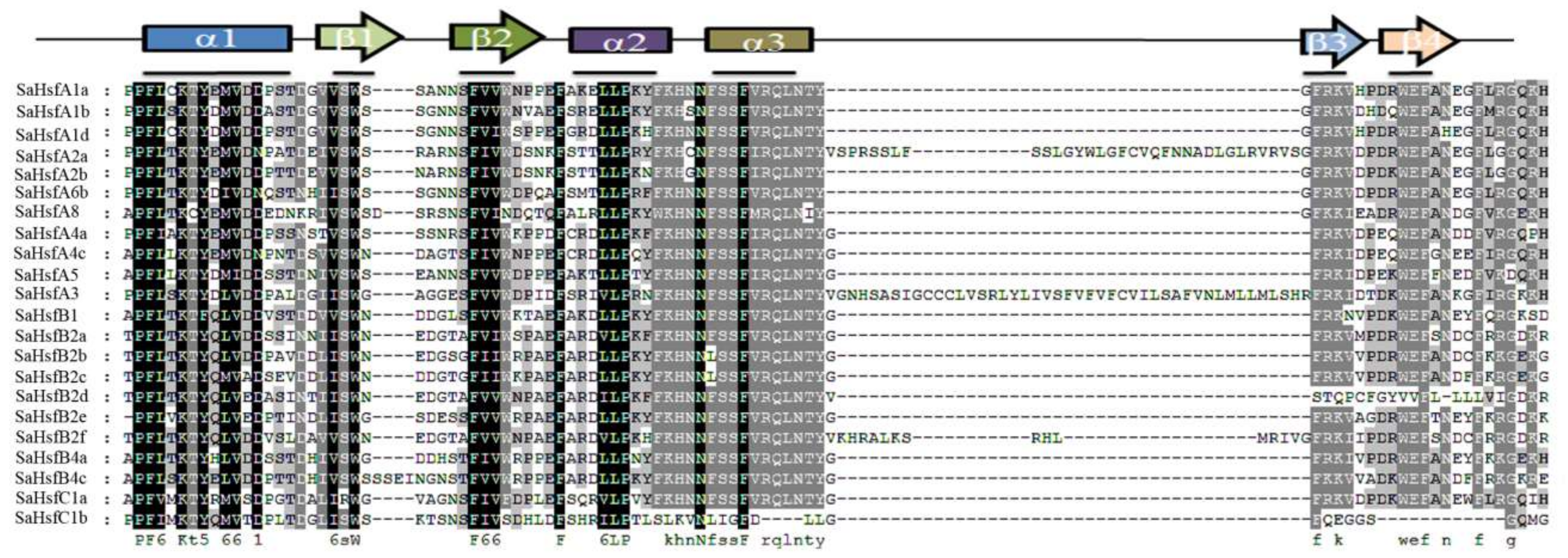
2.3. Conserved Domains and Motifs in SaHsf Proteins
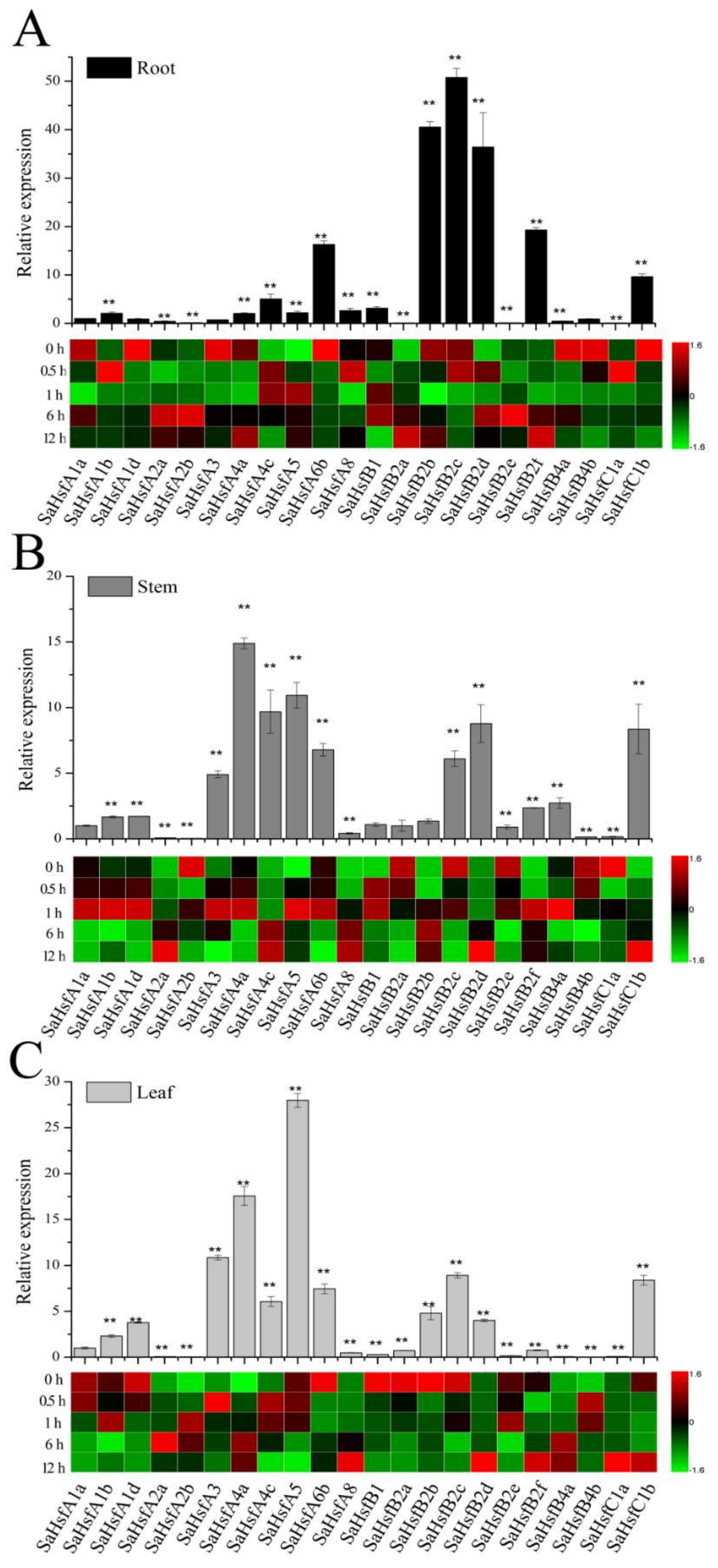
2.4. Expression Profiles of SaHsfs
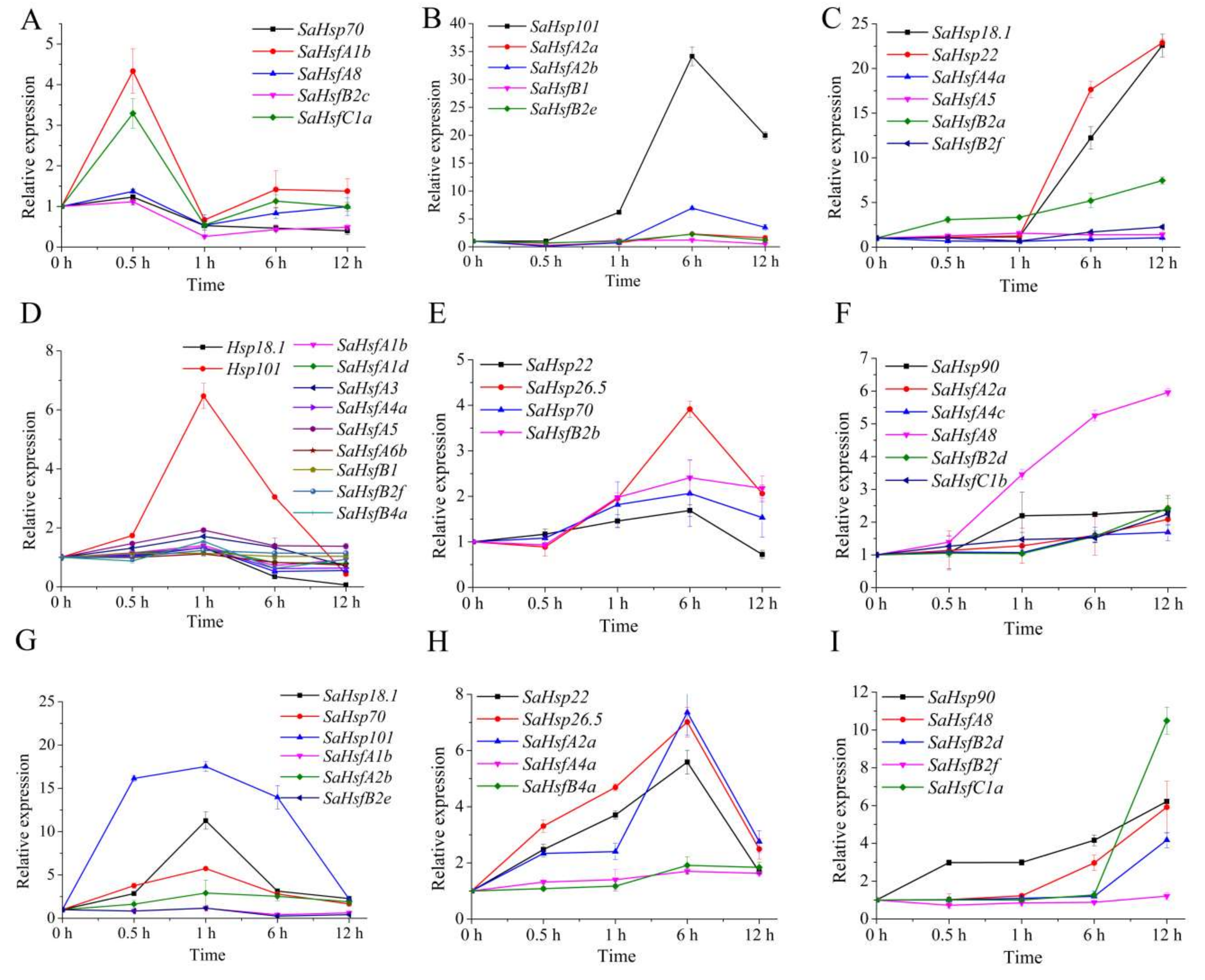
2.5. SaHsfs and Their Downstream Genes, SaHsps, Exhibited Similar Expression Patterns
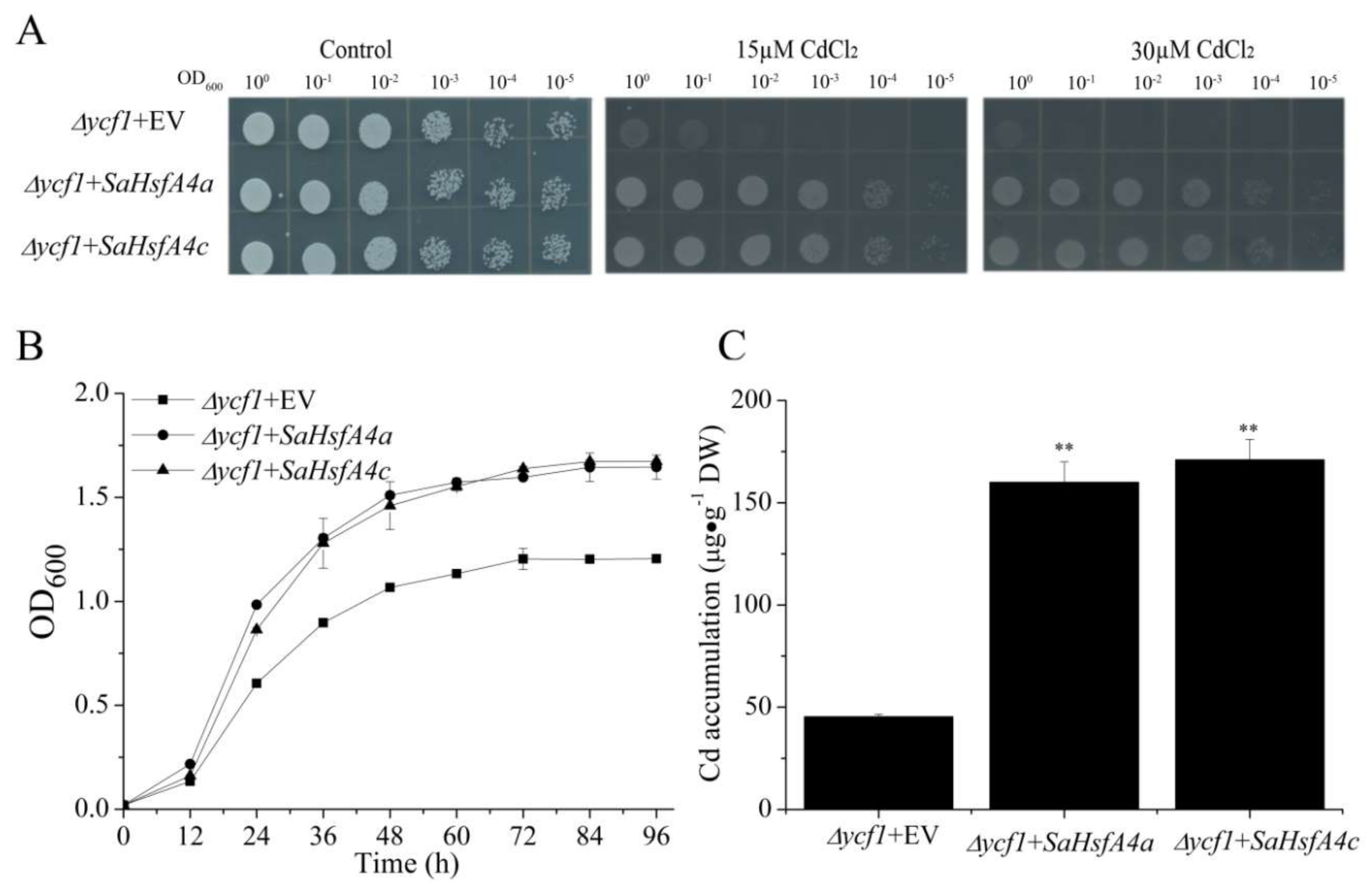
2.6. Class SaHsfA4’ Expression Enhanced Cd Tolerance in Yeast
3. Discussion
4. Methods
4.1. Plant Materials and Stress Treatments
4.2. Identification of SaHsfs in S. alfredii
4.3. Multiple Sequence Alignment, Conserved Motif and Domain Prediction
4.4. Phylogenetic Analysis and Classification of SaHsf Genes
4.5. Total RNA Isolation and Expression Analysis
4.6. Heterologous Expression of SaHsfA4a and SaHsfA4c in Yeast
4.7. Transcriptional Activation Activity Assay of Two SaHsfA4 Members in Yeast
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| aa | amino acid |
| ABA | abscisic acid |
| bp | base pair |
| kDa | kilodalton |
| pI | isoelectric points |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| SG-U | synthetic-galactose-uracil |
| X-α-gal | 5-bromo-4-chloro-3-indoxyl-α-d-galacto-pyranoside |
| SD | synthetic dropout nutrient medium |
| SD/Trp− | SD medium lacking tryptophan |
| SD/Trp−His− | SD medium lacking tryptophan and histidine |
References
- Wang, M.; Vannozzi, A.; Wang, G.; Liang, Y.H.; Tornielli, G.B.; Zenoni, S.; Cavallini, E.; Pezzotti, M.; Cheng, Z.M. Genome and transcriptome analysis of the grapevine (Vitis vinifera L.) WRKY gene family. Hortic. Res. 2014, 1, 14016. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.H.; Ma, X.; Luo, D.X.; Gong, Z.H.; Lu, M.H. The plant heat stress transcription factors (Hsfs): Structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Shi, X.; Chen, S.Y.; Ma, C.; Xu, S.B. Evolutionary origin, gradual accumulation and functional divergence of heat shock factor gene family with plant evolution. Front. Plant Sci. 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Yi, M.; Xu, L.; Zhu, X.W.; Wang, Y.; Guo, J.; Liu, L.W. Genome-wide characterization of differentially expressed genes provides insights into regulatory network of heat stress response in radish (Raphanus sativus L.). Funct. Integr. Genom. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gomez-pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta 2012, 1819, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Ganguli, A.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K.D. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. (Bangalore) 2004, 29, 471–487. [Google Scholar] [CrossRef]
- Nakai, A. Heat Shock Factor; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Sémon, M.; Wolfe, K.H. Consequences of genome duplication. Curr. Opin. Genet. Dev. 2007, 17, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Zhu, W.; Song, X.; Lin, X.; Cai, J.; Wang, M.; Yang, Q. Genome-wide identification and function analyses of heat shock transcription factors in potato. Front. Plant Sci. 2016, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, J.; Ji, Q.; Wang, C.; Luo, L.; Yuan, Y.; Wang, Y.; Wang, J. Genome-wide analysis of heat shock transcription factor families in rice and Arabidopsis. J. Genet. Genom. 2008, 35, 105–118. [Google Scholar] [CrossRef]
- Wang, F.; Dong, Q.; Jiang, H.; Zhu, S.; Chen, B.; Xiang, Y. Genome-wide analysis of the heat shock transcription factors in Populus trichocarpa and Medicago truncatula. Mol. Biol. Rep. 2012, 39, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Kim, K.M.; Lee, J.H. Genome-wide analysis and molecular characterization of heat shock transcription factor family in Glycine max. J. Genet. Genom. 2013, 40, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Jiang, H.Y.; Chu, Z.X.; Tang, X.L.; Zhu, S.W.; Cheng, B.J. Genome-wide identification, classification and analysis of heat shock transcription factor family in maize. BMC Genom. 2011, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.P.; Sadat, S.; Drenth, J.; McIntyre, C.L. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J. Exp. Bot. 2014, 65, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, B.B.; Li, J.B.; Zhang, L.; Wang, Y.; Zheng, H.Q.; Lu, M.Z.; Chen, J. Hsf and Hsp gene families in Populus: Genome-wide identification, organization and correlated expression during development and in stress responses. BMC Genom. 2015, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Nover, L.; Bharti, K.; Döring, P.; Mishra, S.K.; Ganguli, A.; Scharf, K.D. Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperones 2001, 6, 177–189. [Google Scholar] [PubMed]
- Wang, C.; Zhang, Q.; Shou, H.X. Identification and expression analysis of OsHsfs in rice. J. Zhejiang Univ. Sci. B 2009, 10, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, H.; Khurana, N.; Agarwal, P.; Khurana, P. Heat shock factors in rice (Oryza sativa L.): Genome-wide expression analysis during reproductive development and abiotic stress. Mol. Genet. Genom. 2011, 286, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Mittler, R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006, 98, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, N.; Ichikawa, T.; Kondou, Y.; Matsui, M.; Hirochika, H.; Iwabuchi, M.; Oda, K. Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta 2008, 227, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Niu, C.Y.; Yang, C.R.; Jinn, T.L. The heat stress factor HsfA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol. 2016, 172, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.; Hwang, J.U.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 2009, 21, 4031–4043. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.E.; Long, X.X.; Ni, W.Z.; Fu, C.X. Sedum alfredii H: A new Zn hyperaccumulating plant first found in China. Chin. Sci. Bull. 2002, 47, 1634–1637. [Google Scholar] [CrossRef]
- Sun, Q.; Ye, Z.H.; Wang, X.R.; Wong, M.H. Cadmium hyperaccumulation leads to an increase of glutathione rather than phytochelatins in the cadmium hyperaccumulator Sedum alfredii. J. Plant Physiol. 2007, 164, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Tian, S.K.; Yang, X.E.; Wang, X.C.; Brown, P.H.; Li, T.Q.; He, Z.L. Enhanced root-to-shoot translocation of cadmium in the hyperaccumulating ecotype of Sedum alfredii. J. Exp. Bot. 2008, 59, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.K.; Xie, R.H.; Wang, H.X.; Hu, Y.; Ge, J.; Liao, X.C.; Gao, X.Y.; Brown, P.H; Lin, X.Y.; Lu, L.L. Calcium deficiency triggers phloem remobilization of cadmium in a hyperaccumulating species. Plant Physiol. 2016, 172, 2300–2313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Tian, S.K.; Lu, L.L.; Shohag, M.J.I.; Yang, X.E. Metallothionein 2 (SaMT2) from Sedum alfredii Hance confers increased Cd tolerance and accumulation in yeast and tobacco. PLoS ONE 2014, 9, e102750. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, X.J.; Song, X.X.; Zhang, Y.X.; Jiang, J.; Han, Q.; Liu, M.Y.; Qiao, G.R.; Zhuo, R.Y. Overexpressing the Sedum alfredii Cu/Zn superoxide dismutase increased resistance to oxidative stress in transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 1010. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Qiu, W.M.; He, X.L.; Zheng, L.; Song, X.X.; Han, X.J.; Jiang, J.; Qiao, G.R.; Sang, J.; Liu, M.Q.; et al. Functional characterization of a gene in Sedum alfredii Hance resembling rubber elongation factor endowed with functions associated with cadmium tolerance. Front. Plant Sci. 2016, 7, 965. [Google Scholar] [CrossRef] [PubMed]
- Han, X.J.; Yin, H.F.; Song, X.X.; Zhang, Y.X.; Liu, M.Y.; Sang, J.; Jiang, J.; Li, J.H.; Zhuo, R.Y. Integration of small RNAs, degradome and transcriptome sequencing in hyperaccumulator Sedum alfredii uncovers a complex regulatory network and provides insights into cadmium phytoremediation. Plant Biotechnol. J. 2016, 14, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan, P.; Jagannadham, P.T.K.; Satheesh, V.; Kohli, D.; Basavarajappa, S.H.; Chellapilla, B.; Kumar, J.; Jain, P.K.; Srinivasan, R. Genome-wide analysis identifies Chickpea (Cicer arietinum) heat stress transcription factors (Hsfs) responsive to heat stress at the pod development stage. J. Plant Res. 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, J.P.; Zhai, Y.F.; Chai, W.G.; Gong, Z.H.; Lu, M.H. Genome-wide analysis, expression profile of heat shock factor gene family (CaHsfs) and characterisation of CaHsfA2 in pepper (Capsicum annuum L.). BMC Plant Biol. 2015, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Zheng, Q.Q.; Chen, L.; Liang, Y.N.; Wu, J.D. Ectopic overexpression of maize heat shock transcription factor gene ZmHsf04 confers increased thermo and salt-stress tolerance in transgenic Arabidopsis. Acta Physiol. Plant. 2018, 40, 9. [Google Scholar] [CrossRef]
- Lin, Z.; Kong, H.; Nei, M.; Ma, H. Origins and evolution of the recA/RAD51 gene family: Evidence for ancient gene duplication and endosymbiotic gene transfer. Proc. Natl. Acad. Sci. USA 2006, 103, 10328–10333. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.Y.; Lin, L.F.; Jheng, J.L.; Wang, C.C.; Yang, J.H.; Chou, M.L. Identification of heat shock transcription factor genes involved in thermotolerance of octoploid cultivated strawberry. Int. J. Mol. Sci. 2016, 17, 2130. [Google Scholar] [CrossRef] [PubMed]
- von Koskull-Doring, P.; Scharf, K.D.; Nover, L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007, 12, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Hua, J. From freezing to scorching, transcriptional responses to temperature variations in plants. Curr. Opin. Plant Biol. 2009, 12, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Huang, W.L.; Liu, J.; Yang, Z.M.; Huang, B.R. Molecular regulation and physiological functions of a novel FaHsfA2c cloned from tall fescue conferring plant tolerance to heat stress. Plant Biotechnol. J. 2017, 15, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.L.; Cheng, H.; Cheng, S.Y.; Li, L.L.; Xu, F.; Yu, W.W.; Yuan, H.H. Expression of selected ginkgo biloba heat shock protein genes after cold treatment could be induced by other abiotic stress. Int. J. Mol. Sci. 2012, 13, 5768–5788. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, H.R.; Zhao, H.S.; Gao, T.W.; Song, A.P.; Jiang, J.F.; Chen, F.D.; Chen, S.M. Chrysanthemum CmHsfA4 gene positively regulates salt stress tolerance in transgenic chrysanthemum. Plant Biotechnol. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.G.; Ye, C.J.; Lu, H.Y.; Chen, X.J.; Chai, G.H.; Chen, J.N.; Wang, C. Identification and characterization of a novel heat shock transcription factor gene, GmHsfA1, in soybeans (Glycine max). J. Plant Res. 2006, 119, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Davletova, S.; Mittler, R. The zinc-finger protein ZAT12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005, 139, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Personat, J.M.; Tejedor-Cano, J.; Prieto-Dapena, P.; Almoguera, C.; Jordano, J. Co-overexpression of two heat shock factors results in enhanced seed longevity and in synergistic effects on seedling tolerance to severe dehydration and oxidative stress. BMC Plant Biol. 2014, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Xie, Y.B.; Ma, J.Y.; Luo, X.T.; Nie, P.; Zuo, Z.X.; Lahrmann, U.; Zhao, Q.; Zheng, Y.Y.; Zhao, Y.; et al. IBS: An illustrator for the presentation and visualization of biological sequences. Bioinformatics 2015, 31, 3359. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Han, X.J.; Fang, J.; Lu, Z.C.; Qiu, W.M.; Liu, M.Y.; Sang, J.; Jiang, J.; Zhuo, R.Y. Sedum alfredii SaNramp6 metal transporter contributes to cadmium accumulation in transgenic Arabidopsis thaliana. Sci. Rep. 2017, 7, 13318. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Han, X.J.; Liu, M.Y.; Qiao, G.R.; Jiang, J.; Zhuo, R.Y. Selection and validation of reference genes for real-time quantitative PCR in hyperaccumulating ecotype of Sedum alfredii under different heavy metals stresses. PLoS ONE 2013, 8, e82927. [Google Scholar] [CrossRef] [PubMed]
- Szczypka, M.S.; Wemmie, J.A.; Moye-Rowley, W.S.; Thiele, D.J. A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J. Biol. Chem. 1994, 269, 22853–25857. [Google Scholar] [PubMed]

| Number | Gene Name | ORF Length (bp) | No. of AA | Mol. Wt. (kDa) | pI |
|---|---|---|---|---|---|
| 1 | SaHsfA1a | 1482 | 493 | 55.11 | 4.66 |
| 2 | SaHsfA1b | 1491 | 496 | 54.84 | 5.02 |
| 3 | SaHsfA1d | 1563 | 520 | 57.42 | 4.65 |
| 4 | SaHsfA2a | 1221 | 406 | 45.82 | 4.95 |
| 5 | SaHsfA2b | 1122 | 373 | 42.69 | 4.43 |
| 6 | SaHsfA3 | 1545 | 514 | 57.75 | 5.04 |
| 7 | SaHsfA4a | 1332 | 443 | 50.10 | 5.36 |
| 8 | SaHsfA4c | 1302 | 433 | 49.32 | 6.20 |
| 9 | SaHsfA5 | 1284 | 427 | 47.53 | 5.45 |
| 10 | SaHsfA6b | 1143 | 380 | 43.33 | 6.69 |
| 11 | SaHsfA8 | 1263 | 420 | 48.63 | 4.89 |
| 12 | SaHsfB1 | 855 | 284 | 31.83 | 4.50 |
| 13 | SaHsfB2a | 1038 | 345 | 37.49 | 6.91 |
| 14 | SaHsfB2b | 942 | 313 | 34.47 | 4.62 |
| 15 | SaHsfB2c | 993 | 330 | 36.52 | 4.99 |
| 16 | SaHsfB2d | 1047 | 348 | 37.61 | 5.35 |
| 17 | SaHsfB2e | 849 | 282 | 31.99 | 7.89 |
| 18 | SaHsfB2f | 1140 | 379 | 41.08 | 8.15 |
| 19 | SaHsfB4a | 1032 | 343 | 39.57 | 6.74 |
| 20 | SaHsfB4b | 885 | 294 | 33.81 | 7.47 |
| 21 | SaHsfC1a | 930 | 309 | 35.11 | 5.45 |
| 22 | SaHsfC1b | 873 | 290 | 32.64 | 6.73 |
| Class | Subclass | A. thaliana | O. sativa | P. trichocarpa | Z. mays | S. alfredii |
|---|---|---|---|---|---|---|
| A | A1 | 4 | 1 | 3 | 2 | 2 |
| A2 | 1 | 5 | 1 | 2 | 3 | |
| A3 | 1 | 1 | 1 | 1 | 1 | |
| A4 | 2 | 2 | 3 | 3 | 2 | |
| A5 | 1 | 1 | 2 | 1 | 1 | |
| A6 | 2 | 2 | 2 | 2 | 1 | |
| A7 | 2 | 0 | 2 | 2 | 0 | |
| A8 | 1 | 0 | 2 | 2 | 1 | |
| A9 | 1 | 1 | 1 | 0 | 0 | |
| B | B1 | 1 | 1 | 1 | 2 | 1 |
| B2 | 2 | 3 | 3 | 4 | 6 | |
| B3 | 1 | 0 | 2 | 0 | 0 | |
| B4 | 1 | 4 | 4 | 1 | 2 | |
| C | C1 | 1 | 2 | 1 | 2 | 2 |
| C2 | 0 | 2 | 0 | 1 | 0 | |
| Total members | 21 | 25 | 28 | 25 | 22 |
| Name | Domains | |||||
|---|---|---|---|---|---|---|
| DBD | HR-A/B | NLS | AHA | RD | NES | |
| SaHsfA1a | 24–117 | 129–186 | 204–214 (RIIGENNKKRR) | 454–461 (ITDQMELL) | ||
| SaHsfA1b | 20–113 | 133–194 | 215–225 (RRITSSNKKRR) | (AHA2) 431–440 (DVFWEQFLST) | 481–488 (LTSQMGLL) | |
| SaHsfA1d | 37–130 | 156–220 | 238–248 (RRINEANKKQR) | 488–495 (ITDQIGLL) | ||
| SaHsfA2a | 33–159 | 175–239 | 255–268 (RKALDGANVKRKRT) | (AHA1) 314–322 (SLLRAGLES) | ||
| SaHsfA2b | 25–118 | 134–198 | 214–228 (RKALDDAYSKRKRRL) | (AHA1) 313–320 (QMLWDELV) | ||
| SaHsfA3 | 57–194 | 222–268 | 294–303 (KMKRKFITHH) | (AHA3) 472–481 (FTDGWEFGSM) | ||
| (AHA4) 489–495 (LELGSPS) | ||||||
| SaHsfA4a | 11–104 | 130–187 | 205–208 (KKRR) | (AHA1) 253–262 (ITHWEKIIYQ) | 430–437 (LVEQMGHI) | |
| (AHA2) 383–392 (DTFWAQFLTE) | ||||||
| SaHsfA4c | 22–115 | 139–196 | 214–217 (KKRR) | (AHA1) 264–273 (FSYWENILYS) | 420–427 (ITEQMGQL) | |
| (AHA2) 369–378 (DVFWEQYLTE) | ||||||
| SaHsfA5 | 19–112 | 132–189 | 200–215 (TKINSMEFSAYSKKRR) | (AHA) 404–413 (DAFWEQYLTE) | ||
| SaHsfA6b | 48–141 | 162–227 | 250–257 (AAANKRRH) | (AHA) 332–341 (QVFWEGFLNN) | ||
| SaHsfA8 | 13–121 | 154–211 | 326–334 (VDNTWYANH) | (AHA1) 312–321 (DDAILDHFIF) | ||
| SaHsfB1 | 7–100 | 150–187 | 255–261 (KLFGVWL) | |||
| SaHsfB2a | 45–138 | 203–239 | 313–317 (KRART) | 304–310 (KLFGFQL) | ||
| SaHsfB2b | 21–114 | 170–206 | 268–272 (KRVKR) | 259–265 (KLFGVSI) | ||
| SaHsfB2c | 17–110 | 191–227 | 290–294 (KRRKK) | 281–287 (MLFGVSI) | ||
| SaHsfB2d | 27–120 | 202–238 | 320–324 (KRARG) | 311–317 (RLFGFQL) | ||
| SaHsfB2e | 19–112 | 177–213 | ||||
| SaHsfB2f | 41–134 | 229–265 | 355–359 (KRARE) | 346–352 (SLFGYQL) | ||
| SaHsfB4a | 32–125 | 206–242 | 322-325 (SSSG) | 312–318 (RLFGVPL) | 336–338 (NLM) | |
| SaHsfB4b | 49–146 | 202–238 | 286–289 (KRFH) | 276–282 (KLFGVSI) | ||
| SaHsfC1a | 8–102 | 127–170 | 196–201 (DKRRRM) | |||
| SaHsfC1b | 7–101 | 113–156 | 182–187 (NKRRRL) | |||
| Motif | Width | Reference Sequences |
|---|---|---|
| 1 | 29 | QRAAPPPFELTKTYQEMDDPSTDGIVSWSS |
| 2 | 41 | GNSFVVWDPEFARDLLPKYFKHNNFSSFVRQLNTYGFRKV |
| 3 | 6 | EGDCCC |
| 4 | 29 | DPDRWEFANEGFLRGZKHLLKTIKRRKPI |
| 5 | 15 | ACVEVGKYGLEEEVE |
| 6 | 65 | LKPDKNVLMEJVKLKQZQQSSDKQLLMDRLQGMEQRQQQMMSFLAKAVQNPGFLSQFVQQQA |
| 7 | 10 | HHPHLSGRSC |
| 8 | 6 | SPPPPP |
| 9 | 6 | NKKRRL |
| 10 | 16 | TGVNDVFMWEQYLTEHP |
| 11 | 21 | DKNQNINNITDQMGLLTSSAK |
| 12 | 22 | GGQIVKYQPFLDDMPTFFRNMM |
| 13 | 22 | FDIENIPPEHENTDGSAYDDVM |
| 14 | 11 | IASEPIPRPME |
| 15 | 51 | RAGLESDSHNNVDVQQPESVARIEESNLDSVARIEESNLDSVNZKLWDELLAGNLVJDNDDD |
| 16 | 26 | QLLGDIPEAEDLEGQPSDWEEEDLQ |
| 17 | 18 | HWEKIJYQIGRECGEDMF |
| 18 | 6 | HHHHNN |
| 19 | 41 | RAELMEENERLKKEINTQLTSELSHMKLCTNIYSMMSNYNP |
| 20 | 8 | KRFHNNGH |
| 21 | 14 | PKLFGVQIGSKRAR |
| 22 | 41 | QDPGNPSMESKLQLDLLQSEKYLEEQGGSSGGNAGAMDEKEC |
| 23 | 17 | VGCGSSNSSQAESMMNP |
| 24 | 9 | DLLQLQQPG |
| 25 | 14 | LLAAVVETQAIQAA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-S.; Jiang, J.; Han, X.-J.; Zhang, Y.-X.; Zhuo, R.-Y. Identification, Expression Analysis of the Hsf Family, and Characterization of Class A4 in Sedum Alfredii Hance under Cadmium Stress. Int. J. Mol. Sci. 2018, 19, 1216. https://doi.org/10.3390/ijms19041216
Chen S-S, Jiang J, Han X-J, Zhang Y-X, Zhuo R-Y. Identification, Expression Analysis of the Hsf Family, and Characterization of Class A4 in Sedum Alfredii Hance under Cadmium Stress. International Journal of Molecular Sciences. 2018; 19(4):1216. https://doi.org/10.3390/ijms19041216
Chicago/Turabian StyleChen, Shuang-Shuang, Jing Jiang, Xiao-Jiao Han, Yun-Xing Zhang, and Ren-Ying Zhuo. 2018. "Identification, Expression Analysis of the Hsf Family, and Characterization of Class A4 in Sedum Alfredii Hance under Cadmium Stress" International Journal of Molecular Sciences 19, no. 4: 1216. https://doi.org/10.3390/ijms19041216
APA StyleChen, S.-S., Jiang, J., Han, X.-J., Zhang, Y.-X., & Zhuo, R.-Y. (2018). Identification, Expression Analysis of the Hsf Family, and Characterization of Class A4 in Sedum Alfredii Hance under Cadmium Stress. International Journal of Molecular Sciences, 19(4), 1216. https://doi.org/10.3390/ijms19041216






