Separation Options for Phosphorylated Osteopontin from Transgenic Microalgae Chlamydomonas reinhardtii
Abstract
:1. Introduction
2. Results and Discussion
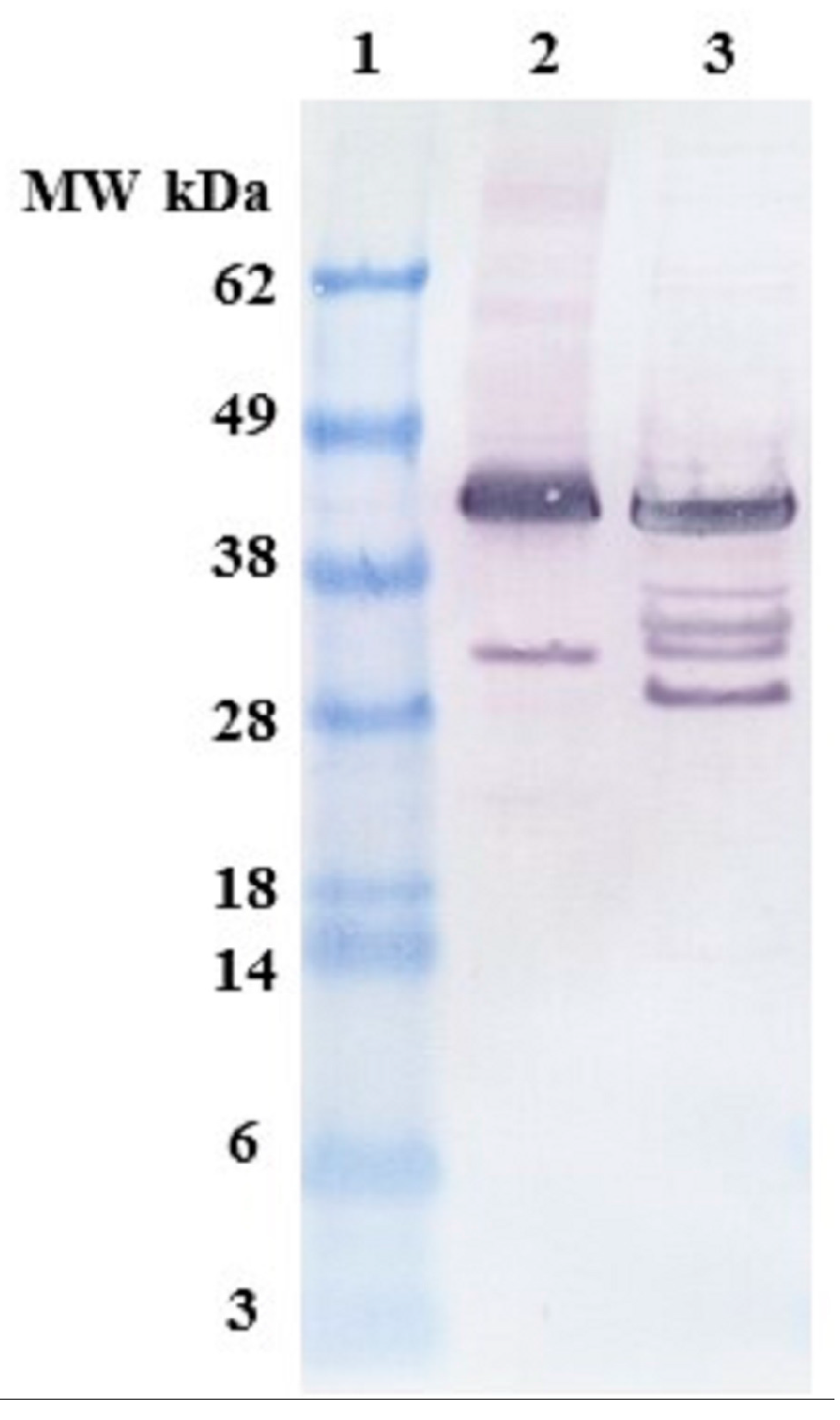
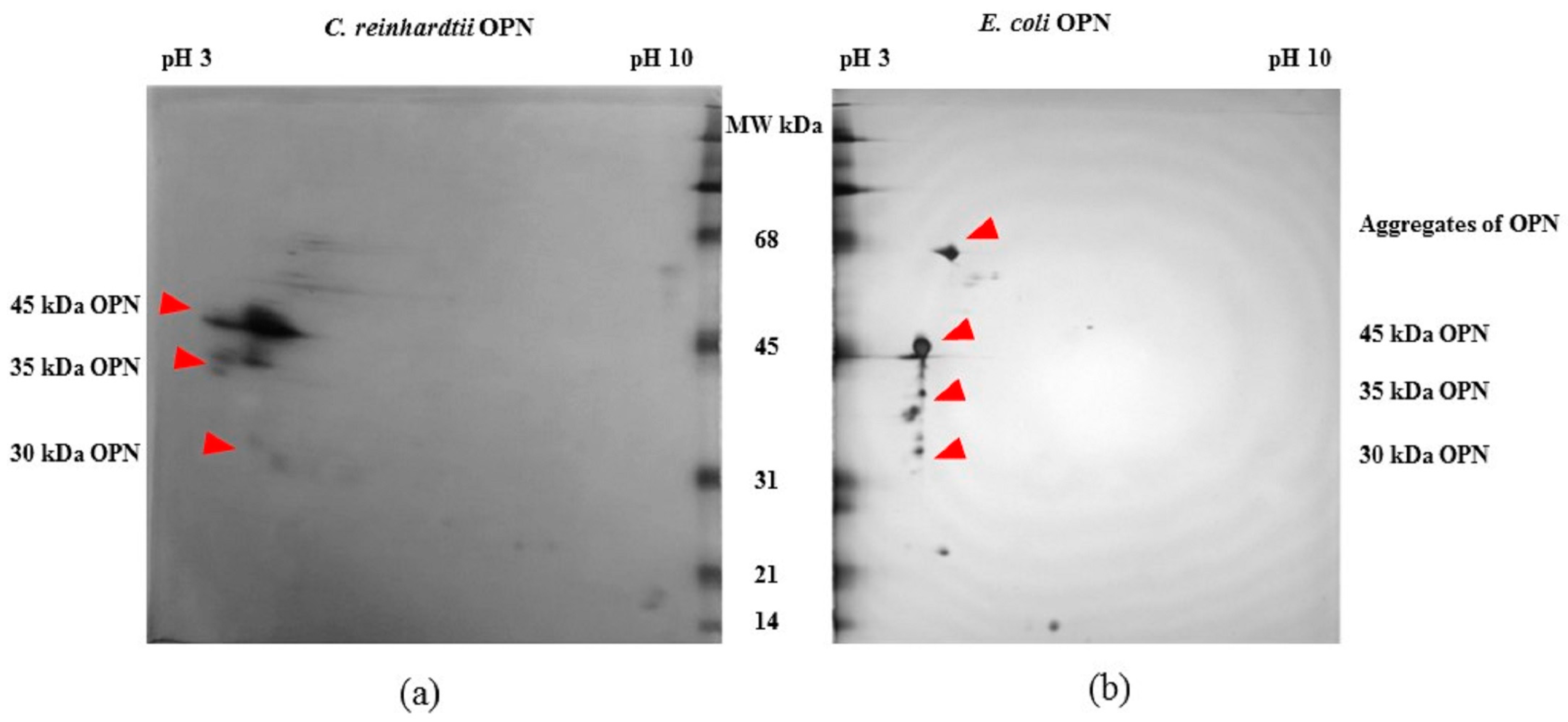
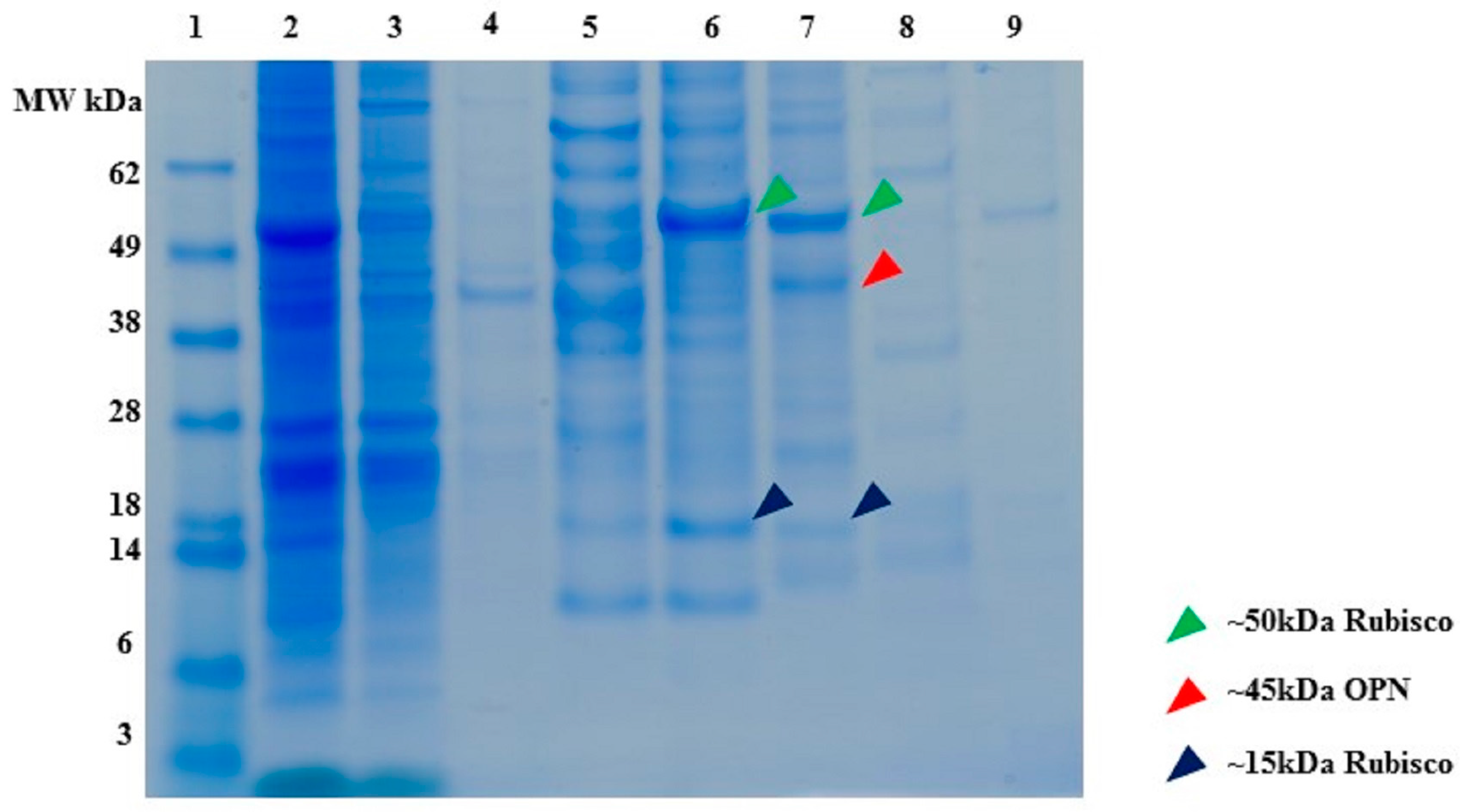
2.1. Expression and Characterization of Recombinant Osteopontin in C. reinhardtii and E. coli
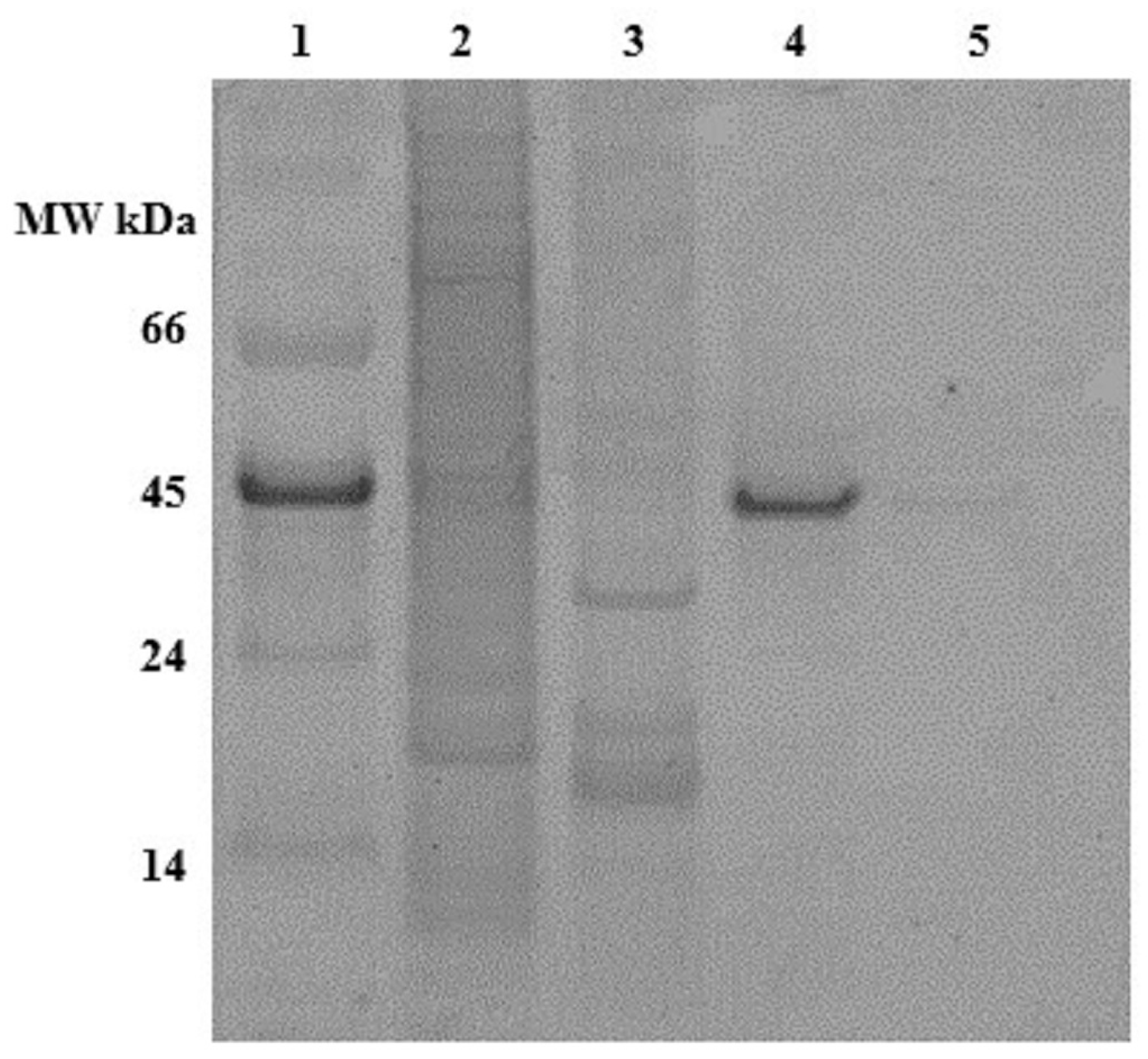
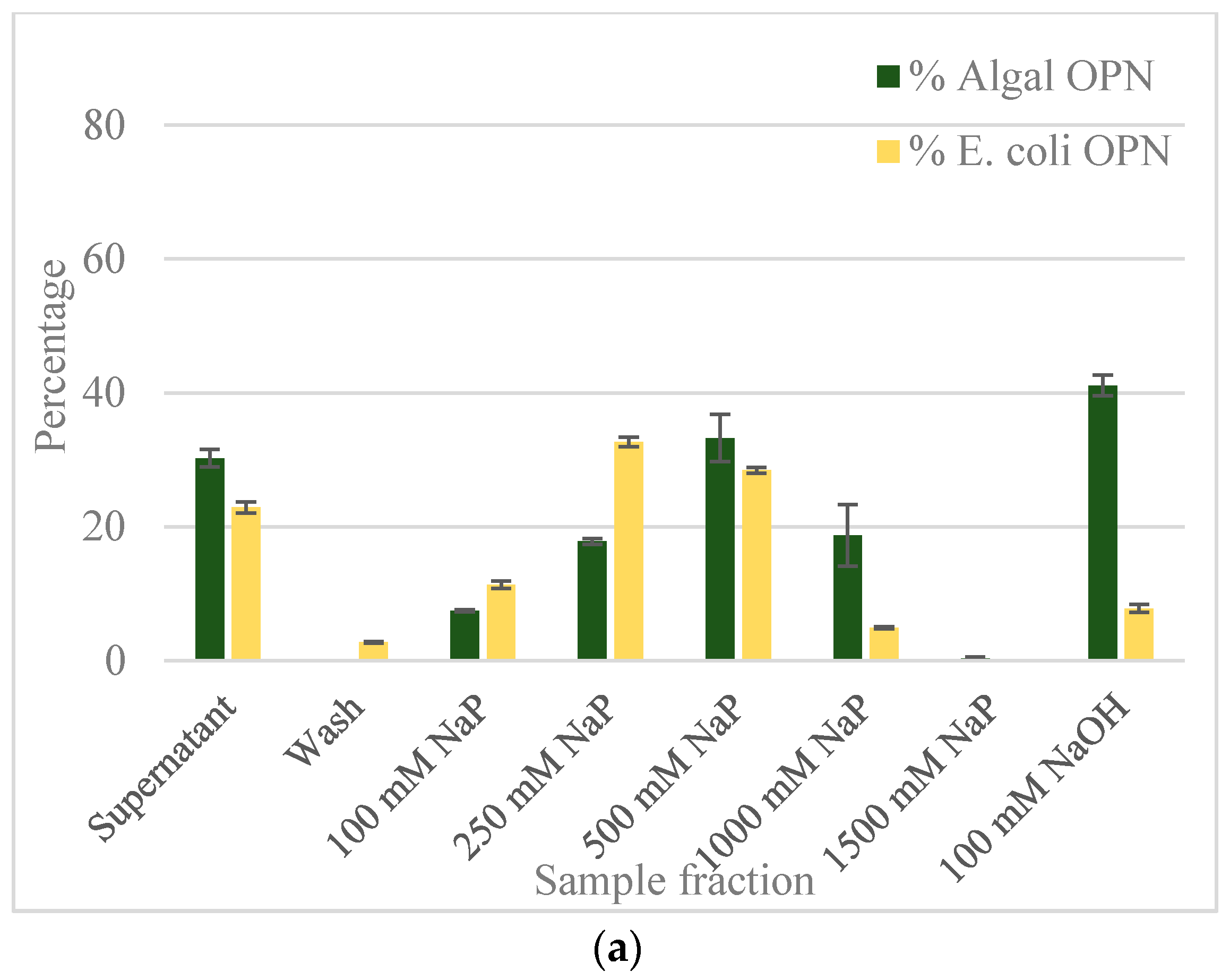
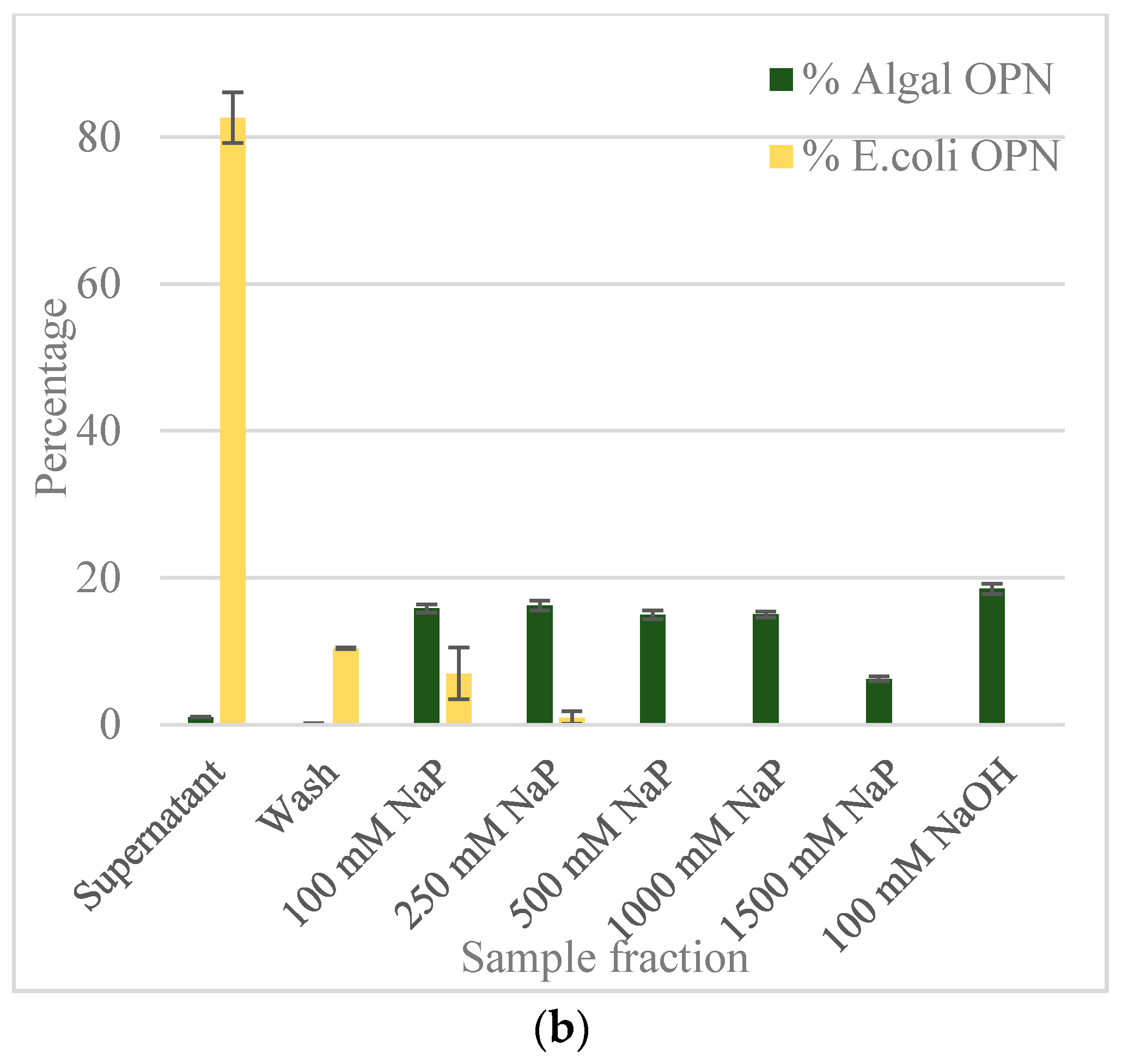
2.2. Ceramic Hydroxyapatite Chromatography
2.3. Gallium-Immobilized Metal Affinity Chromatography (Ga-IMAC)
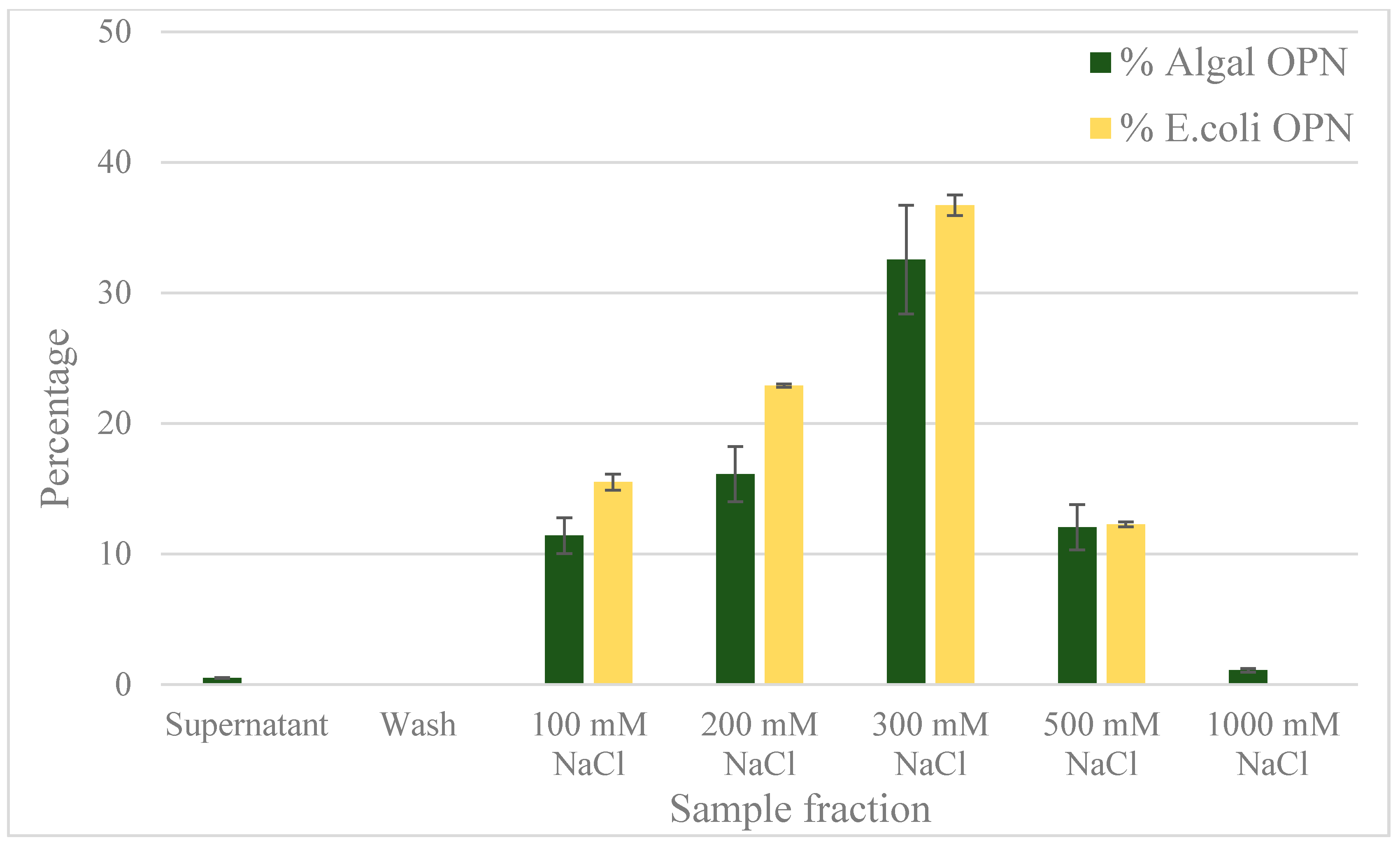
2.4. Anion Exchange Chromatography
3. Materials and Methods
3.1. Cultivation of Recombinant Chlamydomonas reinhardtii Strain Expressing Bovine OPN
3.2. Recombinant E. coli Strain and Cultivation
3.3. Batch Adsorption Chromatography and Purification Studies
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CHO | Chinese hamster ovary |
| PTM | Post-translational modification |
| OPN | Osteopontin |
| BSP | Bone sialoprotein |
| NaP | Sodium phosphate |
| CHT | Ceramic hydroxyapatite |
| Ga-IMAC | Gallium-immobilized metal affinity chromatography |
| Rubisco | Ribulose-1, 5-bisphosphate carboxylase/oxygenase |
| TSP | Total soluble protein |
References
- Baeshen, N.A.; Baeshen, M.N.; Sheikh, A.; Bora, R.S.; Ahmed, M.M.M.; Ramadan, H.A.I.; Saini, K.S.; Redwan, E.M. Cell factories for insulin production. Microb. Cell Fact. 2014, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Muto, M.; Lee, P.A.; Jager, M.; Cardoso, R.M.; Behnke, C.A.; Kirk, P.; Hokanson, C.A.; Crea, R.; Mendez, M. Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol. J. 2010, 8, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hu, H.; Gao, Y.; Xu, X.; Gao, H. Microalgae as platforms for production of recombinant proteins and valuable compounds: Progress and prospects. J. Ind. Microbiol. Biotechnol. 2011, 38, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Specht, E.; Miyake-Stoner, S.; Mayfield, S. Micro-algae come of age as a platform for recombinant protein production. Biotechnol. Lett. 2010, 32, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Munjal, N.; Garzon-Sanabria, A.J.; Quinones, K.W.; Gregory, J.; Nikolov, Z.L. Light-induced production of an antibody fragment and malaria vaccine antigen from Chlamydomonas reinhardtii. Processes 2014, 2, 625–638. [Google Scholar] [CrossRef]
- Sierra, L.S.; Dixon, C.K.; Wilken, L.R. Enzymatic cell disruption of the microalgae Chlamydomonas reinhardtii for lipid and protein extraction. Algal Res. 2017, 25, 149–159. [Google Scholar] [CrossRef]
- Mazzali, M.; Kipari, T.; Ophascharoensuk, V.; Wesson, J.; Johnson, R.; Hughes, J. Osteopontin—A molecule for all seasons. QJM 2002, 95, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Xanthou, G.; Alissafi, T.; Semitekolou, M.; Simoes, D.C.; Economidou, E.; Gaga, M.; Lambrecht, B.N.; Lloyd, C.M.; Panoutsakopoulou, V. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat. Med. 2007, 13, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Kazanecki, C.C.; Uzwiak, D.J.; Denhardt, D.T. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J. Cell. Biochem. 2007, 102, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, E.S.; Petersen, T.E.; Højrup, P. Posttranslational modifications of bovine osteopontin: Identification of twenty-eight phosphorylation and three O-glycosylation sites. Protein Sci. 1995, 4, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Qin, C.; Spevak, L.; Fujimoto, Y.; Butler, W.; Sørensen, E.; Boskey, A. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif. Tissue Int. 2005, 77, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Zawaideh, S.; Hikita, S.; Kumar, V.A.; Cantor, H.; Ashkar, S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J. Leukoc. Biol. 2002, 72, 752–761. [Google Scholar] [PubMed]
- Jono, S.; Peinado, C.; Giachelli, C.M. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J. Biol. Chem. 2000, 275, 20197–20203. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.T. Generation and use of recombinant human bone sialoprotein and osteopontin for hydroxyapatite studies. Connect. Tissue Res. 1996, 35, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.W.; Hota, C.; Chambers, A.F. Recombinant GST-human osteopontin fusion protein is functional in RGD-dependent cell adhesion. J. Cell. Biochem. 1994, 54, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Kim, J.-H. Improved cellular response of osteoblast cells using recombinant human osteopontin protein produced by escherichia coli. Biotechnol. Lett. 2005, 27, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, S.; Teplow, D.; Glimcher, M.; Saavedra, R. In vitro phosphorylation of mouse osteopontin expressed in E. coli. Biochem. Biophys. Res. Commun. 1993, 191, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Perruzzi, C.A.; Papadopoulos, A.; Tenen, D.G. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1989, 996, 43–48. [Google Scholar] [CrossRef]
- Sørensen, S.; Justesen, S.J.; Johnsen, A.H. Purification and characterization of osteopontin from human milk. Protein Expr. Purif. 2003, 30, 238–245. [Google Scholar] [CrossRef]
- Bayless, K.J.; Davis, G.E.; Meininger, G.A. Isolation and biological properties of osteopontin from bovine milk. Protein Expr. Purif. 1997, 9, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Azuma, N.; Maeta, A.; Fukuchi, K.; Kanno, C. A rapid method for purifying osteopontin from bovine milk and interaction between osteopontin and other milk proteins. Int. Dairy J. 2006, 16, 370–378. [Google Scholar] [CrossRef]
- Mayfield, S.P.; Hallgren, A.C.; Rasala, B.A.; Tran, M.; Mayfield, M. Colostrum/Milk Protein Compositions. U.S. Patents US2015/016460, 27 August 2015. [Google Scholar]
- Gao, Y.A.; Agnihotri, R.; Vary, C.P.; Liaw, L. Expression and characterization of recombinant osteopontin peptides representing matrix metalloproteinase proteolytic fragments. Matrix Biol. 2004, 23, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Guo, X. Osteopontin: A protein with diverse functions. FASEB J. 1993, 7, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Miralles, N.; Domingo-Espín, J.; Corchero, J.L.; Vázquez, E.; Villaverde, A. Microbial factories for recombinant pharmaceuticals. Microb. Cell Fact. 2009, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakruddin, M.; Mohammad Mazumdar, R.; Bin Mannan, K.S.; Chowdhury, A.; Hossain, M.N. Critical factors affecting the success of cloning, expression and mass production of enzymes by recombinant E. coli. ISRN Biotechnol. 2013, 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, P.; Latiff, S.M.A.; Cai, C.; Lau, W.; Lim, C.L. IgM purification with hydroxyapatite. Available online: http://www.bioprocessintl.com/upstream-processing/biochemicals-raw-materials/igm-purification-with-hydroxyapatite-349783/ (accessed on 8 February 2018).
- Gagnon, P. Monoclonal antibody purification with hydroxyapatite. New Biotechnol. 2009, 25, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Losh, J.L.; Young, J.N.; Morel, F.M.M. Rubisco is a small fraction of total protein in marine phytoplankton. New Phytol. 2013, 198, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Aryal, U.K.; Olson, D.J.H.; Ross, A.R.S. Optimization of immobilized gallium (iii) ion affinity chromatography for selective binding and recovery of phosphopeptides from protein digests. J. Biomol. Tech. 2008, 19, 296–310. [Google Scholar] [PubMed]
- Machida, M.; Kosako, H.; Shirakabe, K.; Kobayashi, M.; Ushiyama, M.; Inagawa, J.; Hirano, J.; Nakano, T.; Bando, Y.; Nishida, E.; et al. Purification of phosphoproteins by immobilized metal affinity chromatography and its application to phosphoproteome analysis. FEBS J. 2007, 274, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; FitzGerald, R.J. Caseinophosphopeptide enrichment and identification. Int. J. Food Sci. Technol. 2012, 47, 2235–2242. [Google Scholar] [CrossRef]
- Biswas, S.; Sarkar, A.; Misra, R. Iron affinity gel and gallium immobilized metal affinity chromatographic technique for phosphopeptide enrichment: A comparative study. Biotechnol. Biotechnol. Equip. 2017, 31, 639–646. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, X.; Weng, S.; Guan, W.; Xiang, D.; Gao, J.; Li, J.; Han, W.; Yu, Y. Expression and purification of bioactive high-purity recombinant mouse spp1 in escherichia coli. Appl. Biochem. Biotechnol. 2014, 173, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.Y.; Zhou, L.; Guan, W.; Deng, Q.; Chen, H.H.; Han, W.; Yu, Y.; Yuan, Y.S. High-purity recombinant osteopontin n-terminal domain. Acta Biochim. Biophys. Sin. 2015, 47, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Hutner, S.; Provasoli, L.; Schatz, A.; Haskins, C. Some approaches to the study of the role of metals in the metabolism of microorganisms. Proc. Am. Philos. Soc. 1950, 94, 152–170. [Google Scholar]
- Gorman, D.S.; Levine, R. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of chlamydomonas reinhardi. Proc. Natl. Acad. Sci. 1965, 54, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Wessel, D.; Flügge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravi, A.; Guo, S.; Rasala, B.; Tran, M.; Mayfield, S.; Nikolov, Z.L. Separation Options for Phosphorylated Osteopontin from Transgenic Microalgae Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2018, 19, 585. https://doi.org/10.3390/ijms19020585
Ravi A, Guo S, Rasala B, Tran M, Mayfield S, Nikolov ZL. Separation Options for Phosphorylated Osteopontin from Transgenic Microalgae Chlamydomonas reinhardtii. International Journal of Molecular Sciences. 2018; 19(2):585. https://doi.org/10.3390/ijms19020585
Chicago/Turabian StyleRavi, Ayswarya, Shengchun Guo, Beth Rasala, Miller Tran, Stephen Mayfield, and Zivko L. Nikolov. 2018. "Separation Options for Phosphorylated Osteopontin from Transgenic Microalgae Chlamydomonas reinhardtii" International Journal of Molecular Sciences 19, no. 2: 585. https://doi.org/10.3390/ijms19020585
APA StyleRavi, A., Guo, S., Rasala, B., Tran, M., Mayfield, S., & Nikolov, Z. L. (2018). Separation Options for Phosphorylated Osteopontin from Transgenic Microalgae Chlamydomonas reinhardtii. International Journal of Molecular Sciences, 19(2), 585. https://doi.org/10.3390/ijms19020585



