Serum Is Not Necessary for Prior Pharmacological Activation of AMPK to Increase Insulin Sensitivity of Mouse Skeletal Muscle
Abstract
:1. Introduction
2. Results
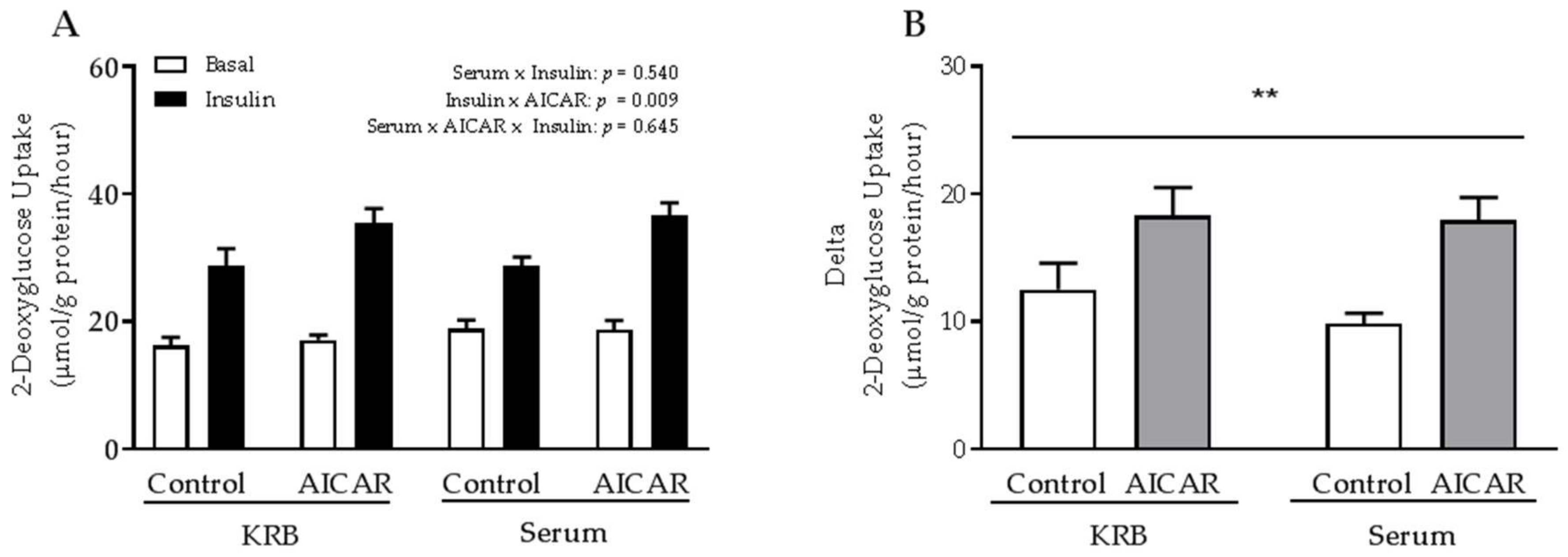
2.1. Acute Serum Stimulation Does Not Affect Basal or AICAR-Stimulated Glucose Uptake in Mouse Skeletal Muscle
2.2. The Absence of Serum Does Not Influence the Ability of Prior AICAR Stimulation to Increase Mouse Muscle Insulin Sensitivity
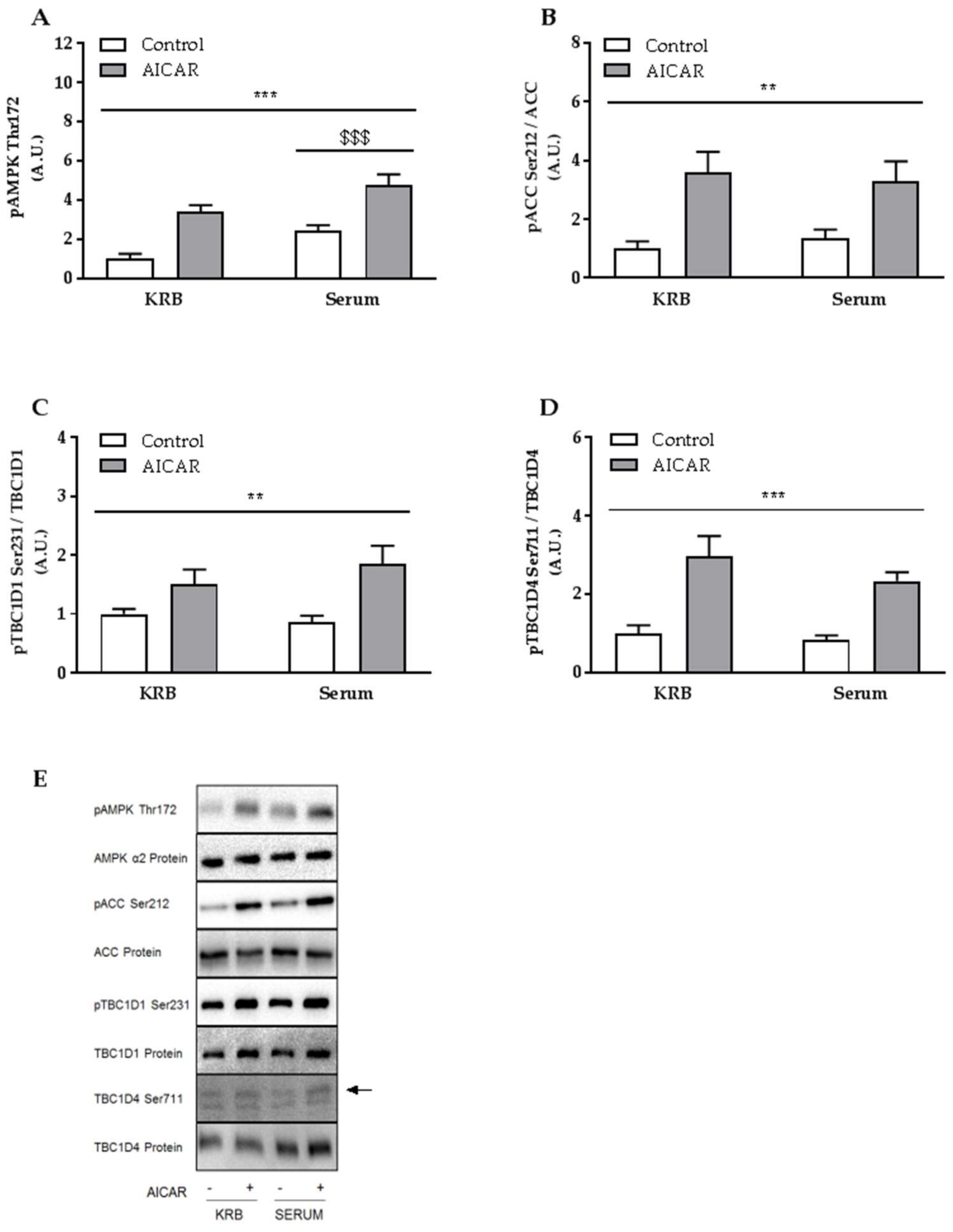
2.3. Increased Muscle Insulin Sensitivity Coincides with Elevated AMPK Signaling
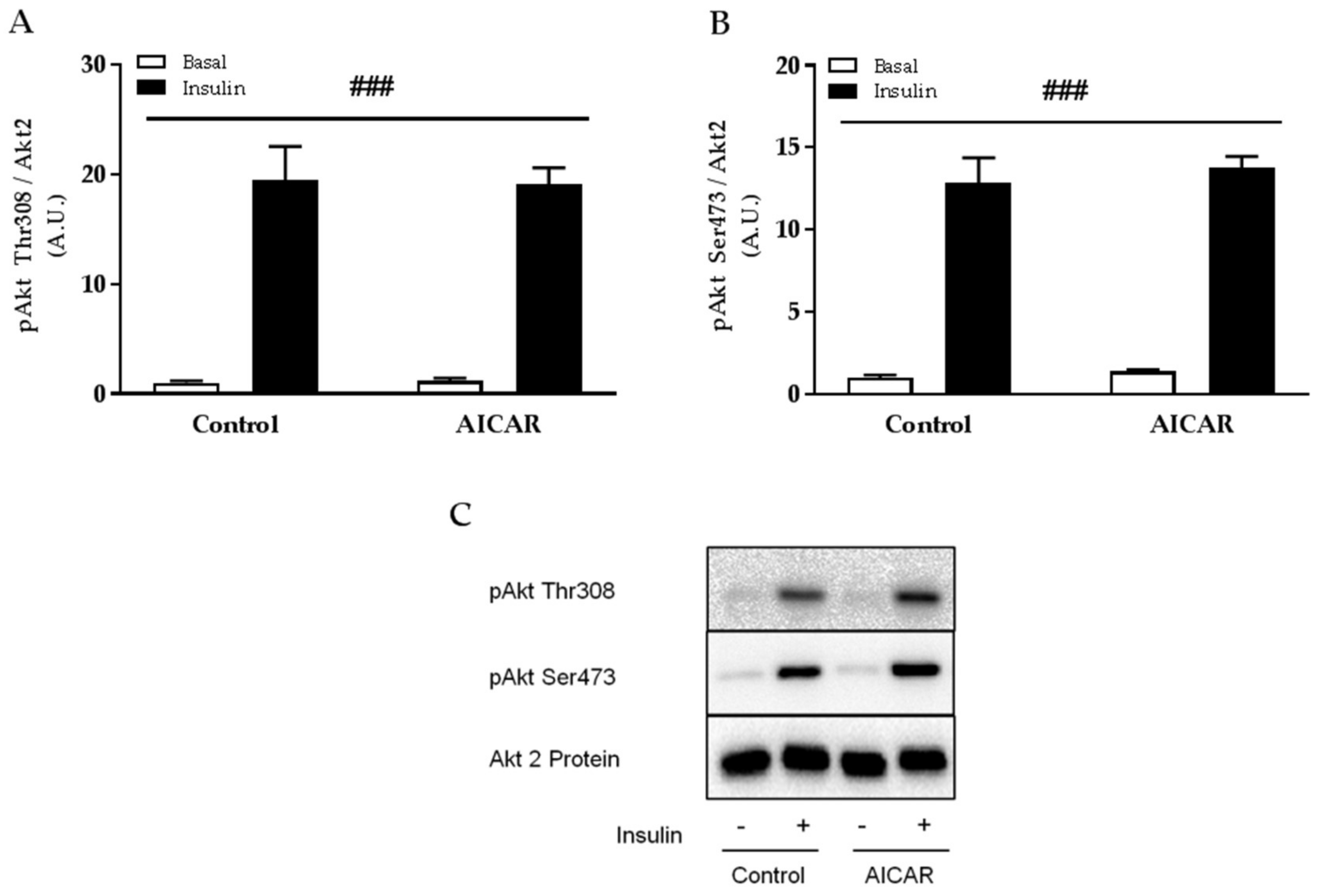
2.4. Insulin-Stimulated Phosphorylation of Akt Thr308 and Ser473 Is Not Affected by Prior AICAR Stimulation
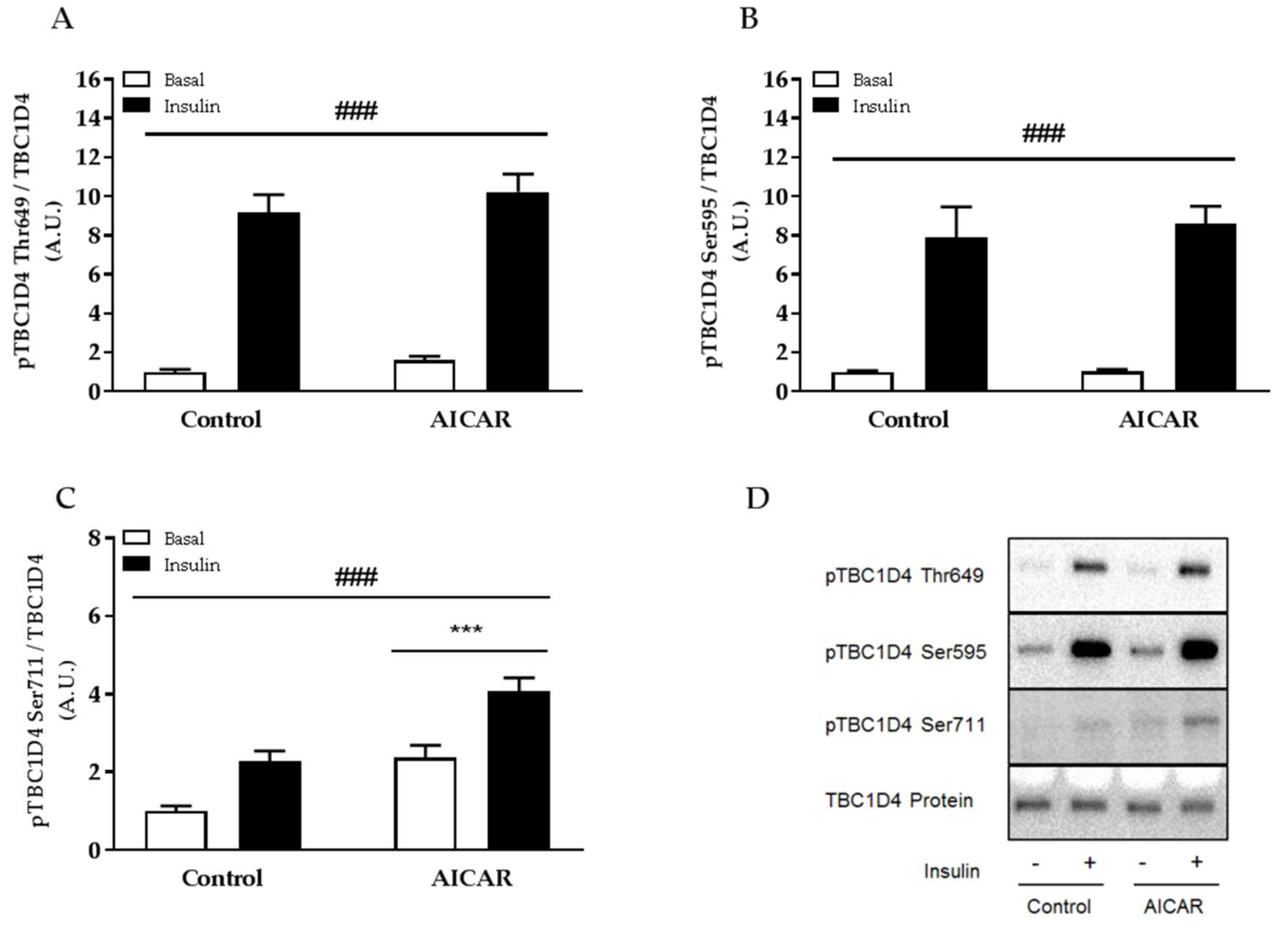
2.5. Insulin-Stimulated Phosphorylation of TBC1D4 Ser711 Is Elevated in Prior AICAR-Stimulated Muscle
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Muscle Incubations
4.3. Muscle Processing, Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), and Western Blot Analyses
4.4. Antibodies
4.5. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2-DG | 2-deoxyglucose |
| ACC | Acetyl-CoA carboxylase |
| AICAR | 5-aminoimidazole-4-carboxamide ribonucleotide |
| AMPK | AMP-activated protein kinase |
| ANOVA | Analysis of variance |
| A.U. | Arbitrary units |
| EDL | Extensor digitorum longus |
| GLUT4 | Glucose transporter 4 |
| HK-II | Hexokinase II |
| KRB | Krebs Ringer buffer |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TBC1D1 | Tre-2/BUB2/CDC16-domain family member 1 |
| TBC1D4 | Tre-2/BUB2/CDC16-domain family member 4 |
References
- DeFronzo, R.A. The triumvirate: Beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988, 37, 667–687. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Garetto, L.P.; Goodman, M.N.; Ruderman, N.B. Enhanced muscle glucose metabolism after exercise: Modulation by local factors. Am. J. Physiol. 1984, 246, E476–E482. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Young, D.A.; Sleeper, M.D.; Zierath, J.; Wallberg-Henriksson, H.; Holloszy, J.O. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am. J. Physiol. 1989, 256, E494–E499. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Mikines, K.J.; Galbo, H.; Kiens, B. Effect of exercise on insulin action in human skeletal muscle. J. Appl. Physiol. 1989, 66, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszewski, J.F.P.; Hansen, B.F.; Kiens, B.; Richter, E.A. Insulin signaling in human skeletal muscle: Time course and effect of exercise. Diabetes 1997, 46, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszewski, J.F.; Hansen, B.F.; Gade; Kiens, B.; Markuns, J.F.; Goodyear, L.J.; Richter, E.A. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 2000, 49, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Pehmøller, C.; Brandt, N.; Birk, J.B.; Høeg, L.D.; Sjøberg, K.A.; Goodyear, L.J.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F.P. Exercise alleviates lipid-induced insulin resistance in human skeletal muscle-signaling interaction at the level of TBC1 domain family member 4. Diabetes 2012, 61, 2743–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castorena, C.M.; Arias, E.B.; Sharma, N.; Cartee, G.D. Post-exercise Improvement in Insulin-Stimulated Glucose Uptake Occurs Concomitant with Greater AS160 Phosphorylation in Muscle from Normal and Insulin Resistant Rats. Diabetes 2014, 63, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Arias, E.B.; Cartee, G.D. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. J. Appl. Physiol. 2006, 101, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Kjøbsted, R.; Munk-Hansen, N.; Birk, J.B.; Foretz, M.; Viollet, B.; Björnholm, M.; Zierath, J.R.; Treebak, J.T.; Wojtaszewski, J.F.P. Enhanced Muscle Insulin Sensitivity After Contraction/Exercise Is Mediated by AMPK. Diabetes 2017, 66, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Kjøbsted, R.; Treebak, J.T.; Fentz, J.; Lantier, L.; Viollet, B.; Birk, J.B.; Schjerling, P.; Björnholm, M.; Zierath, J.R.; Wojtaszewski, J.F.P. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes 2015, 64, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Holloszy, J.O. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am. J. Physiol. 1990, 258, E390–E393. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.S.; Gao, J.; Han, D.-H.; Holloszy, J.O.; Nolte, L.A. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E18–E23. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gulve, E.A.; Holloszy, J.O. Contraction-induced increase in muscle insulin sensitivity: Requirement for a serum factor. Am. J. Physiol. 1994, 266, E186–E192. [Google Scholar] [CrossRef] [PubMed]
- Dumke, C.L.; Kim, J.; Arias, E.B.; Cartee, G.D. Role of kallikrein-kininogen system in insulin-stimulated glucose transport after muscle contractions. J. Appl. Physiol. 2002, 92, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, G.G.; Castorena, C.M.; Hamada, T.; Funai, K.; Arias, E.B.; Cartee, G.D. The B2 receptor of bradykinin is not essential for the post-exercise increase in glucose uptake by insulin-stimulated mouse skeletal muscle. Physiol. Res. 2011, 60, 511–519. [Google Scholar] [PubMed]
- Schweitzer, G.G.; Cartee, G.D. Postexercise skeletal muscle glucose transport is normal in kininogen-deficient rats. Med. Sci. Sports Exerc. 2011, 43, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hiam, D.; Hong, Y.H.; Zulli, A.; Hayes, A.; Rattigan, S.; McConell, G.K. Nitric oxide is required for the insulin sensitizing effects of contraction in mouse skeletal muscle. J. Physiol. 2017, 595, 7427–7439. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Arias, E.B.; Kanzaki, M.; Cartee, G.D. Prior treatment with the AMPK activator AICAR induces subsequently enhanced glucose uptake in isolated skeletal muscles from 24 month-old rats. Appl. Physiol. Nutr. Metab. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bonen, A.; Tan, M.H.; Watson-Wright, W.M. Effects of exercise on insulin binding and glucose metabolism in muscle. Can. J. Physiol. Pharmacol. 1984, 62, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Funai, K.; Schweitzer, G.G.; Sharma, N.; Kanzaki, M.; Cartee, G.D. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E242–E251. [Google Scholar] [CrossRef] [PubMed]
- Funai, K.; Schweitzer, G.G.; Castorena, C.M.; Kanzaki, M.; Cartee, G.D. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E999–E1010. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.F.; Witczak, C.A.; Taylor, E.B.; Fujii, N.; Hirshman, M.F.; Goodyear, L.J. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J. Biol. Chem. 2006, 281, 31478–31485. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wasserman, D.H.; MacKintosh, C.; Sakamoto, K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab. 2011, 13, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Burrows, S. Insulin response in glucose-tolerance tests. Am. J. Clin. Pathol. 1967, 47, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.C. Serum insulin concentration, insulin release and degradation, glucose tolerance and in vivo insulin sensitivity in cholesterol-fed rats. J. Nutr. 1977, 107, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Wojtaszewski, J.F.P. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl. Physiol. Nutr. Metab. 2007, 32, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.F.; Witczak, C.A.; Fujii, N.; Jessen, N.; Taylor, E.B.; Arnolds, D.E.; Sakamoto, K.; Hirshman, M.F.; Goodyear, L.J. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 2006, 55, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Treebak, J.T.; Taylor, E.B.; Witczak, C.A.; An, D.; Toyoda, T.; Koh, H.-J.; Xie, J.; Feener, E.P.; Wojtaszewski, J.F.P.; Hirshman, M.F.; et al. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am. J. Physiol. Cell Physiol. 2010, 298, C377–C385. [Google Scholar] [CrossRef] [PubMed]
- Treebak, J.T.; Pehmøller, C.; Kristensen, J.M.; Kjøbsted, R.; Birk, J.B.; Schjerling, P.; Richter, E.A.; Goodyear, L.J.; Wojtaszewski, J.F.P. Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle. J. Physiol. 2014, 592, 351–375. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jørgensen, N.O.; Wojtaszewski, J.F.P.; Kjøbsted, R. Serum Is Not Necessary for Prior Pharmacological Activation of AMPK to Increase Insulin Sensitivity of Mouse Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 1201. https://doi.org/10.3390/ijms19041201
Jørgensen NO, Wojtaszewski JFP, Kjøbsted R. Serum Is Not Necessary for Prior Pharmacological Activation of AMPK to Increase Insulin Sensitivity of Mouse Skeletal Muscle. International Journal of Molecular Sciences. 2018; 19(4):1201. https://doi.org/10.3390/ijms19041201
Chicago/Turabian StyleJørgensen, Nicolas O., Jørgen F. P. Wojtaszewski, and Rasmus Kjøbsted. 2018. "Serum Is Not Necessary for Prior Pharmacological Activation of AMPK to Increase Insulin Sensitivity of Mouse Skeletal Muscle" International Journal of Molecular Sciences 19, no. 4: 1201. https://doi.org/10.3390/ijms19041201



