Cys Site-Directed Mutagenesis of the Human SLC1A5 (ASCT2) Transporter: Structure/Function Relationships and Crucial Role of Cys467 for Redox Sensing and Glutamine Transport
Abstract
:1. Introduction
1.1. Relevance of ASCT2 to Metabolism
1.2. Functional and Kinetic Characterization of ASCT2
1.3. Regulatory Properties of ASCT2
2. Results
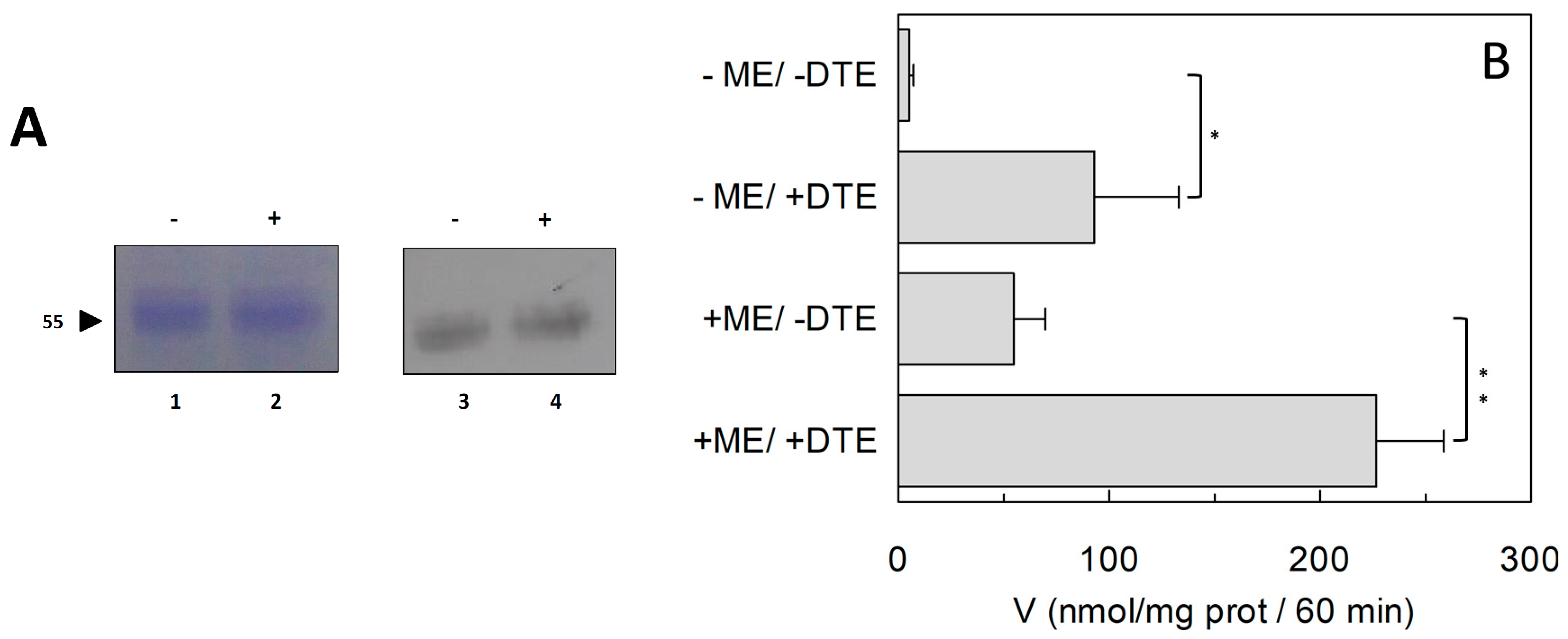
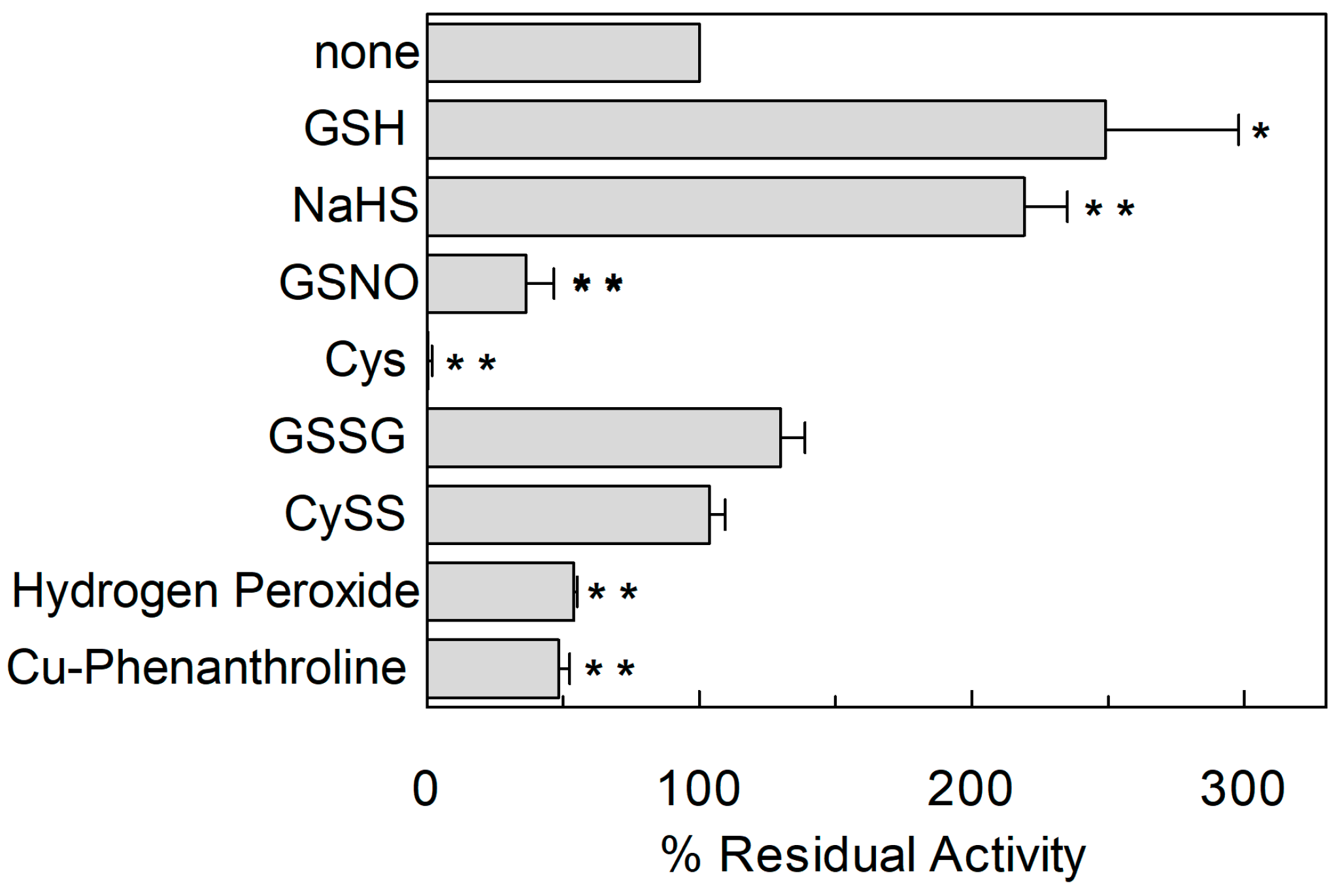
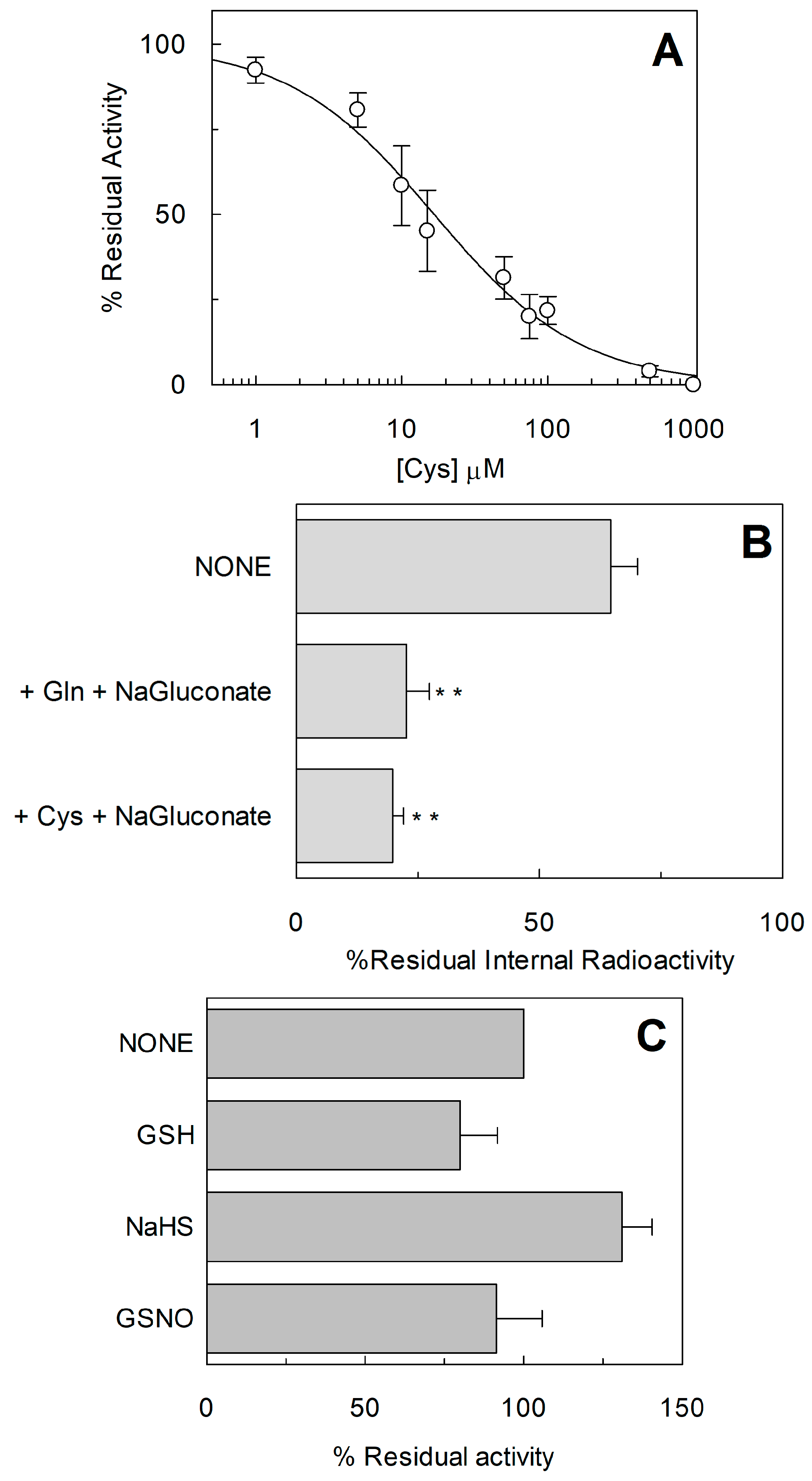
2.1. Effect of Reducing Agents on Wild Type hASCT2
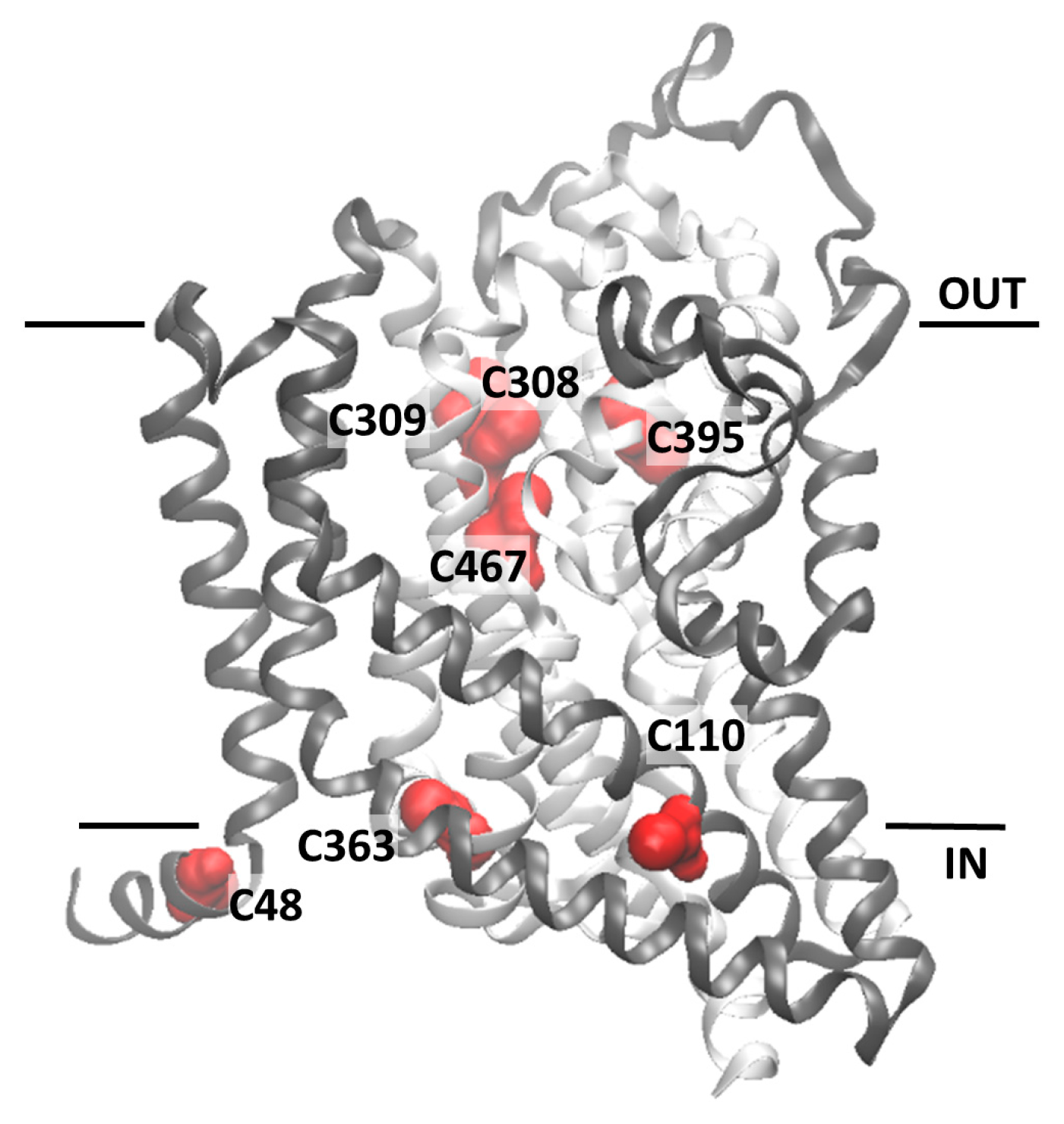
2.2. Homology Structural Model of hASCT2 and Effects of SH-Reagents on WT
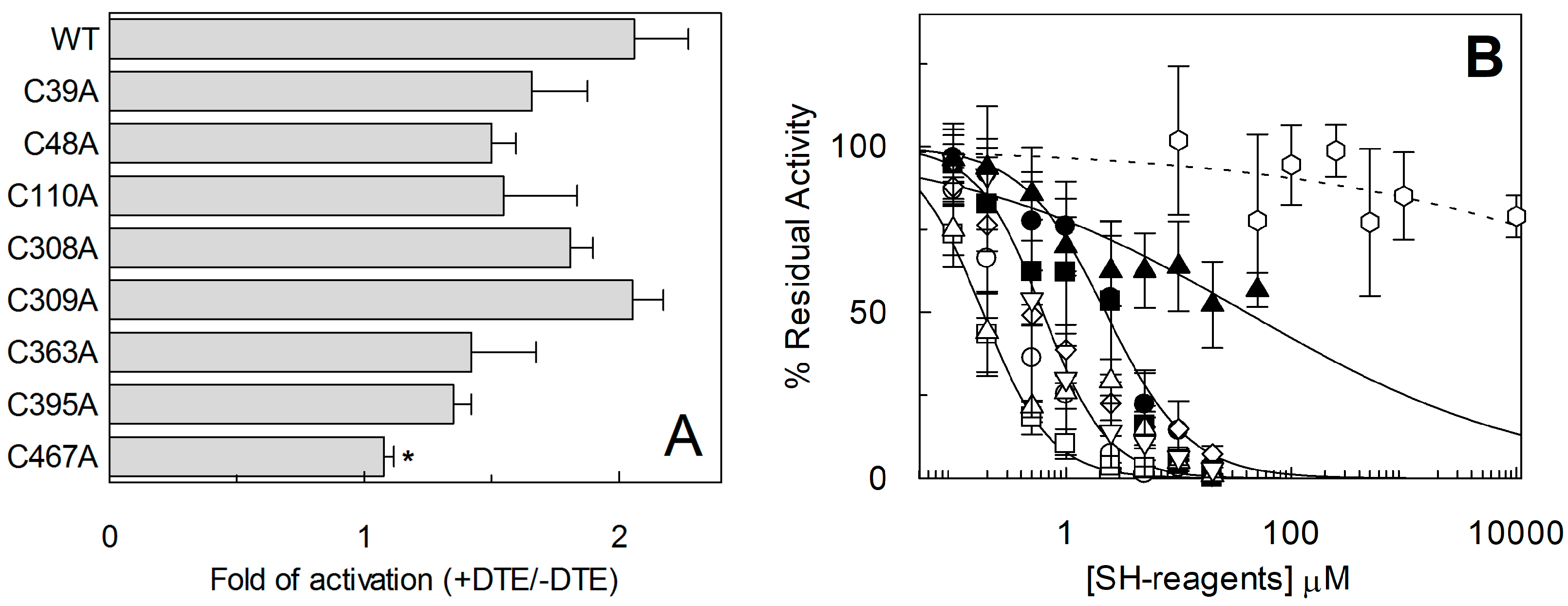
2.3. Site-Directed Mutagenesis and Transport Activity of Cys-Ala Mutants of hASCT2
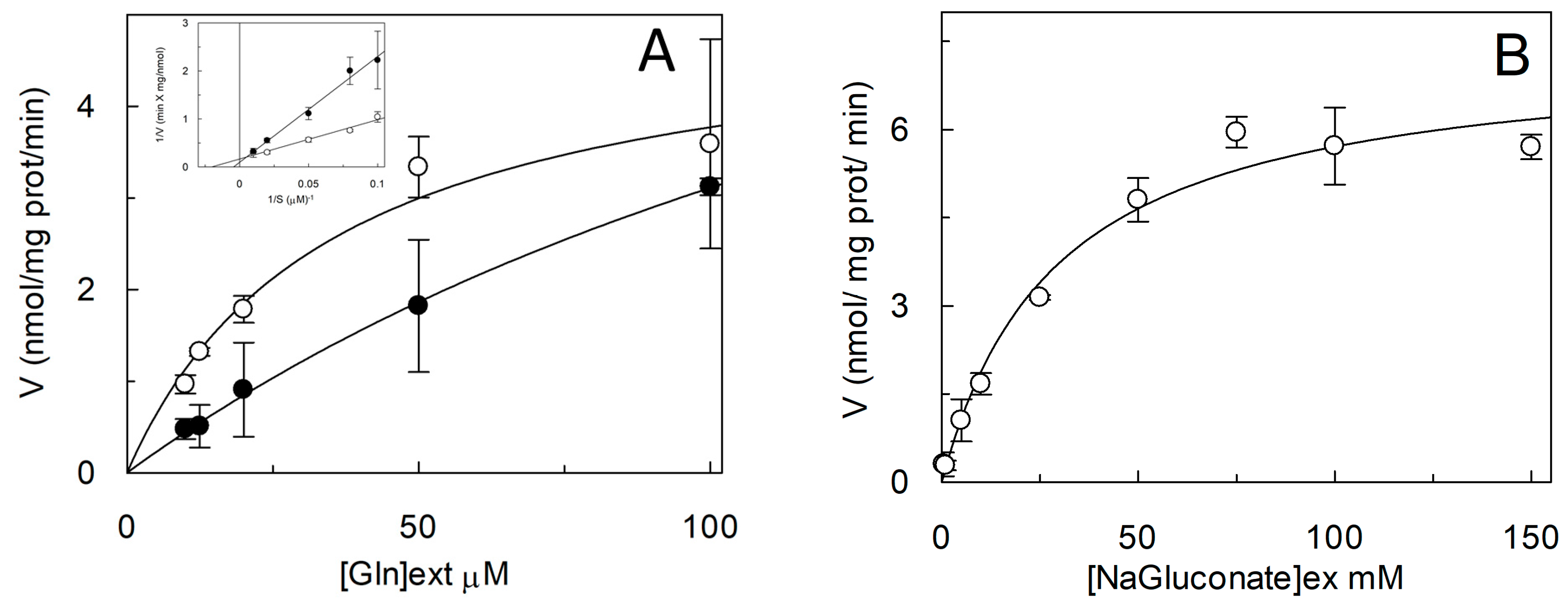
2.4. Functional and Kinetic Characterization of C467A Mutant
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Generation and Cloning of hASCT2 Mutants
- C39A Forward GTGCTGCCGCAGGTGGATACGCTGGATCCAGAGATCAAGTCAGAAG
- C39A Reverse CTTCTGACTTGATCTCTGGATCCAGCGTATCCACCTGCGGCAGCAC
- C48A Forward GATCAAGTCAGAAGAGCTTTGAGAGCTAACTTGC
- C48A Reverse GCAAGTTAGCTCTCAAAGCTCTTCTGACTTGATC
- C110A Forward CTTCCTTTGGTTGTCGCTAGTTTGATTGGTGGAGCTG
- C11A0 Reverse CAGCTCCACCAATCAAACTAGCGACAACCAAAGGAAG
- C308A Forward GGGAAAATATATCCTTGCTTGCCTTTTGGGTCATGCC
- C308A Reverse GGCATGACCCAAAAGGCAAGCAAGGATATATTTTCCC
- C309A Forward GGGAAAATATATCCTTTGTGCTCTTTTGGGTCATGCC
- C309A Reverse GGCATGACCCAAAAGAGCACAAAGGATATATTTTCCC
- C363A Forward CCTCTTATGATGAAGGCTGTTGAAGAGAACAATGGTGTC
- C363A Reverse GACACCATTGTTCTCTTCAACAGCCTTCATCATAAGAGG
- C395A Forward CGCATTGTTTCAAgcaGTCGCTGCCGTTTTCATTGC
- C395A Reverse GCAATGAAAACGGCAGCGACtgcTTGAAACAATGCG
- C467A Forward GGTTGACAGATCTGCTACCGTCTTGAACG
- C467A Reverse CGTTCAAGACGGTAGCAGATCTGTCAACC
4.3. Recombinant Production of hASCT2 WT and Mutants
4.4. Solubilization and Purification of hASCT2 WT and Mutants
4.5. Reconstitution of the hASCT2 into Liposomes
4.6. Transport Measurements
4.7. Homology Modelling of hASCT2
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| C12E8 | Octaethylene glycol monododecyl ether |
| YPDS | Yeast Extract Peptone Dextrose Sorbitol |
| BMGY | Buffered Glycerol-complex Medium |
| BMMY | Buffered Methanol-complex Medium |
| DTE | DiThioErythritol |
| MTSES | Sodium (2-sulfonatoethyl)methane thiosulfonate |
| NEM | N-ethylmaleimide |
| TCA | Tricarboxylic acid cycle |
| CySS | Cystine |
| GSH | Reduced Glutathione |
| GSSG | Oxidized Glutathione |
| NaHS | Sodium hydrosulfide |
| GSNO | S-nitrosoglutathione |
References
- Kanai, Y.; Clemencon, B.; Simonin, A.; Leuenberger, M.; Lochner, M.; Weisstanner, M.; Hediger, M.A. The slc1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp. Med. 2013, 34, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Broer, A.; Brookes, N.; Ganapathy, V.; Dimmer, K.S.; Wagner, C.A.; Lang, F.; Broer, S. The astroglial asct2 amino acid transporter as a mediator of glutamine efflux. J. Neurochem. 1999, 73, 2184–2194. [Google Scholar] [PubMed]
- Oppedisano, F.; Pochini, L.; Galluccio, M.; Cavarelli, M.; Indiveri, C. Reconstitution into liposomes of the glutamine/amino acid transporter from renal cell plasma membrane: Functional characterization, kinetics and activation by nucleotides. Biochim. Biophys. Acta 2004, 1667, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Pochini, L.; Panni, S.; Pingitore, P.; Hedfalk, K.; Indiveri, C. Transport mechanism and regulatory properties of the human amino acid transporter asct2 (slc1a5). Amino Acids 2014, 46, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Ganapathy, V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015, 75, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Bode, B.P. Amino acid transporters asct2 and lat1 in cancer: Partners in crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Pochini, L.; Galluccio, M.; Indiveri, C. Glutamine transport. From energy supply to sensing and beyond. Biochim. Biophys. Acta 2016, 1857, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, P.; Pochini, L.; Scalise, M.; Galluccio, M.; Hedfalk, K.; Indiveri, C. Large scale production of the active human asct2 (slc1a5) transporter in pichia pastoris—functional and kinetic asymmetry revealed in proteoliposomes. Biochim. Biophys. Acta 2013, 1828, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Pochini, L.; Pingitore, P.; Hedfalk, K.; Indiveri, C. Cysteine is not a substrate but a specific modulator of human asct2 (slc1a5) transporter. FEBS Lett. 2015, 589, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Zander, C.B.; Albers, T.; Grewer, C. Voltage-dependent processes in the electroneutral amino acid exchanger asct2. J. Gen. Physiol. 2013, 141, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Console, L.; Scalise, M.; Tarmakova, Z.; Coe, I.R.; Indiveri, C. N-linked glycosylation of human slc1a5 (asct2) transporter is critical for trafficking to membrane. Biochim. Biophys. Acta 2015, 1853, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Tonazzi, A.; Giangregorio, N.; Console, L.; De Palma, A.; Indiveri, C. Nitric oxide inhibits the mitochondrial carnitine/acylcarnitine carrier through reversible s-nitrosylation of cysteine 136. Biochim. Biophys. Acta 2017, 1858, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Shanmughapriya, S.; Tomar, D.; Siddiqui, N.; Lynch, S.; Nemani, N.; Breves, S.L.; Zhang, X.; Tripathi, A.; Palaniappan, P.; et al. Mitochondrial ca2+ uniporter is a mitochondrial luminal redox sensor that augments mcu channel activity. Mol. Cell 2017, 65, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, N.; Tonazzi, A.; Console, L.; Lorusso, I.; De Palma, A.; Indiveri, C. The mitochondrial carnitine/acylcarnitine carrier is regulated by hydrogen sulfide via interaction with c136 and c155. Biochim. Biophys. Acta 2016, 1860, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, N.; Palmieri, F.; Indiveri, C. Glutathione controls the redox state of the mitochondrial carnitine/acylcarnitine carrier Cys residues by glutathionylation. Biochim. Biophys. Acta 2013, 1830, 5299–5304. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Seifert, E.L.; Bouillaud, F.; Aguer, C.; Collins, S.; Harper, M.E. Glutathionylation acts as a control switch for uncoupling proteins ucp2 and ucp3. J. Biol. Chem. 2011, 286, 21865–21875. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, L.; Scalise, M.; Koyioni, M.; Koutentis, P.; Catto, M.; Eberini, I.; Parravicini, C.; Palazzolo, L.; Pisani, L.; Galluccio, M.; et al. Potent inhibitors of human lat1 (slc7a5) transporter based on dithiazole and dithiazine compounds for development of anticancer drugs. Biochem. Pharmacol. 2017, 143, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Canul-Tec, J.C.; Assal, R.; Cirri, E.; Legrand, P.; Brier, S.; Chamot-Rooke, J.; Reyes, N. Structure and allosteric inhibition of excitatory amino acid transporter 1. Nature 2017, 544, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, N.; Georgieva, E.R.; Zhou, Z.; Stolzenberg, S.; Cuendet, M.A.; Khelashvili, G.; Altman, R.B.; Terry, D.S.; Freed, J.H.; Weinstein, H.; et al. Transport domain unlocking sets the uptake rate of an aspartate transporter. Nature 2015, 518, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, L.M.; Huang, C.J.; Batt, C.A. Protein secretion in pichia pastoris and advances in protein production. Appl. Microbiol. Biotechnol. 2012, 93, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, A.J.; Ryan, R.M.; Vandenberg, R.J. Molecular determinants for functional differences between alanine-serine-cysteine transporter 1 and other glutamate transporter family members. J. Biol. Chem. 2013, 288, 8250–8257. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- Oberg, F.; Sjohamn, J.; Conner, M.T.; Bill, R.M.; Hedfalk, K. Improving recombinant eukaryotic membrane protein yields in pichia pastoris: The importance of codon optimization and clone selection. Mol. Membr. Biol. 2011, 28, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Torchetti, E.M.; Brizio, C.; Colella, M.; Galluccio, M.; Giancaspero, T.A.; Indiveri, C.; Roberti, M.; Barile, M. Mitochondrial localization of human fad synthetase isoform 1. Mitochondrion 2010, 10, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Galluccio, M.; Pochini, L.; Peta, V.; Ianni, M.; Scalise, M.; Indiveri, C. Functional and molecular effects of mercury compounds on the human octn1 cation transporter: C50 and c136 are the targets for potent inhibition. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 144, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Indiveri, C. Studying amino acid transport using liposomes. Methods Mol. Biol. 2010, 606, 55–68. [Google Scholar] [PubMed]
- Palmieri, F.; Indiveri, C.; Bisaccia, F.; Iacobazzi, V. Mitochondrial metabolite carrier proteins: Purification, reconstitution, and transport studies. Methods Enzymol. 1995, 260, 349–369. [Google Scholar] [PubMed]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The swiss-model workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalise, M.; Pochini, L.; Console, L.; Pappacoda, G.; Pingitore, P.; Hedfalk, K.; Indiveri, C. Cys Site-Directed Mutagenesis of the Human SLC1A5 (ASCT2) Transporter: Structure/Function Relationships and Crucial Role of Cys467 for Redox Sensing and Glutamine Transport. Int. J. Mol. Sci. 2018, 19, 648. https://doi.org/10.3390/ijms19030648
Scalise M, Pochini L, Console L, Pappacoda G, Pingitore P, Hedfalk K, Indiveri C. Cys Site-Directed Mutagenesis of the Human SLC1A5 (ASCT2) Transporter: Structure/Function Relationships and Crucial Role of Cys467 for Redox Sensing and Glutamine Transport. International Journal of Molecular Sciences. 2018; 19(3):648. https://doi.org/10.3390/ijms19030648
Chicago/Turabian StyleScalise, Mariafrancesca, Lorena Pochini, Lara Console, Gilda Pappacoda, Piero Pingitore, Kristina Hedfalk, and Cesare Indiveri. 2018. "Cys Site-Directed Mutagenesis of the Human SLC1A5 (ASCT2) Transporter: Structure/Function Relationships and Crucial Role of Cys467 for Redox Sensing and Glutamine Transport" International Journal of Molecular Sciences 19, no. 3: 648. https://doi.org/10.3390/ijms19030648
APA StyleScalise, M., Pochini, L., Console, L., Pappacoda, G., Pingitore, P., Hedfalk, K., & Indiveri, C. (2018). Cys Site-Directed Mutagenesis of the Human SLC1A5 (ASCT2) Transporter: Structure/Function Relationships and Crucial Role of Cys467 for Redox Sensing and Glutamine Transport. International Journal of Molecular Sciences, 19(3), 648. https://doi.org/10.3390/ijms19030648







