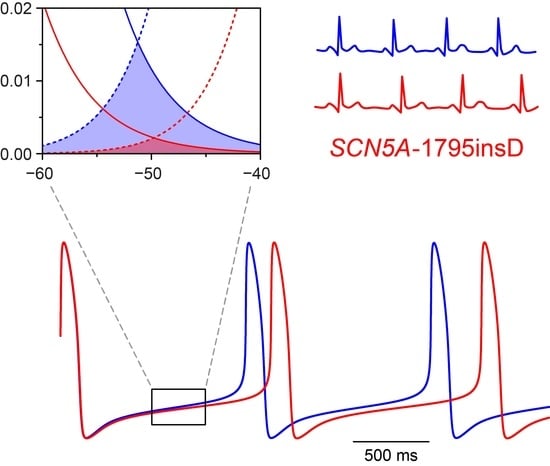
Sinus Bradycardia in Carriers of the SCN5A-1795insD Mutation: Unraveling the Mechanism through Computer Simulations
Abstract
:1. Introduction
2. Results
2.1. Implementation of the 1795insD Mutation
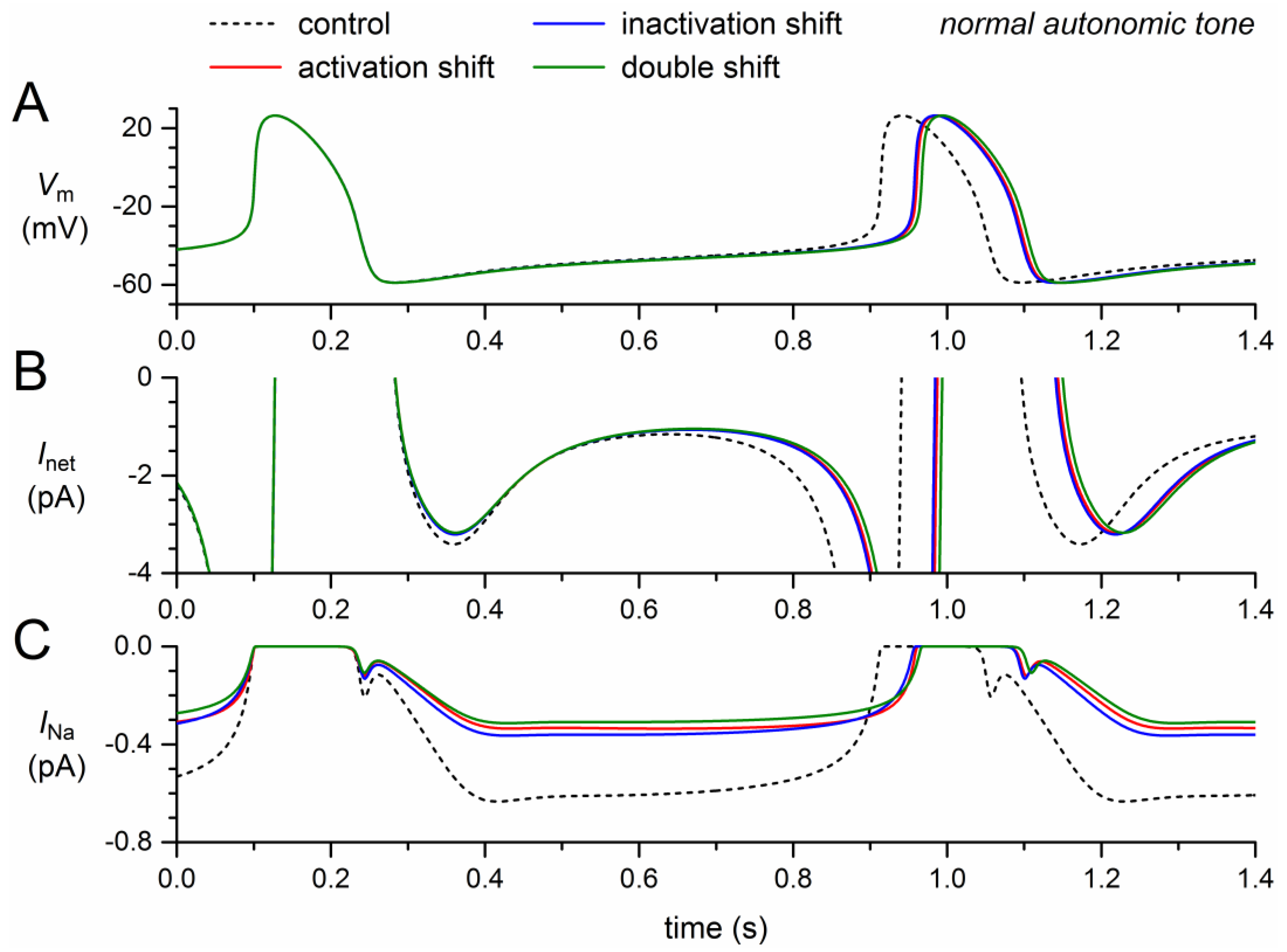
2.2. Effects of the Shifts in Steady-State Activation and Inactivation
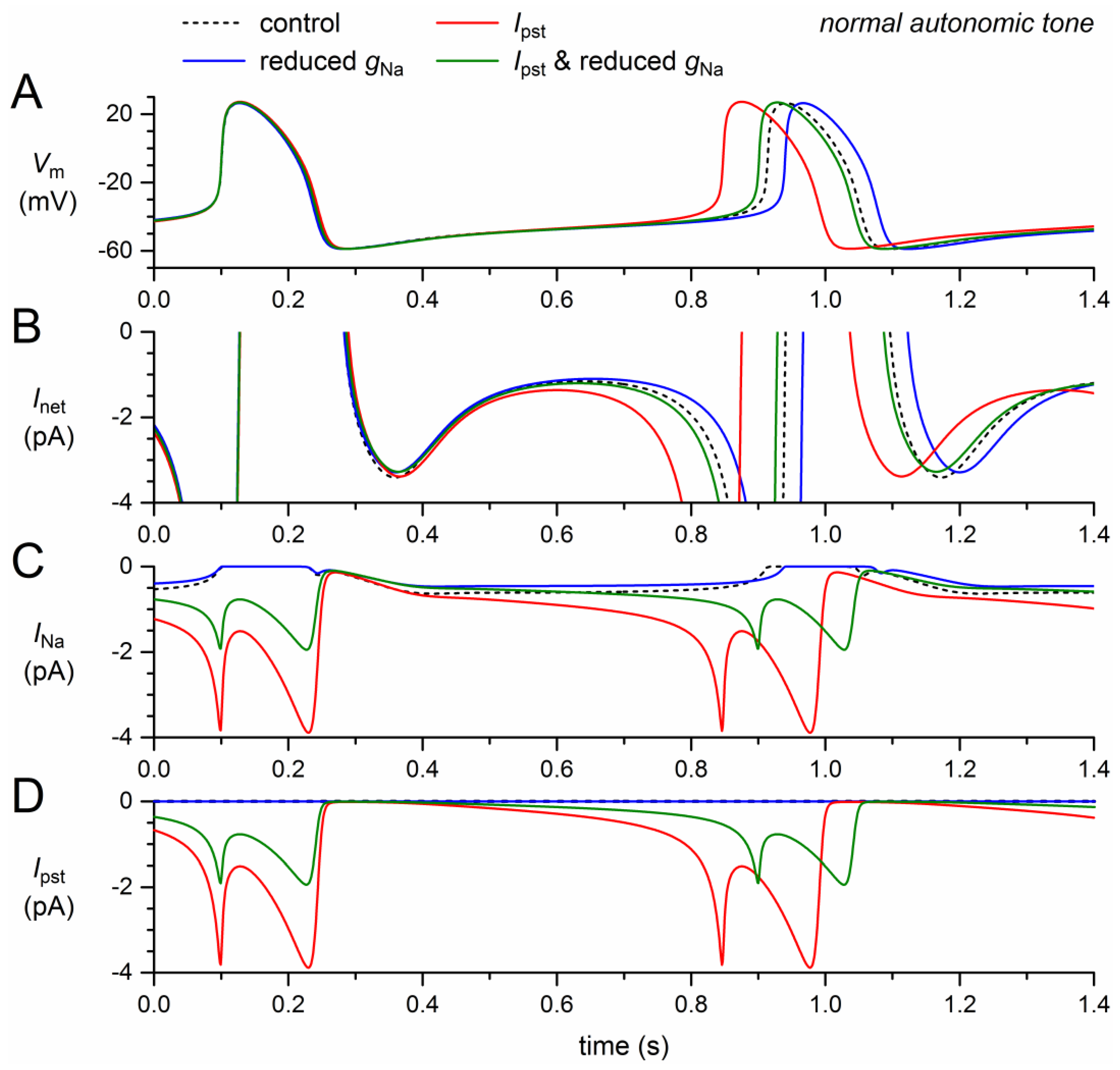
2.3. Effects of the Reduction in Fully-Activated Conductance and the Incorporation of a Persistent Current
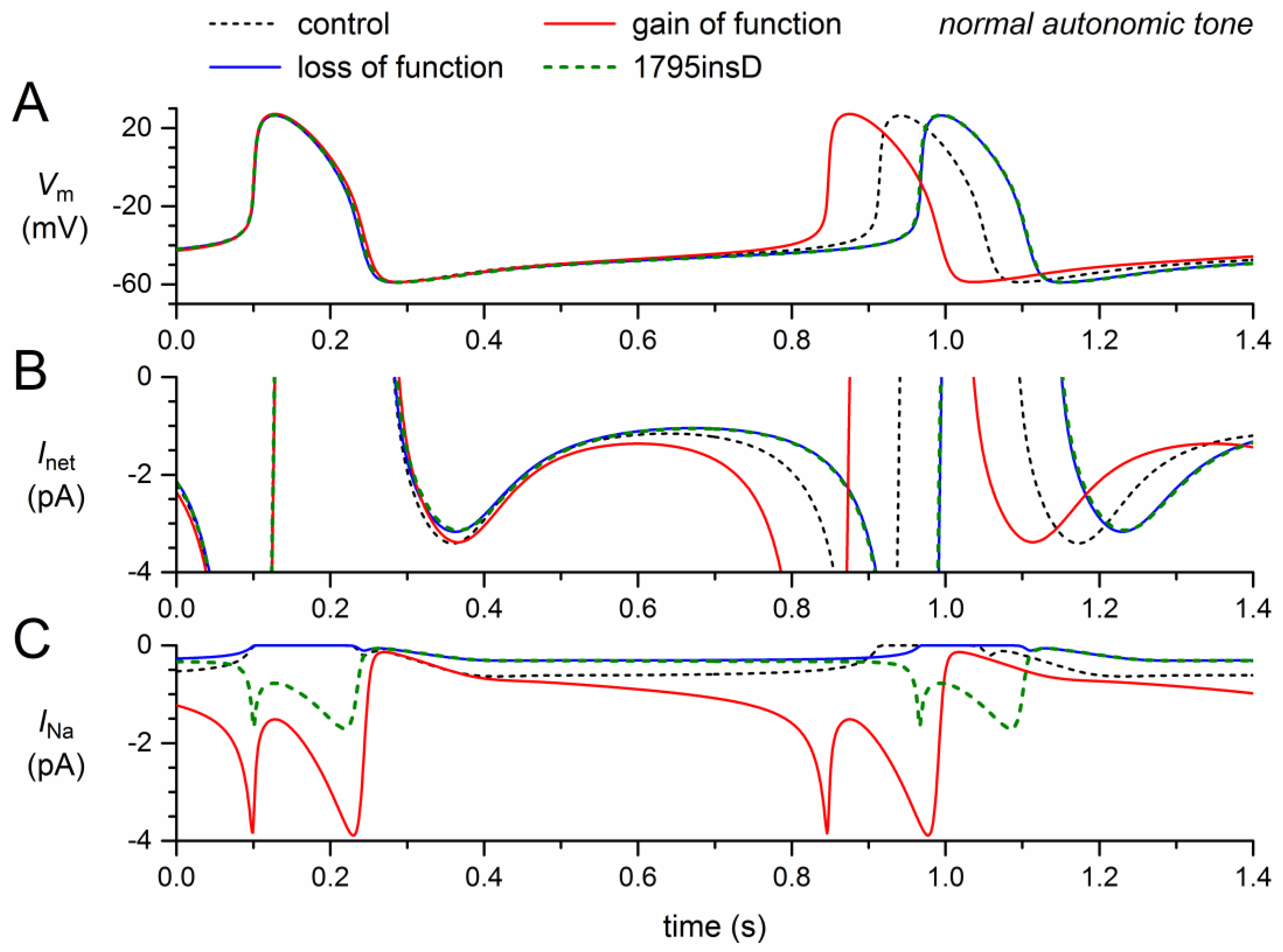
2.4. Net Effect: Loss of Function Versus Gain of Function
2.5. Net Effect at Low Heart Rate
2.6. Sinus Bradycardia
3. Discussion
3.1. Applicability of the Simulation Results
3.2. Parameter Settings
3.3. Previous Simulation Studies
3.4. Future Directions
4. Materials and Methods
4.1. Computational Model of a Single Human SA Nodal Pacemaker Cell
4.2. Implementation of the 1795insD Mutation
4.3. Implementation of Vagal and β-Adrenergic Tone
4.4. Computer Simulations
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetyl-choline |
| gNa | Conductance of INa |
| INa | Fast sodium current |
| Inet | Net membrane current |
| Ipst | Persistent, non-inactivating component of INa |
| Iso | Isoprenaline |
| LQT3 | Long-QT syndrome type 3 |
| LQTS | Long QT syndrome |
| NaV1.5 | Pore-forming α-subunit of the cardiac voltage-gated sodium channel |
| SCN5A | Gene encoding the NaV1.5 protein |
| Vm | Membrane potential |
References
- Grant, A.O. Cardiac ion channels. Circ. Arrhythm. Electrophysiol. 2009, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.S.; Tan, H.L.; Wilde, A.A.M. Cardiac ion channels in health and disease. Heart Rhythm 2010, 7, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bartos, D.C.; Grandi, E.; Ripplinger, C.M. Ion channels in the heart. Compr. Physiol. 2015, 5, 1423–1464. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, R.E. Cardiac electrophysiology: Normal and ischemic ionic currents and the ECG. Adv. Physiol. Educ. 2017, 41, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lieve, K.V.V.; Wilde, A.A.M. Inherited ion channel diseases: A brief review. Europace 2015, 17, ii1–ii6. [Google Scholar] [CrossRef] [PubMed]
- Abriel, H.; Zaklyazminskaya, E.V. Cardiac channelopathies: Genetic and molecular mechanisms. Gene 2013, 517, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Stramba-Badiale, M.; Crotti, L.; Pedrazzini, M.; Besana, A.; Bosi, G.; Gabbarini, F.; Goulene, K.; Insolia, R.; Mannarino, S.; et al. Prevalence of the congenital long-QT syndrome. Circulation 2009, 120, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Hocini, M.; Pison, L.; Proclemer, A.; Larsen, T.B.; Madrid, A.; Blomström-Lundqvist, C. Diagnosis and management of patients with inherited arrhythmia syndromes in Europe: Results of the European Heart Rhythm Association Survey. Europace 2014, 16, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Liu, N.; Priori, S.G. Sodium channel mutations and arrhythmias. Nat. Rev. Cardiol. 2009, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Abriel, H. Cardiac sodium channel NaV1.5 and interacting proteins: Physiology and pathophysiology. J. Mol. Cell. Cardiol. 2010, 48, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Remme, C.A.; Bezzina, C.R. Sodium channel (dys)function and cardiac arrhythmias. Cardiovasc. Ther. 2010, 28, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.S.; Asghari-Roodsari, A.; Tan, H.L. Cardiac sodium channelopathies. Pflügers Arch. 2010, 460, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, K.C.; Dudley, S.C., Jr. Cardiac sodium channel mutations: Why so many phenotypes? Nat. Rev. Cardiol. 2014, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Detta, N.; Frisso, G.; Salvatore, F. The multi-faceted aspects of the complex cardiac Nav1.5 protein in membrane function and pathophysiology. Biochim. Biophys. Acta 2015, 1854, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Veerman, C.C.; Wilde, A.A.M.; Lodder, E.M. The cardiac sodium channel gene SCN5A and its gene product NaV1.5: Role in physiology and pathophysiology. Gene 2015, 573, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Crotti, L.; Celano, G.; Dagradi, F.; Schwartz, P.J. Congenital long QT syndrome. Orphanet J. Rare Dis. 2008, 3, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeyesekere, M.N.; Antzelevitch, C.; Krahn, A.D. Management of ventricular arrhythmias in suspected channelopathies. Circ. Arrhythm. Electrophysiol. 2015, 8, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.; Veldkamp, M.W.; Van den Berg, M.P.; Postma, A.V.; Rook, M.B.; Viersma, J.W.; Van Langen, I.M.; Tan-Sindhunata, G.; Bink-Boelkens, M.T.E.; Van der Hout, A.H.; et al. A single Na+ channel mutation causing both long-QT and Brugada syndromes. Circ. Res. 1999, 85, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Remme, C.A.; Wilde, A.A.M.; Bezzina, C.R. Cardiac sodium channel overlap syndromes: Different faces of SCN5A mutations. Trends Cardiovasc. Med. 2008, 18, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Postema, P.G.; Van den Berg, M.P.; Van Tintelen, J.P.; Van den Heuvel, F.; Grundeken, M.; Hofman, N.; Van der Roest, W.P.; Nannenberg, E.A.; Krapels, I.P.C.; Bezzina, C.R.; et al. Founder mutations in the Netherlands: SCN5a 1795insD, the first described arrhythmia overlap syndrome and one of the largest and best characterised families worldwide. Neth. Heart J. 2009, 17, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.P.; Viersma, J.W.; Beaufort-Krol, G.C.M.; Bink-Boelkens, M.T.E.; Bezzina, C.R.; Veldkamp, M.W.; Brouwer, J.; Haaksma, J.; Van Tintelen, J.P.; Van Langen, I.M.; et al. A large family characterised by nocturnal sudden death. Neth. Heart J. 2002, 10, 304–312. [Google Scholar] [PubMed]
- Moss, A.J.; Zareba, W.; Benhorin, J.; Locati, E.H.; Hall, W.J.; Robinson, J.L.; Schwartz, P.J.; Towbin, J.A.; Vincent, G.M.; Lehmann, M.H.; et al. ECG T-wave patterns in genetically distinct forms of the hereditary long QT syndrome. Circulation 1995, 92, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Wilders, R. Pacemaker activity of the human sinoatrial node: An update on the effects of mutations in HCN4 on the hyperpolarization-activated current. Int. J. Mol. Sci. 2015, 16, 3071–3094. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Priori, S.G.; Locati, E.H.; Napolitano, C.; Cantù, F.; Towbin, J.A.; Keating, M.T.; Hammoude, H.; Brown, A.M.; Chen, L.S.K.; Colatsky, T.J. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate: Implications for gene-specific therapy. Circulation 1995, 92, 3381–3386. [Google Scholar] [CrossRef] [PubMed]
- Tobé, T.J.M.; De Langen, C.D.J.; Bink-Boelkens, M.T.E.; Mook, P.H.; Viersma, J.W.; Lie, K.I.; Wesseling, H. Late potentials in a bradycardia-dependent long QT syndrome associated with sudden death during sleep. J. Am. Coll. Cardiol. 1992, 19, 541–549. [Google Scholar] [CrossRef]
- Beaufort-Krol, G.C.M.; Van den Berg, M.P.; Wilde, A.A.M.; Van Tintelen, J.P.; Viersma, J.W.; Bezzina, C.R.; Bink-Boelkens, M.T.E. Developmental aspects of long QT syndrome type 3 and Brugada syndrome on the basis of a single SCN5A mutation in childhood. J. Am. Coll. Cardiol. 2005, 46, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, M.W.; Viswanathan, P.C.; Bezzina, C.; Baartscheer, A.; Wilde, A.A.M.; Balser, J.R. Two distinct congenital arrhythmias evoked by a multidysfunctional Na+ channel. Circ. Res. 2000, 86, e91–e97. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, M.W.; Wilders, R.; Baartscheer, A.; Zegers, J.G.; Bezzina, C.R.; Wilde, A.A.M. Contribution of sodium channel mutations to bradycardia and sinus node dysfunction in LQT3 families. Circ. Res. 2003, 92, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhang, H.; Grace, A.A.; Huang, C.L.H. SCN5A and sinoatrial node pacemaker function. Cardiovasc. Res. 2007, 74, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Huang, C.L.H.; Zhang, Y. Genetic Na+ channelopathies and sinus node dysfunction. Prog. Biophys. Mol. Biol. 2008, 98, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chandler, N.J.; Greener, I.D.; Tellez, J.O.; Inada, S.; Musa, H.; Molenaar, P.; DiFrancesco, D.; Baruscotti, M.; Longhi, R.; Anderson, R.H.; et al. Molecular architecture of the human sinus node: Insights into the function of the cardiac pacemaker. Circulation 2009, 119, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Wilders, R.; Van Borren, M.M.G.J.; Tan, H.L. Is sodium current present in human sinoatrial node cells? Int. J. Biol. Sci. 2009, 5, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.; Fantini, M.; Wilders, R.; Severi, S. Computational analysis of the human sinus node action potential: Model development and effects of mutations. J. Physiol. 2017, 595, 2365–2396. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Hucker, W.J.; Dobrzynski, H.; Rosenshtraukh, L.V.; Efimov, I.R. Postganglionic nerve stimulation induces temporal inhibition of excitability in rabbit sinoatrial node. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H612–H623. [Google Scholar] [CrossRef] [PubMed]
- Abramochkin, D.V.; Kuzmin, V.S.; Sukhova, G.S.; Rosenshtraukh, L.V. Modulation of rabbit sinoatrial node activation sequence by acetylcholine and isoproterenol investigated with optical mapping technique. Acta Physiol. 2009, 196, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Boyett, M.R.; Honjo, H.; Kodama, I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc. Res. 2000, 47, 658–687. [Google Scholar] [CrossRef]
- Monfredi, O.; Dobrzynski, H.; Mondal, T.; Boyett, M.R.; Morris, G.M. The anatomy and physiology of the sinoatrial node—A contemporary review. Pacing Clin. Electrophysiol. 2010, 33, 1392–1406. [Google Scholar] [CrossRef] [PubMed]
- Chandler, N.; Aslanidi, O.; Buckley, D.; Inada, S.; Birchall, S.; Atkinson, A.; Kirk, D.; Monfredi, O.; Molenaar, P.; Anderson, R.; et al. Computer three-dimensional anatomical reconstruction of the human sinus node and a novel paranodal area. Anat. Rec. 2011, 294, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Glukhov, A.V.; Chang, R. Conduction barriers and pathways of the sinoatrial pacemaker complex: Their role in normal rhythm and atrial arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1773–H1783. [Google Scholar] [CrossRef] [PubMed]
- Csepe, T.A.; Zhao, J.; Hansen, B.J.; Li, N.; Sul, L.V.; Lim, P.; Wang, Y.; Simonetti, O.P.; Kilic, A.; Mohler, P.J.; et al. Human sinoatrial node structure: 3D microanatomy of sinoatrial conduction pathways. Prog. Biophys. Mol. Biol. 2016, 120, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hansen, B.J.; Csepe, T.A.; Zhao, J.; Ignozzi, A.J.; Sul, L.V.; Zakharkin, S.O.; Kalyanasundaram, A.; Davis, J.P.; Biesiadecki, B.J.; et al. Redundant and diverse intranodal pacemakers and conduction pathways protect the human sinoatrial node from failure. Sci. Transl. Med. 2017, 9, eaam5607. [Google Scholar] [CrossRef] [PubMed]
- Makita, N. Phenotypic overlap of cardiac sodium channelopathies: Individual-specific or mutation-specific? Circ. J. 2009, 73, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.P.P.; Koopmann, T.T.; Wilders, R.; Veldkamp, M.W.; Opthof, T.; Bhuiyan, Z.A.; Mannens, M.M.A.M.; Balser, J.R.; Tan, H.L.; Bezzina, C.R.; et al. A mutation in the human cardiac sodium channel (E161K) contributes to sick sinus syndrome, conduction disease and Brugada syndrome in two families. J. Mol. Cell. Cardiol. 2005, 38, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Rivolta, I.; Abriel, H.; Tateyama, M.; Liu, H.; Memmi, M.; Vardas, P.; Napolitano, C.; Priori, S.G.; Kass, RS. Inherited Brugada and long QT-3 syndrome mutations of a single residue of the cardiac sodium channel confer distinct channel and clinical phenotypes. J. Biol. Chem. 2001, 276, 30623–30630. [Google Scholar] [CrossRef] [PubMed]
- Dumaine, R.; Towbin, J.A.; Brugada, P.; Vatta, M.; Nesterenko, D.V.; Nesterenko, V.V.; Brugada, J.; Brugada, R.; Antzelevitch, C. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ. Res. 1999, 85, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Wang, T.; Jones, R.P.; Trump, D.; Zimmer, T.; Lei, M. Multiple loss-of-function mechanisms contribute to SCN5A-related familial sick sinus syndrome. PLoS ONE 2010, 5, e10985. [Google Scholar] [CrossRef]
- Wei, J.; Wang, D.W.; Alings, M.; Fish, F.; Wathen, M.; Roden, D.M.; George, A.L., Jr. Congenital long-QT syndrome caused by a novel mutation in a conserved acidic domain of the cardiac Na+ channel. Circulation 1999, 99, 3165–3171. [Google Scholar] [CrossRef] [PubMed]
- Remme, C.A.; Verkerk, A.O.; Nuyens, D.; Van Ginneken, A.C.G.; Van Brunschot, S.; Belterman, C.N.W.; Wilders, R.; Van Roon, M.A.; Tan, H.L.; Wilde, A.A.M.; et al. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation 2006, 114, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Remme, C.A.; Scicluna, B.P.; Verkerk, A.O.; Amin, A.S.; Van Brunschot, S.; Beekman, L.; Deneer, V.H.M.; Chevalier, C.; Oyama, F.; Miyazaki, H.; et al. Genetically determined differences in sodium current characteristics modulate conduction disease severity in mice with cardiac sodium channelopathy. Circ. Res. 2009, 104, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.P.; Casini, S.; Van den Berg, C.W.; Hoekstra, M.; Remme, C.A.; Dambrot, C.; Salvatori, D.; Ward-van Oostwaard, D.; Wilde, A.A.M.; Bezzina, C.R.; et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 2012, 125, 3079–3091. [Google Scholar] [CrossRef] [PubMed]
- Rook, M.B.; Evers, M.M.; Vos, M.A.; Bierhuizen, M.F.A. Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc. Res. 2012, 93, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Shy, D.; Gillet, L.; Abriel, H. Cardiac sodium channel NaV1.5 distribution in myocytes via interacting proteins: The multiple pool model. Biochim. Biophys. Acta 2013, 1833, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Adsit, G.S.; Vaidyanathan, R.; Galler, C.M.; Kyle, J.W.; Makielski, J.C. Channelopathies from mutations in the cardiac sodium channel protein complex. J. Mol. Cell. Cardiol. 2013, 61, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.E.; Rudy, Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature 1999, 400, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, X.H.T.; Abriel, H.; Cabo, C.; Benhorin, J.; Kass, R.S. Arrhythmogenic mechanism of an LQT-3 mutation of the human heart Na+ channel α-subunit: A computational analysis. Circulation 2000, 102, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Bink-Boelkens, M.T.E.; Bezzina, C.R.; Viswanathan, P.C.; Beaufort-Krol, G.C.M.; Van Tintelen, P.J.; Van den Berg, M.P.; Wilde, A.A.M.; Balser, J.R. A sodium-channel mutation causes isolated cardiac conduction disease. Nature 2001, 409, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Viswanathan, P.C.; Balser, J.R.; George, A.L., Jr.; Benson, D.W. Clinical, genetic, and biophysical characterization of SCN5A mutations associated with atrioventricular conduction block. Circulation 2002, 105, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.E.; Rudy, Y. Na+ channel mutation that causes both Brugada and long-QT syndrome phenotypes: A simulation study of mechanism. Circulation 2002, 105, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Rivolta, I.; Clancy, C.E.; Tateyama, M.; Liu, H.; Priori, S.G.; Kass, R.S. A novel SCN5A mutation associated with long QT-3: Altered inactivation kinetics and channel dysfunction. Physiol. Genom. 2002, 10, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.I.; Rougier, J.S.; Kucera, J.P.; Benammar, N.; Fressart, V.; Guicheney, P.; Madle, A.; Fromer, M.; Schläpfer, J.; Abriel, H. Brugada syndrome and fever: Genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc. Res. 2005, 67, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Vecchietti, S.; Grandi, E.; Severi, S.; Rivolta, I.; Napolitano, C.; Priori, S.G.; Cavalcanti, S. In silico assessment of Y1795C and Y1795H SCN5A mutations: Implication for inherited arrhythmogenic syndromes. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H56–H65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Tranquillo, J.; Neplioueva, V.; Bursac, N.; Grant, A.O. Sodium channel kinetic changes that produce Brugada syndrome or progressive cardiac conduction system disease. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H399–H407. [Google Scholar] [CrossRef] [PubMed]
- Flaim, S.N.; Giles, W.R.; McCulloch, A.D. Arrhythmogenic consequences of Na+ channel mutations in the transmurally heterogeneous mammalian left ventricle: Analysis of the I1768V SCN5A mutation. Heart Rhythm 2007, 4, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, S.; Jespersen, T.; Pruvot, E.; Keller, D.I.; Corbaz, C.; Schläpfer, J.; Abriel, H.; Kucera, J.P. Analyses of a novel SCN5A mutation (C1850S): Conduction vs. repolarization disorder hypotheses in the Brugada syndrome. Cardiovasc. Res. 2008, 78, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Bébarová, M.; O’Hara, T.; Geelen, J.L.M.C.; Jongbloed, R.J.; Timmermans, C.; Arens, Y.H.; Rodriguez, L.M.; Rudy, Y.; Volders, P.G.A. Subepicardial phase 0 block and discontinuous transmural conduction underlie right precordial ST-segment elevation by a SCN5A loss-of-function mutation. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H48–H58. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Denegri, M.; Liu, N.; Bachetti, T.; Seregni, M.; Morotti, S.; Severi, S.; Napolitano, C.; Priori, S.G. Trafficking defects and gating abnormalities of a novel SCN5A mutation question gene-specific therapy in long QT syndrome type 3. Circ. Res. 2010, 106, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Laurent, G.; Saal, S.; Amarouch, M.Y.; Béziau, D.M.; Marsman, R.F.J.; Faivre, L.; Barc, J.; Dina, C.; Bertaux, G.; Barthez, O.; et al. Multifocal ectopic Purkinje-related premature contractions: A new SCN5A-related cardiac channelopathy. J. Am. Coll. Cardiol. 2012, 60, 144–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, S.A.; Castro, M.L.; Ohanian, M.; Guo, G.; Zodgekar, P.; Sheu, A.; Stockhammer, K.; Thompson, T.; Playford, D.; Subbiah, R.; et al. R222Q SCN5A mutation is associated with reversible ventricular ectopy and dilated cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Ziyadeh-Isleem, A.; Clatot, J.; Duchatelet, S.; Gandjbakhch, E.; Denjoy, I.; Hidden-Lucet, F.; Hatem, S.; Deschênes, I.; Coulombe, A.; Neyroud, N.; Guicheney, P. A truncating SCN5A mutation combined with genetic variability causes sick sinus syndrome and early atrial fibrillation. Heart Rhythm 2014, 11, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Béziau, D.M.; Barc, J.; O’Hara, T.; Le Gloan, L.; Amarouch, M.Y.; Solnon, A.; Pavin, D.; Lecointe, S.; Bouillet, P.; Gourraud, J.B.; et al. Complex Brugada syndrome inheritance in a family harbouring compound SCN5A and CACNA1C mutations. Basic Res. Cardiol. 2014, 109, 446. [Google Scholar] [CrossRef] [PubMed]
- Swan, H.; Amarouch, M.Y.; Leinonen, J.; Marjamaa, A.; Kucera, J.P.; Laitinen-Forsblom, P.J.; Lahtinen, A.M.; Palotie, A.; Kontula, K.; Toivonen, L.; et al. Gain-of-function mutation of the SCN5A gene causes exercise-induced polymorphic ventricular arrhythmias. Circ. Cardiovasc. Genet. 2014, 7, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Butters, T.D.; Aslanidi, O.V.; Inada, S.; Boyett, M.R.; Hancox, J.C.; Lei, M.; Zhang, H. Mechanistic links between Na+ channel (SCN5A) mutations and impaired cardiac pacemaking in sick sinus syndrome. Circ. Res. 2010, 107, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Zhang, X.; Cheng, L.; Lammers, W.J.; Grace, A.A.; Fraser, J.A.; Zhang, H.; Huang, C.L.H.; Lei, M. Altered sinoatrial node function and intra-atrial conduction in murine gain-of-function Scn5a+/ΔKPQ hearts suggest an overlap syndrome. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1510–H1523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Ma, A.; Zhou, X.; Gui, J.; Wan, H.; Shi, R.; Huang, C.; Grace, A.A.; Huang, C.L.H.; et al. Correlations between clinical and physiological consequences of the novel mutation R878C in a highly conserved pore residue in the cardiac Na+ channel. Acta Physiol. 2008, 194, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Holden, A.V.; Kodama, I.; Honjo, H.; Lei, M.; Varghese, T.; Boyett, M.R. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H397–H421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Holden, A.V.; Noble, D.; Boyett, M.R. Analysis of the chronotropic effect of acetylcholine on sinoatrial node cells. J. Cardiovasc. Electrophysiol. 2002, 13, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Wilders, R.; Jongsma, H.J.; Van Ginneken, A.C.G. Pacemaker activity of the rabbit sinoatrial node: A comparison of mathematical models. Biophys. J. 1991, 60, 1202–1216. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Wilders, R.; Van Borren, M.M.G.J.; Peters, R.J.G.; Broekhuis, E.; Lam, K.; Coronel, R.; De Bakker, J.M.T.; Tan, H.L. Pacemaker current (If) in the human sinoatrial node. Eur. Heart J. 2007, 28, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, A.A.; Lloyd, C.M.; Nielsen, P.F.; Bullivant, D.P.; Nickerson, D.P.; Hunter, P.J. An overview of CellML 1.1, a biological model description language. Simulation 2003, 79, 740–747. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Lawson, J.R.; Hunter, P.J.; Nielsen, P.F. The CellML Model Repository. Bioinformatics 2008, 24, 2122–2123. [Google Scholar] [CrossRef] [PubMed]
- CellML Model Repository. Available online: https://models.cellml.org/ (accessed on 13 February 2018).
- Garny, A.; Kohl, P.; Noble, D. Cellular open resource (COR): A public CellML based environment for modelling biological function. Int. J. Bifurcat. Chaos 2003, 13, 3579–3590. [Google Scholar] [CrossRef]


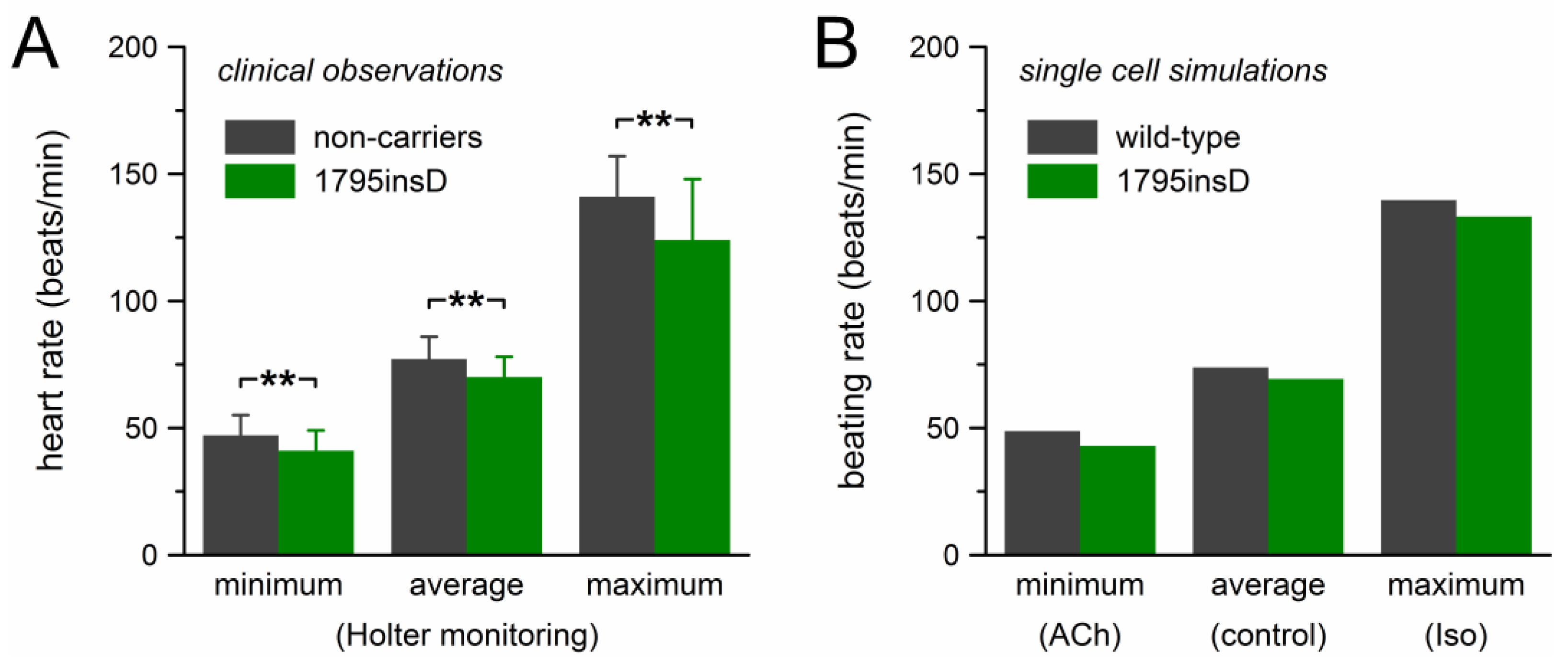
| Group | Heart Rate in 24-h Holter Recordings (beats/min) | n | ||
|---|---|---|---|---|
| Minimum | Average | Maximum | ||
| Mutation carriers | 41 ± 8 ** | 70 ± 8 ** | 124 ± 24 ** | 54 |
| Non-carriers | 47 ± 8 | 77 ± 9 | 141 ± 16 | 40 |
| Condition | CL (ms) | ∆CL (ms) | APD90 (ms) | ∆APD90 (ms) |
|---|---|---|---|---|
| Control | 813.4 | 151.0 | ||
| Activation shift | 859.8 | +46 | 150.7 | −0.2 |
| Inactivation shift | 857.0 | +44 | 150.7 | −0.2 |
| Double shift | 866.3 | +53 | 150.7 | −0.3 |
| Reduced gNa | 839.8 | +26 | 150.8 | −0.2 |
| Ipst | 747.4 | −66 | 157.4 | +6.5 |
| Ipst & reduced gNa | 800.9 | −12 | 154.0 | +3.1 |
| Loss of function | 867.1 | +54 | 150.6 | −0.3 |
| Gain of function | 747.4 | −66 | 157.4 | +6.5 |
| 1795insD | 866.3 | +53 | 153.0 | +2.0 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilders, R. Sinus Bradycardia in Carriers of the SCN5A-1795insD Mutation: Unraveling the Mechanism through Computer Simulations. Int. J. Mol. Sci. 2018, 19, 634. https://doi.org/10.3390/ijms19020634
Wilders R. Sinus Bradycardia in Carriers of the SCN5A-1795insD Mutation: Unraveling the Mechanism through Computer Simulations. International Journal of Molecular Sciences. 2018; 19(2):634. https://doi.org/10.3390/ijms19020634
Chicago/Turabian StyleWilders, Ronald. 2018. "Sinus Bradycardia in Carriers of the SCN5A-1795insD Mutation: Unraveling the Mechanism through Computer Simulations" International Journal of Molecular Sciences 19, no. 2: 634. https://doi.org/10.3390/ijms19020634