The Significance of MMP-1 in EGFR-TKI–Resistant Lung Adenocarcinoma: Potential for Therapeutic Targeting
Abstract
:1. Introduction
2. Results
2.1. mRNA Expression of MMPs and Related Genes
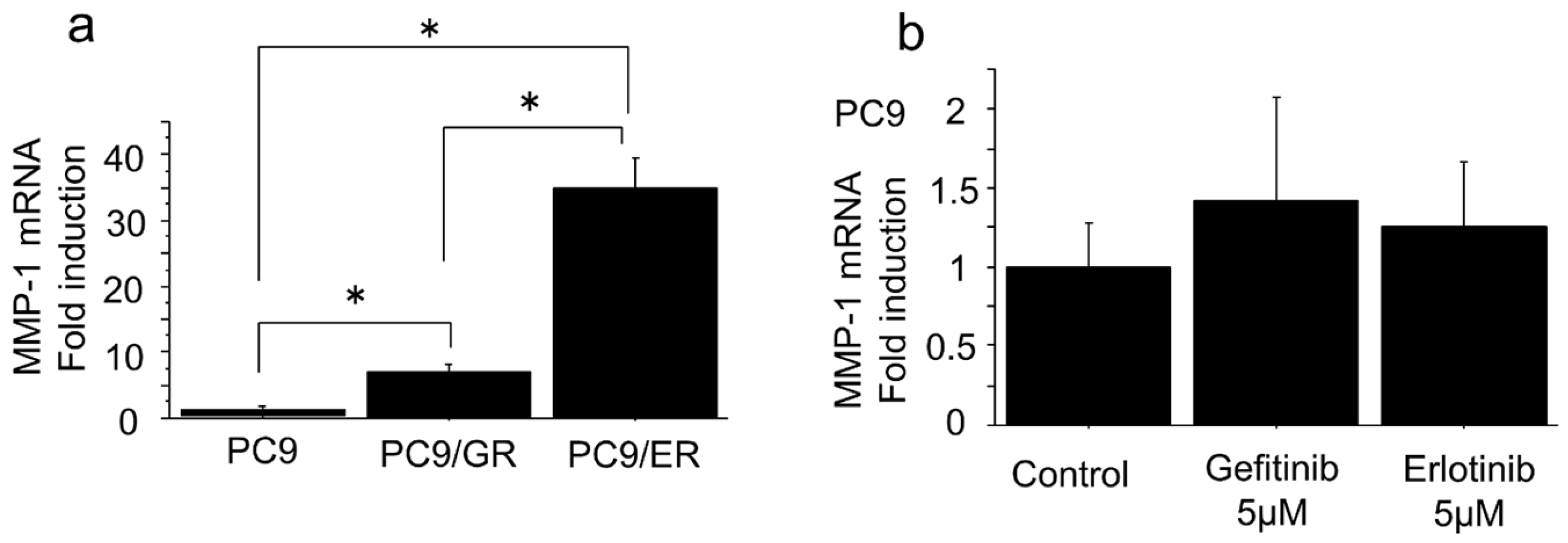
2.2. mRNA Expression of MMP-1 Gene in EGFR-TKI–Resistant Lung Adenocarcinoma Cells Was Higher than in EGFR-TKI–Sensitive Cells
2.3. MMP-1 Induced Migration and Invasion in EGFR-TKI–Resistant Lung Adenocarcinoma Cells with High Expression of MMP-1
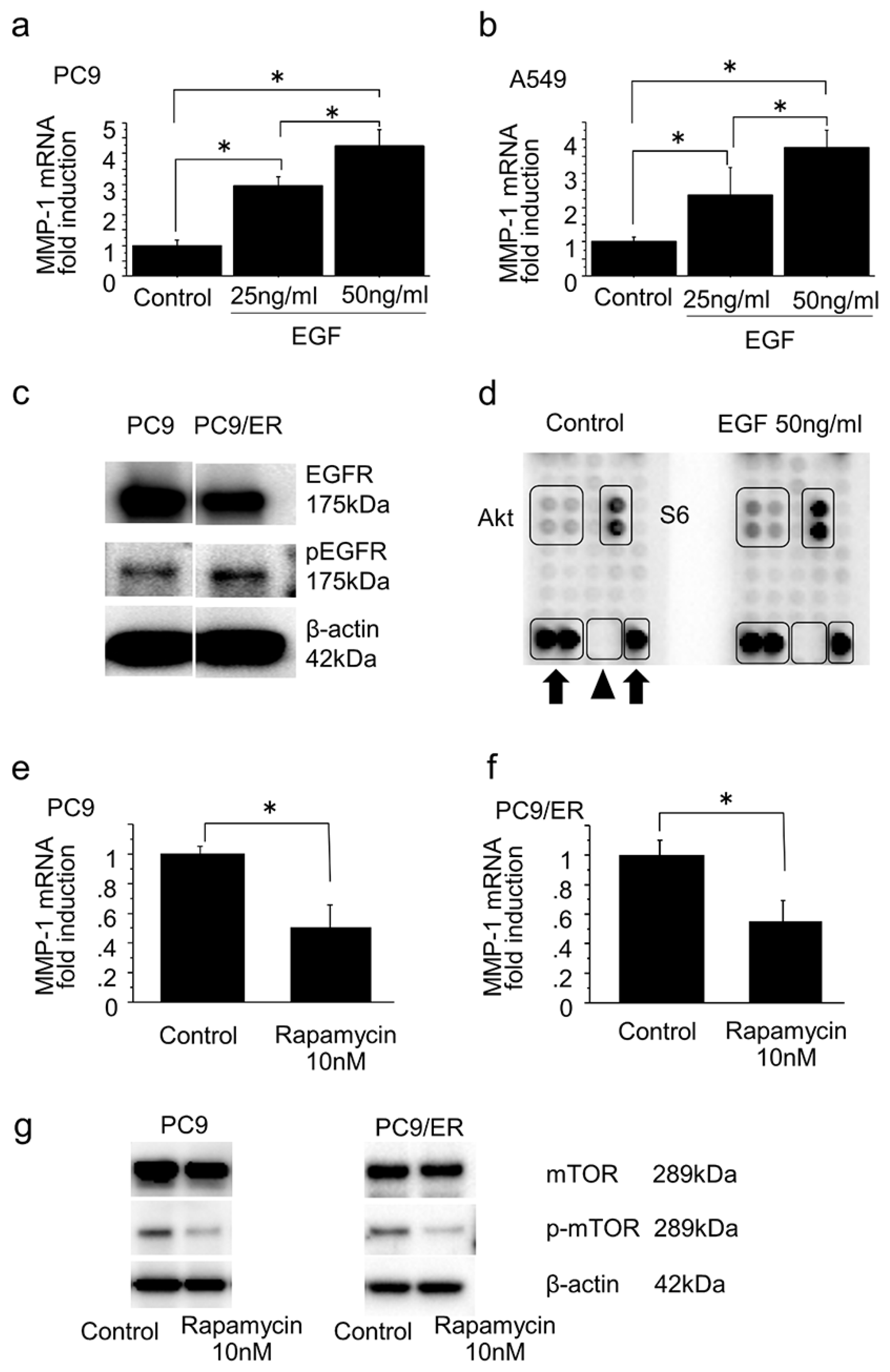
2.4. Mammalian Target of Rapamycin Pathway in the Induction of MMP-1 Expression in Lung Adenocarcinoma
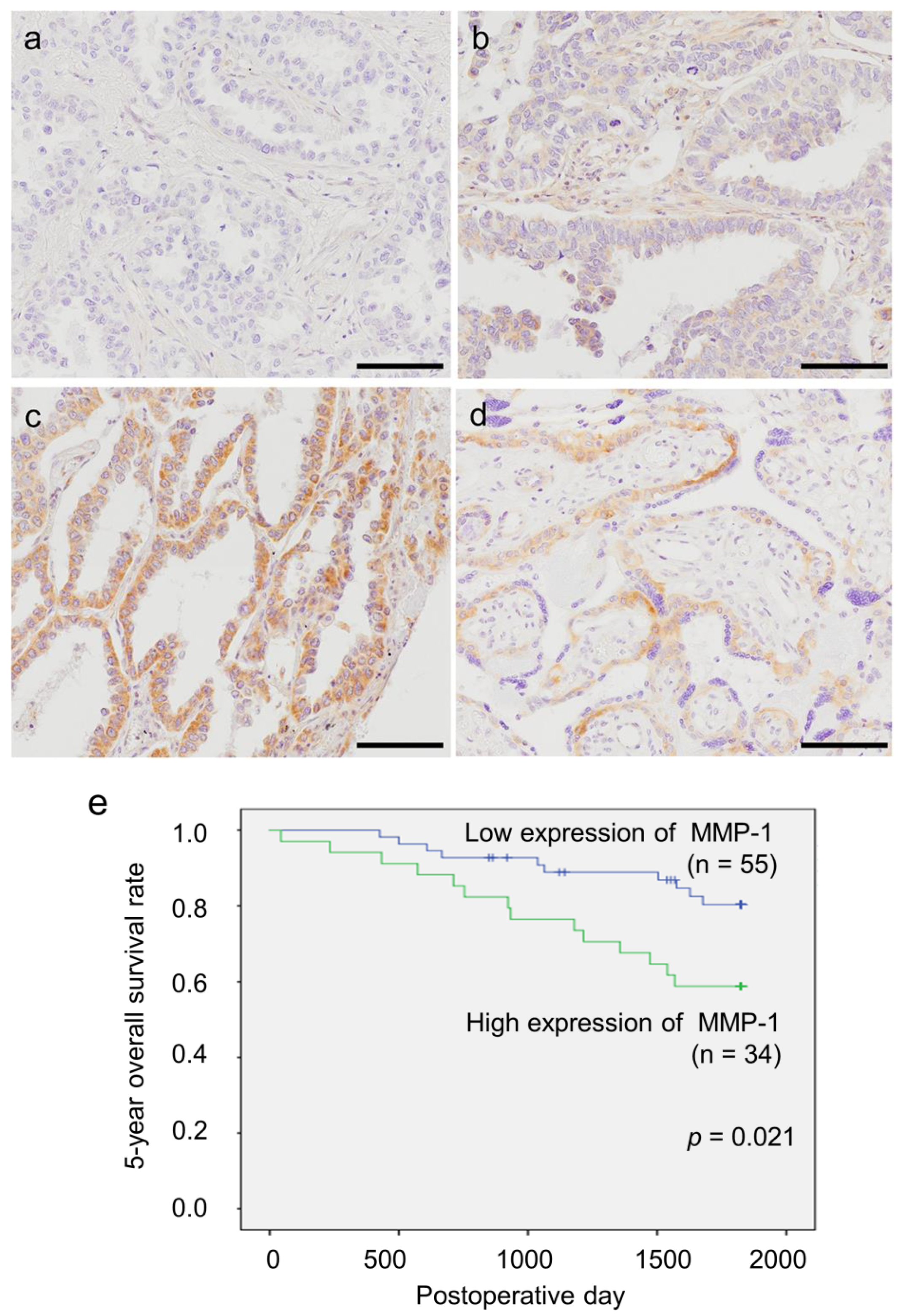
2.5. Status of MMP-1 Immunoreactivity in Patients with Lung Adenocarcinoma
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Microarray
4.3. Cell Culture
4.4. Real-Time RT-PCR
4.5. Western Blotting
4.6. RNA Interference
4.7. Wound Healing Assay
4.8. Migration Assay
4.9. Invasion Assay
4.10. Phosphorylation Antibody Array
4.11. Patients and Tissue Preparation
4.12. Immunohistochemistry
4.13. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohashi, K.; Maruvka, Y.E.; Michor, F.; Pao, W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J. Clin. Oncol. 2013, 31, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, G.; Kang, Y.; Dong, Z.; Qian, Q.; Ma, X. N-cadherin expression is associated with acquisition of EMT phenotype and with enhanced invasion in erlotinib-resistant lung cancer cell lines. PLoS ONE 2013, 8, e57692. [Google Scholar] [CrossRef] [PubMed]
- Breindel, J.L.; Haskins, J.W.; Cowell, E.P.; Zhao, M.; Nguyen, D.X.; Stern, D.F. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res. 2013, 73, 5053–5065. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, S.; Mohammad, K.S.; Pires, R.; Tato-Costa, J.; Alho, I.; Teixeira, R.; Carvalho, A.; Ribeiro, S.; Lipton, A.; Guise, T.A.; et al. RANKL/RANK/MMP-1 molecular triad contributes to the metastatic phenotype of breast and prostate cancer cells in vitro. PLoS ONE 2013, 8, e63153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, Y.; Liao, Q.; Ouyang, H. Helicobacter pylori infection promotes the invasion and metastasis of gastric cancer through increasing the expression of matrix metalloproteinase-1 and matrix metalloproteinase-10. Exp. Ther. Med. 2014, 8, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, Y.; Mimori, K.; Fukagawa, T.; Ishikawa, K.; Etoh, T.; Katai, H.; Sano, T.; Watanabe, M.; Sasako, M.; Mori, M. Clinical significance of molecular detection of matrix metalloproteinase-1 in bone marrow and peripheral blood in patients with gastric cancer. Ann. Surg. Oncol. 2012, 19, S430–S437. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.B.; Abel, E.V.; Mayberry, M.M.; Basile, K.J.; Berger, A.C.; Aplin, A.E. TWIST1 is an ERK1/2 effector that promotes invasion and regulates MMP-1 expression in human melanoma cells. Cancer Res. 2012, 72, 6382–6392. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiao, T.; Zhang, Y.; Feng, L.; Lin, D.; Liu, Y.; Mao, Y.; Guo, S.; Han, N.; Di, X.; et al. Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumour tissues of lung cancer patients. Lung Cancer 2010, 69, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tai, H.H. Increased expression of matrix metalloproteinases mediates thromboxane A2-induced invasion in lung cancer cells. Curr. Cancer Drug Targets 2012, 12, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Murata, T.; Suzuki, T.; Shindoh, M.; Nakajima, K.; Imai, K.; Yoshida, K. Requirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cells. Oncogene 2006, 25, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Pao, W.; Miller, V.; Zakowski, M.; Doherty, J.; Politi, K.; Sarkaria, I.; Singh, B.; Heelan, R.; Rusch, V.; Fulton, L.; et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA 2004, 101, 13306–13311. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Maehara, N.; Su, G.H.; Goggins, M. Effects of 5-aza-2′-deoxycytidine on matrix metalloproteinase expression and pancreatic cancer cell invasiveness. J. Natl, Cancer Inst. 2003, 95, 327–330. [Google Scholar] [CrossRef]
- Park, S.; Jung, H.H.; Park, Y.H.; Ahn, J.S.; Im, Y.H. ERK/MAPK pathways play critical roles in EGFR ligands-induced MMP1 expression. Biochem. Biophys. Res. Commun. 2011, 407, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chao, A.; Wang, T.H.; Hsueh, S.; Lee, Y.S.; Wu, T.I.; Chao, A.S.; Huang, H.J.; Chou, H.H.; Chang, T.C.; et al. A dual tyrosine kinase inhibitor lapatinib suppresses overexpression of matrix metallopeptidase 1 (MMP1) in endometrial cancer. J. Mol. Med. (Berl.) 2014, 92, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Nakayama, Y.; Umeda, M.; Miyazaki, K. Induction of 103-kDa gelatinase/type IV collagenase by acidic culture conditions in mouse metastatic melanoma cell lines. J. Biol. Chem. 1992, 267, 11424–11430. [Google Scholar] [PubMed]
- Memmott, R.M.; Dennis, P.A. The role of the Akt/mTOR pathway in tobacco carcinogen-induced lung tumorigenesis. Clin. Cancer Res. 2010, 16, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Matsukuma, S.; Yoshihara, M.; Nakamura, Y.; Noda, K.; Nakayama, H.; Kameda, Y.; Tsuchiya, E.; Miyagi, Y. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am. J. Clin. Pathol. 2007, 128, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kaseda, K.; Asakura, K.; Kazama, A.; Ozawa, Y. Clinicopathological and prognostic features of surgically resected pathological stage I lung adenocarcinoma harboring epidermal growth factor receptor and K-ras mutation. Thorac. Cancer. 2017, 8, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Nihira, K.; Miki, Y.; Iida, S.; Narumi, S.; Ono, K.; Iwabuchi, E.; Ise, K.; Mori, K.; Saito, M.; Ebina, M.; et al. An activation of LC3A-mediated autophagy contributes to de novo and acquired resistance to EGFR tyrosine kinase inhibitors in lung adenocarcinoma. J. Pathol. 2014, 234, 277–288. [Google Scholar] [PubMed]
- Prasad, N.B.; Fischer, A.C.; Chuang, A.Y.; Wright, J.M.; Yang, T.; Tsai, H.L.; Westra, W.H.; Liegeois, N.J.; Hess, A.D.; Tufaro, A.P. Differential expression of degradome components in cutaneous squamous cell carcinomas. Mod. Pathol. 2014, 27, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Bianco, B.C.; Scotti, F.M.; Vieira, D.S.; Biz, M.T.; Castro, R.G.; Modolo, F. Immunohistochemical expression of matrix metalloproteinase-1, matrix metalloproteinase-2 and matrix metalloproteinase-9, myofibroblasts and Ki-67 in actinic cheilitis and lip squamous cell carcinoma. Int. J. Exp. Pathol. 2015, 96, 311–318. [Google Scholar] [CrossRef] [PubMed]

| Change of mRNA Levels in PC9/GR and PC9/ER Compared to PC9/6m | Gene Name | |
|---|---|---|
| Up | >10-fold | MMP-1, 23, 24 |
| Up | ≦10-fold | MMP-2, 3, 8, 10, 11, 12, 13, 14, 15, 16, 17, 19, 20, 21, 26, 27, TIMP-1, 3, 4 |
| No change | MMP-4, 5, 6, 18, 22 | |
| Down | MMP-7, 9, 25, 28, TIMP-2 | |
| MMP-1 immunoreactivity | |||
|---|---|---|---|
| Low (n = 55) | High (n = 34) | p Value | |
| Age | 65.2 ± 11.1 | 65.1 ± 9.7 | 0.972 |
| Sex | 0.121 | ||
| Male | 28 | 23 | |
| Female | 27 | 11 | |
| Smoking Status | 0.016 * | ||
| Nonsmoker | 27 | 8 | |
| Smoker | 28 | 26 | |
| Brinkmann Index | 432.9 ± 552.9 | 688.3 ± 560.9 | 0.038 * |
| pStage | 0.060 | ||
| I | 40 | 18 | |
| II | 5 | 5 | |
| III–IV | 10 | 11 | |
| pT | 0.029 * | ||
| 1–2 | 52 | 27 | |
| 3–4 | 3 | 7 | |
| pN | 0.947 | ||
| 0 | 44 | 27 | |
| 1–3 | 11 | 7 | |
| Tumor Size | 24.1 ± 9.2 | 33.3 ± 17.5 | 0.007 * |
| Dominant Histological Subtype | |||
| Lepidic | 17 | 5 | 0.085 |
| Papillary | 25 | 10 | 0.132 |
| Acinar | 6 | 3 | 0.751 |
| Solid | 6 | 2 | 0.420 |
| Mucinous | 1 | 12 | <0.001 * |
| Pleomorphic | 0 | 2 | 0.069 |
| Univariate | Multivariate | ||
|---|---|---|---|
| p Value | p Value | Relative Risk (95% CI) | |
| MMP-1 (low/high) | 0.021 * | 0.020 * | 2.722 (1.170–6.330) |
| pT (1–2/3–4) | 0.019 * | 0.031 * | 8.049 (1.211–53.510) |
| pN (0/1–3) | 0.006 * | 0.012 * | 12.429 (1.721–89.738) |
| pM (0/1) | 0.292 | 0.189 | 3.227 (0.558–19.251) |
| pStage (I/II–IV) | 0.003 * | 0.217 | 0.272 (0.034–2.151) |
| Smoking Status (nonsmoker/smoker) | 0.211 | 0.736 | 0.805 (0.228–2.842) |
| Sex (male/female) | 0.040 * | 0.135 | 0.378 (0.106–1.355) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, R.; Miki, Y.; Ishida, N.; Inoue, C.; Kobayashi, M.; Hata, S.; Yamada-Okabe, H.; Okada, Y.; Sasano, H. The Significance of MMP-1 in EGFR-TKI–Resistant Lung Adenocarcinoma: Potential for Therapeutic Targeting. Int. J. Mol. Sci. 2018, 19, 609. https://doi.org/10.3390/ijms19020609
Saito R, Miki Y, Ishida N, Inoue C, Kobayashi M, Hata S, Yamada-Okabe H, Okada Y, Sasano H. The Significance of MMP-1 in EGFR-TKI–Resistant Lung Adenocarcinoma: Potential for Therapeutic Targeting. International Journal of Molecular Sciences. 2018; 19(2):609. https://doi.org/10.3390/ijms19020609
Chicago/Turabian StyleSaito, Ryoko, Yasuhiro Miki, Naoya Ishida, Chihiro Inoue, Masayuki Kobayashi, Shuko Hata, Hisafumi Yamada-Okabe, Yoshinori Okada, and Hironobu Sasano. 2018. "The Significance of MMP-1 in EGFR-TKI–Resistant Lung Adenocarcinoma: Potential for Therapeutic Targeting" International Journal of Molecular Sciences 19, no. 2: 609. https://doi.org/10.3390/ijms19020609
APA StyleSaito, R., Miki, Y., Ishida, N., Inoue, C., Kobayashi, M., Hata, S., Yamada-Okabe, H., Okada, Y., & Sasano, H. (2018). The Significance of MMP-1 in EGFR-TKI–Resistant Lung Adenocarcinoma: Potential for Therapeutic Targeting. International Journal of Molecular Sciences, 19(2), 609. https://doi.org/10.3390/ijms19020609





