Sea Buckthorn Pomace Supplementation in the Diet of Growing Pigs—Effects on Fatty Acid Metabolism, HPA Activity and Immune Status
Abstract
:1. Introduction
2. Results
2.1. Fatty Acid Profiling
2.2. Biochemical Parameters in Blood Plasma
2.3. Immune Parameters in Peripheral Blood
2.4. Fatty Acid Concentration in Hypothalamus Tissue
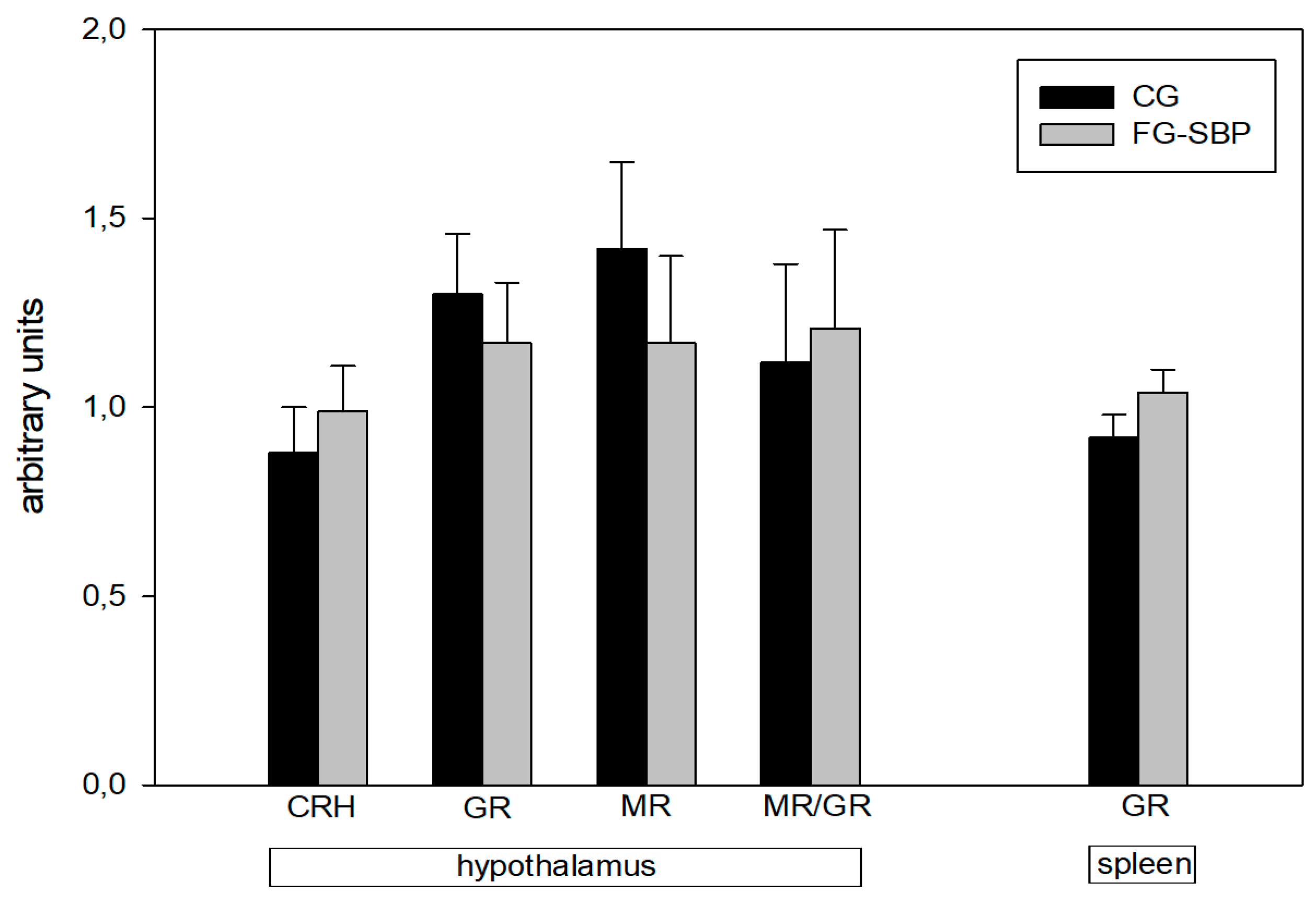
2.5. Hypothalamus mRNA Expression Levels
3. Discussion
4. Materials and Methods
4.1. Animal Study
4.2. Sample Preparation for Fatty Acid Analysis—Blood Plasma
4.3. Sample Preparation for Fatty Acid Analysis—Hypothalamus
4.4. Analysis of Fatty Acids
4.5. Biochemical Analyses
4.6. RNA Isolation and Quantification of Transcripts
4.7. Immune Parameter Analysis
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SBP | Sea buckthorn pomace |
| n-3 PUFA | n-3 polyunsaturated fatty acids |
| SFA | Saturated fatty acids |
| MUFA | Mono unsaturated fatty acids |
| CRH | Corticotrophin releasing hormone |
| MR | Mineralocorticoid receptor |
| GR | Glucocorticoid receptor |
| HPA | Hypothalamic–pituitary–adrenal |
References
- Krylova, S.G.; Konovalova, O.N.; Zueva, E.P. Correction by common sea buckthorn bark and sprout extracts of hormonal and metabolic disturbances during stress in rats. Eksp. Klin. Farmakol. 2000, 63, 70–73. [Google Scholar] [PubMed]
- Zeb, A. Important therapeutic uses of Sea Buckthorn (Hippophae): A review. J. Biol. Sci. 2004, 4, 687–693. [Google Scholar]
- Korekar, G.; Stobdan, T.; Singh, H.; Chaurasia, O.P.; Singh, S.B. Phenolic content and antioxidant capacity of various solvent extracts from sea buckthorn (Hippophae rhamnoides L.) fruit pulp, seeds, leaves and stem bark. Acta Aliment. Hung. 2011, 40, 449–458. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef]
- Vashishtha, V.; Barhwal, K.; Kumar, A.; Hota, S.K.; Chaurasia, O.P.; Kumar, B. Effect of seabuckthorn seed oil in reducing cardiovascular risk factors: A longitudinal controlled trial on hypertensive subjects. Clin. Nutr. 2017, 36, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, T.; Hilal, L.M.; Fourrier, C.; De Smed-Peyrusse, V.; Sans, N.; Capuron, L.; Laye, S. Nutritional omega-3 modulates neuronal morphology in the prefreontal cortex along with depression-related behavior through corticosterone secretion. Transl. Psychiatry 2014, 4, e437. [Google Scholar] [CrossRef] [PubMed]
- Lanfranco, F.; Giordano, R.; Pellegrino, M.; Gianotti, L.; Ramunni, J.; Picu, A.; Baldi, M.; Ghigo, E.; Arvat, E. Free fatty acids exert an inhibitory effect on adrenocorticotropin and cortisol secretion in humans. J. Clin. Endocrinol. Metab. 2004, 89, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Vallette, G.; Vanet, A.; Sumida, C.; Nunez, E.A. Modulatory effects of unsaturated fatty acids on binding of glucocorticoids to rat liver glucocorticoid receptors. Endocrinology 1991, 129, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, P.; Calder, P.C. Fatty acids and immune function: new insights into mechanisms. J. Nutr. 2007, 98, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Nuernberg, K.; Nuernberg, G.; Priepke, A.; Dannenberger, D. Sea buckthorn pomace supplementation in the finishing diets of pigs—Are there effects on meat quality and muscle fatty acids? Arch. Anim. Breed. 2015, 58, 107–113. [Google Scholar] [CrossRef]
- Swiatkiewicz, S.; Arczewska-Wlosek, A.; Jozefiak, D. The relationship between dietary fat sources and immune response in poultry and pigs: An updated review. Livest. Sci. 2015, 180, 237–246. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways inweaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Gras, M.; Pistol, G.C.; Motiu, M.; Marin, D.E.; Lefter, N.; Ropota, M.; Habeanu, M. ω-3 PUFA rich Camelina oil by-products improve the systemic metabolism and spleen cell functions in fattening pigs. PLoS ONE 2014, 9, e110186. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.P.; Huang, F.R.; Luo, J.; Dai, J.J.; Yan, X.H.; Peng, J. Duration of feeding linseed diet influences expression of inflammation-related genes and growth performance of growing-finishing barrows. J. Anim. Sci. 2009, 87, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Dullemeijer, C.; Zock, P.L.; Coronel, R.; Den Ruijter, H.M.; Katan, M.B.; Brummer, R.J.M.; Kok, F.J.; Beekman, J.; Brouwer, I.A. Differences in fatty acid composition between cerebral brain lobes in juvenile pigs after fish oil feeding. Br. J. Nutr. 2008, 100, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, J.; Wang, J.; Pang, X.; Zhuang, X.; Zhu, X.; Qu, W. Hypoglycemic effect of aqueous extract of seabuckthorn (Hippophae rhamnoides L.) seed residues in streptozotocin-induced diabetic rats. Phytother. Res. 2010, 24, 228–232. [Google Scholar] [PubMed]
- Zakynthinos, G.; Varzakas, T.; Petsios, D. Sea buckthorn (Hippophae rhamnoides) lipids and their functionality on health aspects. Curr. Res. Nutr. Food Sci. 2016, 4, 182–194. [Google Scholar] [CrossRef]
- Dubey, G.P.; Agrawal, A.; Dixit, S.P. Role of sea buckthorn (Hippophae rhamnoides) in the maintenance of cardiovascular homeostasis following cold stress. J. Nat. Rem. 2003, 3, 36–40. [Google Scholar]
- Diandong, H.; Feng, G.; Zaifu, L.; Helland, T.; Weixin, F.; Liping, C. Sea buckthorn (Hippophae rhamnoides L.) oil protects against chronic stress-induced inhibitory function of natural killer cells in rats. Int. J. Immunpath. Pharmacol. 2016, 29, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Habenau, M.; Hebean, V.; Nagy, A.; Taranu, I.; Lefter, N.; Marin, D.; Grosu, H. The dietary omega-3 PUFA alter the metabolic and the immunologic serum profile in Mangalitza pigs in extensive rearing system. Arch. Zootechn. 2011, 14, 5–12. [Google Scholar]
- Yao, W.; Li, J.; Wang, J.J.; Zhou, W.; Wang, Q.; Zhu, R.; Wang, F.; Thacker, P. Effects of dietary ratio of n-6 to n-3 polyunsaturated fatty acids on immunoglobulins, cytokines, fatty acid composition, and performance of lactating sows and suckling piglets. J. Anim. Sci. Biotechnol. 2012, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. Effect of maternal fish oil and seaweed extract supplementation on colostrum and milk composition, humoral immune response, and performance of suckled piglets. J. Anim. Sci. 2010, 88, 2988–2997. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Brendemuhl, J.H.; Jeong, K.C.; Badinga, L. Effects of dietary omega-3 polyunsaturated fatty acids on growth and immune response of weanling pigs. J. Anim. Sci. Technol. 2014, 56, 7. [Google Scholar] [CrossRef] [PubMed]
- Puppe, B.; Tuchscherer, M.; Tuchscherer, A. The effect of housing conditions and social environment immediatly after weaning on the agonistic behaviour, neutrophil/lymphocyte ratio, and plasma glucose level in pigs. Livest. Prod. Sci. 1997, 48, 157–164. [Google Scholar] [CrossRef]
- Tuchscherer, M.; Otten, W.; Kanitz, E.; Gräbner, M.; Tuchscherer, A.; Bellmann, O.; Rehfeldt, C.; Metges, C. Effect of inadequate maternal dietary protein:carbohydrate ratios during pregnancy on offspring immunity in pigs. BMC Vet. Res. 2012, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, J.W.T.; Dobbing, J. Prenetal and postnatal growth and development of the nervous system of the pig. Proc. R. Soc. (B) 1967, 166, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, L.; Fan, J.; Sun, X.; Yin, Y. n-6:n-3 PUFA ratio is involved in regulating lipid metabolism and inflammation in pigs. Br. J. Nutr. 2014, 111, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Dannenberger, D.; Nuernberg, G.; Nuernberg, K.; Will, K.; Schauer, N.; Schmicke, M. Effects of diets supplemented with n-3 or n-6 PUFA on pig muscle lipid metabolites measured by non-targeted LC–MS lipidomic profiling. J. Food Compos. Anal. 2017, 56, 47–54. [Google Scholar] [CrossRef]
- Dannenberger, D.; Nuernberg, K.; Nuernberg, G.; Priepke, A. Different dietary protein and PUFA intervention alters the fatty acid concentrations, but not the meat quality of porcine muscle. Nutrients 2012, 4, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Piechotta, M.; Mysegades, W.; Ligges, U.; Lilienthal, J.; Hoeflich, A.; Miyamoto, A.; Bollwein, H. Antepartal insulin-like growth factor 1 and insulin-like growth factor binding protein 2 concentrations are indicative of ketosis in dairy cows. J. Dairy Sci. 2015, 98, 3100–3109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanitz, E.; Otten, W.; Tuchscherer, M.; Gräbner, M.; Brüssow, K.P.; Rehfeldt, C.; Metges, C.C. High and low protein: carbohydrate dietary ratios during gestation alter maternal-fetal cortisol regulation in pigs. PLoS ONE 2012, 7, e52748. [Google Scholar] [CrossRef] [PubMed]
- Paffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002. [Google Scholar] [CrossRef] [Green Version]
- Tuchscherer, M.; Kanitz, E.; Puppe, B.; Tuchscherer, A. Altered immunomodulation by glucocorticoids in neonatal pigs exposed to a psychosocial stressor. Pediatr. Res. 2010, 68, 473–478. [Google Scholar] [CrossRef] [PubMed]
| CG | FG-SBP | CG | FG-SBP | p-Value | p-Value | p-Value | |
|---|---|---|---|---|---|---|---|
| Timepoint 0 (TP0) § | Timepoint 1 (TP1) § | CG vs. FG-SBP | TP 0 vs. TP 1 | SBP * TP | |||
| LSMSEM (n = 10) | LSMSEM (n = 10) | LSMSEM (n = 10) | LSMSEM (n = 10) | ||||
| SFA | |||||||
| C12:0 | 0.590.08 a | 0.640.08 a | 0.090.01 b | 0.090.01 b | 0.7680 | <0.0001 | 0.6479 |
| C14:0 | 0.980.03 a | 0.930.03 a,b | 0.650.03 b | 0.580.03 b | 0.0297 | <0.0001 | 0.8261 |
| C15:0 | 0.460.05 a | 0.330.05 a | 0.360.02 a,b | 0.280.02 b | 0.0162 | 0.0786 | 0.5120 |
| C16:0 | 15.720.32 | 15.330.32 | 16.220.34 | 15.760.34 | 0.2908 | 0.0867 | 0.8897 |
| C18:0 | 10.700.20 a | 11.150.20 a | 11.980.17 b | 11.920.17 b | 0.2652 | <0.0001 | 0.2202 |
| Sum SFA* | 39.860.79 a | 38.440.79 a | 34.300.40 b | 33.830.40 b | 0.1261 | <0.0001 | 0.4788 |
| MUFA | |||||||
| C16:1cis-9 | 1.260.05 a | 1.260.05 a | 1.630.10 b | 1.600.10 b | 0.8417 | 0.0016 | 0.8493 |
| C18:1cis-9 | 16.380.45 a | 16.410.45 a | 18.810.49 b | 17.870.49 b | 0.2557 | 0.0021 | 0.3828 |
| C18:1cis-11 | 1.410.05 a | 1.370.05 a | 1.720.08 b | 1.720.08 b | 0.7300 | 0.0001 | 0.7724 |
| Sum MUFA # | 20.860.63 a | 20.330.63 a | 23.880.52 b | 22.580.52 b | 0.0689 | 0.0009 | 0.5690 |
| PUFA | |||||||
| C18:2n-6 | 25.020.68 a | 25.600.68 a | 25.590.75 a | 27.570.75 b | 0.1226 | 0.0578 | 0.2790 |
| C18:3n-3 | 0.720.04 a | 0.710.04 a | 0.930.09 b | 0.990.08 b | 0.7748 | 0.0003 | 0.4793 |
| C20:4n-6 | 9.310.41 | 10.270.41 | 10.160.33 | 9.860.33 | 0.3996 | 0.5468 | 0.0912 |
| C20:5n-3 | 0.560.03 a | 0.630.03 a | 0.870.04 b | 0.800.04 b | 0.9143 | <0.0001 | 0.0934 |
| C22:5n-3 | 1.260.05 a | 1.370.05 a | 1.640.05 b | 1.530.05 b | 0.9677 | <0.0001 | 0.0470 |
| C22:6n-3 | 0.920.09 a | 0.980.09 a | 0.710.04 b | 0.600.04 b | 0.7586 | 0.0009 | 0.2987 |
| Sum PUFA + | 39.280.91 a | 41.230.91 a | 41.820.60 b | 43.560.60 b | 0.0225 | 0.0062 | 0.9129 |
| n-6/n-3 PUFA ratio | 10.300.30 a | 10.150.30 a | 9.070.30 b | 10.100.30 a | 0.2110 | 0.0281 | 0.0416 |
| CG | FG-SBP | CG | FG-SBP | p-Value | p-Value | p-Value | |
|---|---|---|---|---|---|---|---|
| Timepoint 0 (TP0) § | Timepoint 1 (TP1) § | CG vs. FG-SBP | TP 0 vs. TP 1 | SBP * time point | |||
| LSMSEM (n = 10) | LSMSEM (n = 10) | LSMSEM (n = 10) | LSMSEM (n = 10) | ||||
| Triglycerides (mmol/L) | 0.420.05 | 0.460.05 | 0.330.03 | 0.360.03 | 0.4844 | 0.0298 | 0.8820 |
| Cholesterol (mmol/L) | 2.340.06 | 2.310.06 | 2.270.06 | 2.340.06 | 0.7855 | 0.7476 | 0.4222 |
| Glucose (mmol/L) | 3.830.20 a | 5.510.20 b | 4.430.22 b | 4.830.22 b | 0.0003 | 0.8110 | 0.0032 |
| FFA (mmol/L) | 57.619.01 | 62.369.01 | 108.626.65 | 45.0026.65 | 0.1883 | 0.3648 | 0.0750 |
| Lactate (mmol/L) | 2.940.46 | 2.150.46 | 2.540.62 | 2.350.62 | 0.4217 | 0.8450 | 0.5513 |
| Cortisol (ng/mL) | 34.372.94a | 14.612.94b | 24.023.39c | 12.793.39b | 0.0007 | 0.0188 | 0.0870 |
| Protein (mg/mL) | 69.820.94 a | 70.410.94 a | 72.021.07 b | 72.881.07 b | 0.4979 | 0.0260 | 0.8926 |
| IgG (mg/mL) | 10.920.64 a | 12.610.64 b | 10.100.45 a | 13.550.45 b | 0.0005 | 0.9091 | 0.0925 |
| IgM (mg/mL) | 5.230.47 | 5.510.47 | 6.110.58 | 5.570.58 | 0.8461 | 0.1912 | 0.2464 |
| CG | FG-SBP | p-Value (p < 0.05) | |
|---|---|---|---|
| LSMSEM (n = 10) | LSMSEM (n = 10) | ||
| PBMC × 106 | 8.310.67 | 8.810.67 | 0.6072 |
| PI-ConA | 3.360.20 | 3.360.20 | 0.9972 |
| PI-LPS | 2.080.11 | 2.030.11 | 0.7598 |
| Lymphocytes (%) | 45.762.33 | 44.992.33 | 0.8187 |
| Monocytes (%) | 8.140.56 | 8.660.54 | 0.5224 |
| Neutrophils (%) | 27.212.34 | 30.832.36 | 0.2911 |
| Neutrophil to lymphocyte (N/L) ratio | 0.620.08 | 0.730.08 | 0.3773 |
| CG | FG-SBP | p-Value (p < 0.05) | |
|---|---|---|---|
| LSMSEM (n =10) | LSMSEM (n = 10) | ||
| Sum fatty acids | 35.042.79 | 37.682.79 | 0.5118 |
| SFA | |||
| C12:0 | 0.010.001 | 0.010.001 | 0.8066 |
| C14:0 | 0.090.01 | 0.090.01 | 0.7045 |
| C15:0 | 0.030.003 | 0.030.003 | 0.5327 |
| C16:0 | 5.510.43 | 5.840.43 | 0.5964 |
| C18:0 | 6.000.50 | 6.440.50 | 0.5389 |
| C20:0 | 0.080.01 | 0.090.01 | 0.3140 |
| Sum SFA | 12.411.00 | 13.210.97 | 0.5653 |
| MUFA | |||
| C16:1 | 0.240.02 | 0.260.02 | 0.5384 |
| C18:1 cis-9 | 6.730.57 | 7.540.57 | 0.3253 |
| C18:1 cis-11 | 1.700.14 | 1.880.14 | 0.4091 |
| Sum MUFA | 10.090.87 | 11.290.87 | 0.3422 |
| PUFA | |||
| C18:2 n-6 | 0.240.02 | 0.250.02 | 0.7032 |
| C18:3 n-3 | 0.170.02 | 0.210.02 | 0.2637 |
| C20:4 n-6 | 4.160.32 | 4.380.32 | 0.6368 |
| C20:5 n-3 | 0.320.03 | 0.360.03 | 0.3234 |
| C22:5 n-3 | 0.140.01 | 0.130.01 | 0.6499 |
| C22:6 n-3 | 4.740.38 | 4.890.38 | 0.7726 |
| Sum PUFA | 12.240.95 | 12.840.95 | 0.6661 |
| n-6/n-3 PUFA ratio | 1.250.02 | 1.270.02 | 0.3655 |
| Control Group (CG) | Feeding Group (FG-SBP) | |
|---|---|---|
| Feed components (%) | ||
| SBP | - | 12.0 |
| Wheat | 26.2 | 53.4 |
| Barley | 20.0 | - |
| Triticale | 20.0 | - |
| Rye | 15.0 | 15.0 |
| Soybean meal | 11.8 | 12.5 |
| Soybean skin | 2.5 | - |
| Beet molasses | 0.75 | - |
| Dextrose | - | 3.3 |
| Nutrient composition | ||
| Crude fat (%) | 2.7 | 3.6 |
| Crude protein (%) | 15.4 | 15.6 |
| Energy (MJ) | 13.4 | 12.2 |
| Lysine (%) | 0.68 | 0.60 |
| Fatty acid profile (%) | ||
| C14:0 | 0.43 | 0.45 |
| C16:0 | 20.5 | 21.5 |
| C16:1 | 1.2 | 5.0 |
| C18:0 | 2.4 | 2.3 |
| C18:1cis-9 | 17.5 | 19.5 |
| C18:2n-6 | 47.4 | 39.4 |
| C18:3n-3 | 5.8 | 5.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dannenberger, D.; Tuchscherer, M.; Nürnberg, G.; Schmicke, M.; Kanitz, E. Sea Buckthorn Pomace Supplementation in the Diet of Growing Pigs—Effects on Fatty Acid Metabolism, HPA Activity and Immune Status. Int. J. Mol. Sci. 2018, 19, 596. https://doi.org/10.3390/ijms19020596
Dannenberger D, Tuchscherer M, Nürnberg G, Schmicke M, Kanitz E. Sea Buckthorn Pomace Supplementation in the Diet of Growing Pigs—Effects on Fatty Acid Metabolism, HPA Activity and Immune Status. International Journal of Molecular Sciences. 2018; 19(2):596. https://doi.org/10.3390/ijms19020596
Chicago/Turabian StyleDannenberger, Dirk, Margret Tuchscherer, Gerd Nürnberg, Marion Schmicke, and Ellen Kanitz. 2018. "Sea Buckthorn Pomace Supplementation in the Diet of Growing Pigs—Effects on Fatty Acid Metabolism, HPA Activity and Immune Status" International Journal of Molecular Sciences 19, no. 2: 596. https://doi.org/10.3390/ijms19020596





