Cytokeratin-8 in Anaplastic Thyroid Carcinoma: More Than a Simple Structural Cytoskeletal Protein
Abstract
:1. Introduction
2. Results
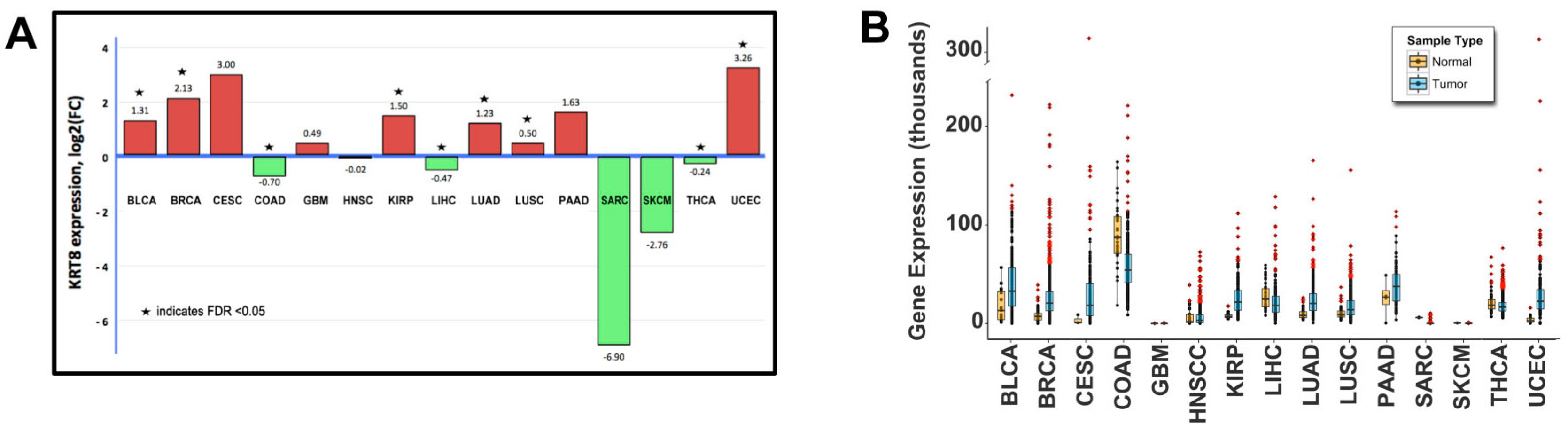
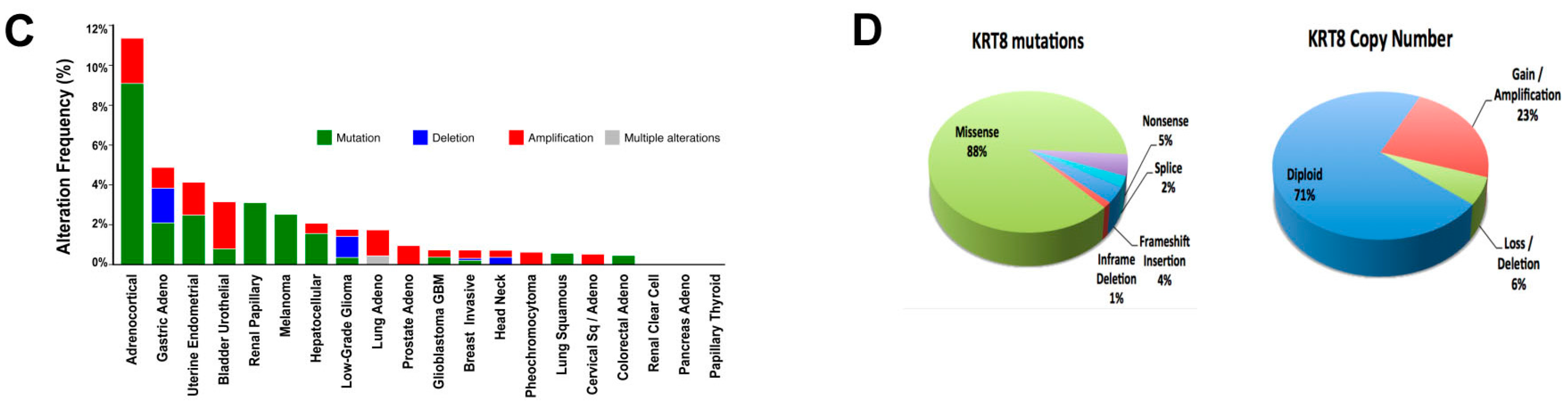
2.1. Genomic Analysis
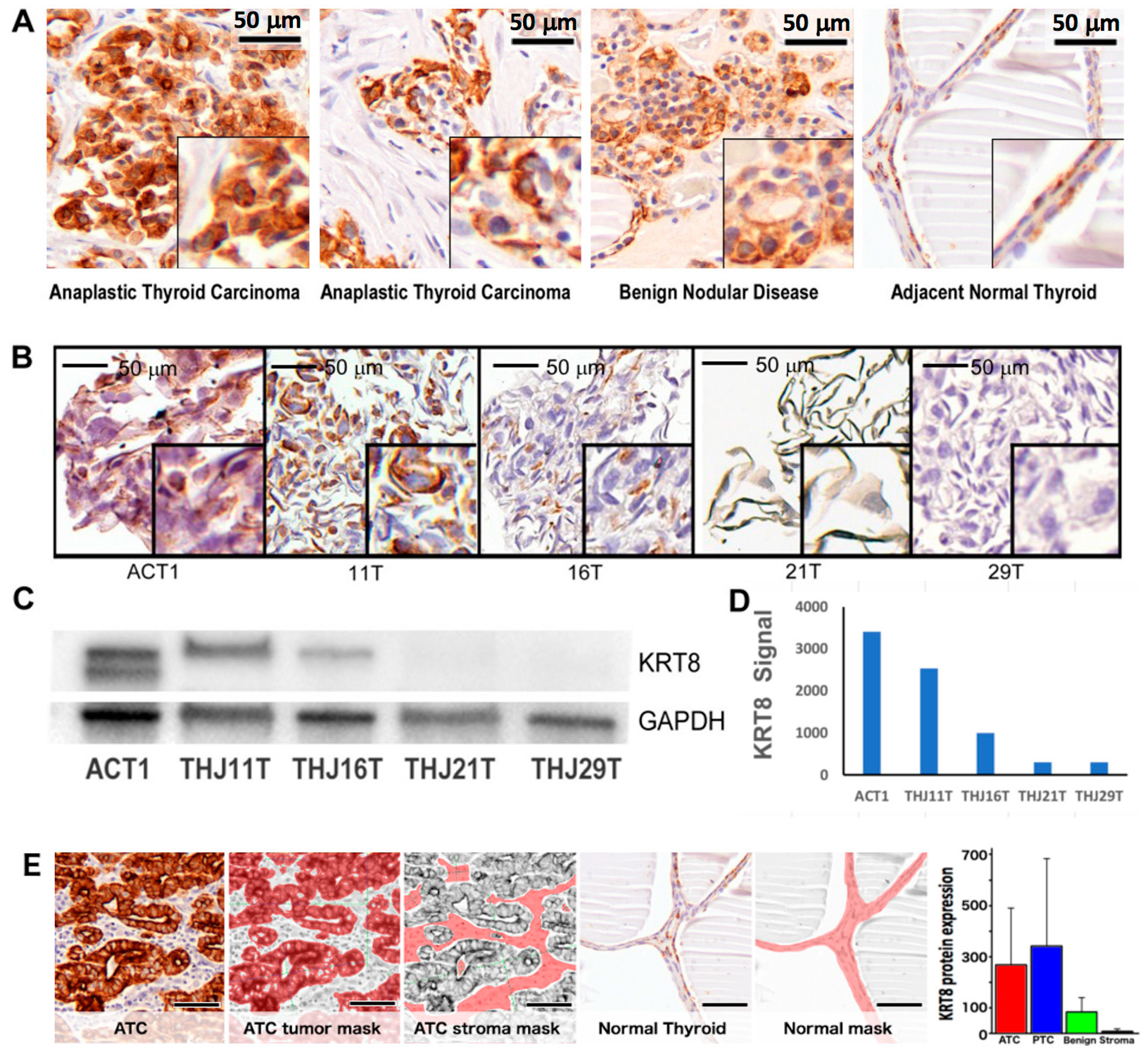
2.2. Patient Demographics and Keratin-8 (KRT8) Expression
2.3. KRT8 Expression in Cell Lines
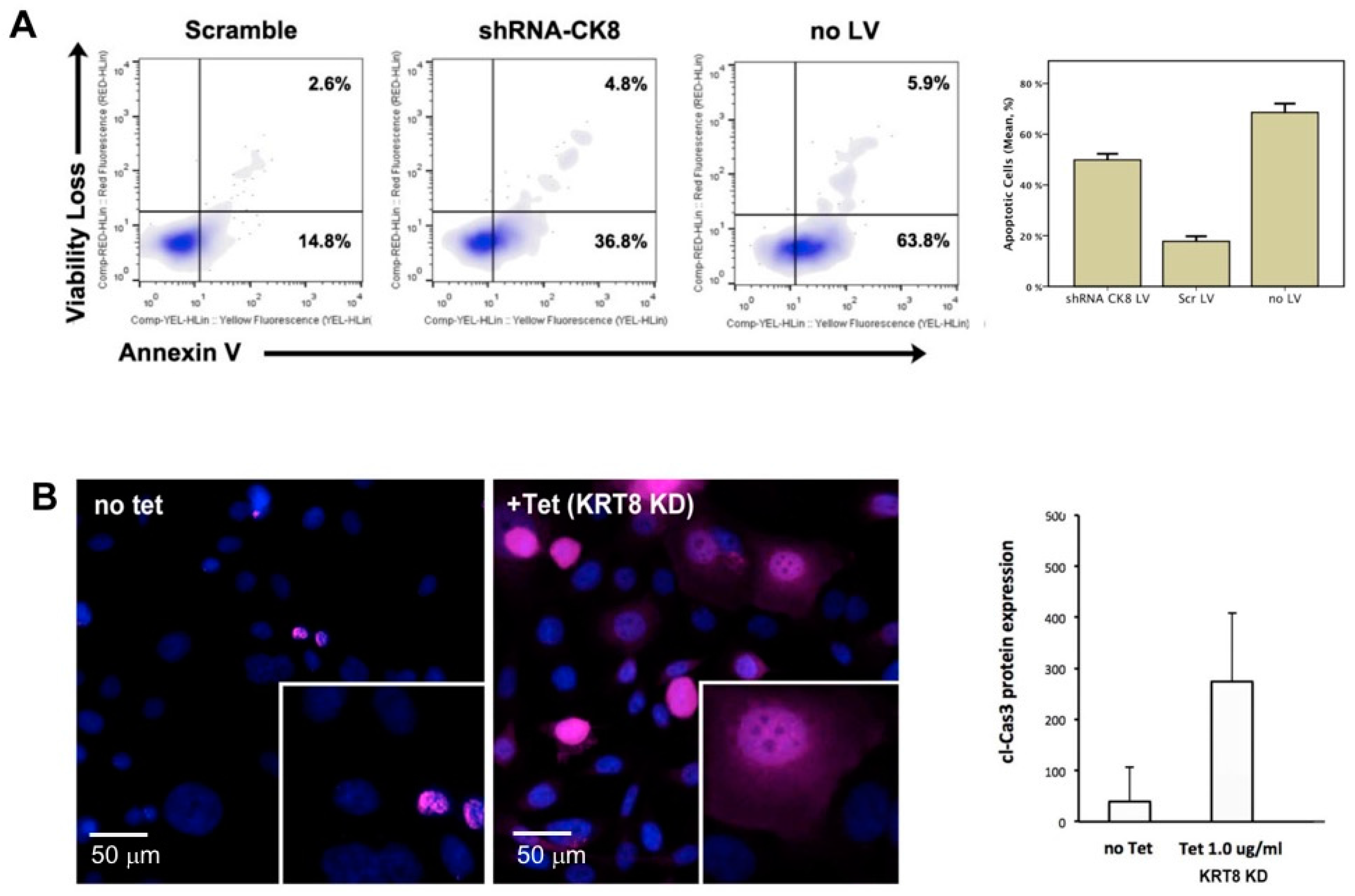
2.4. RNA-Interference-Mediated Knockdown of KRT8
2.5. Tet-Inducible KRT8 Knockdown and Apoptosis
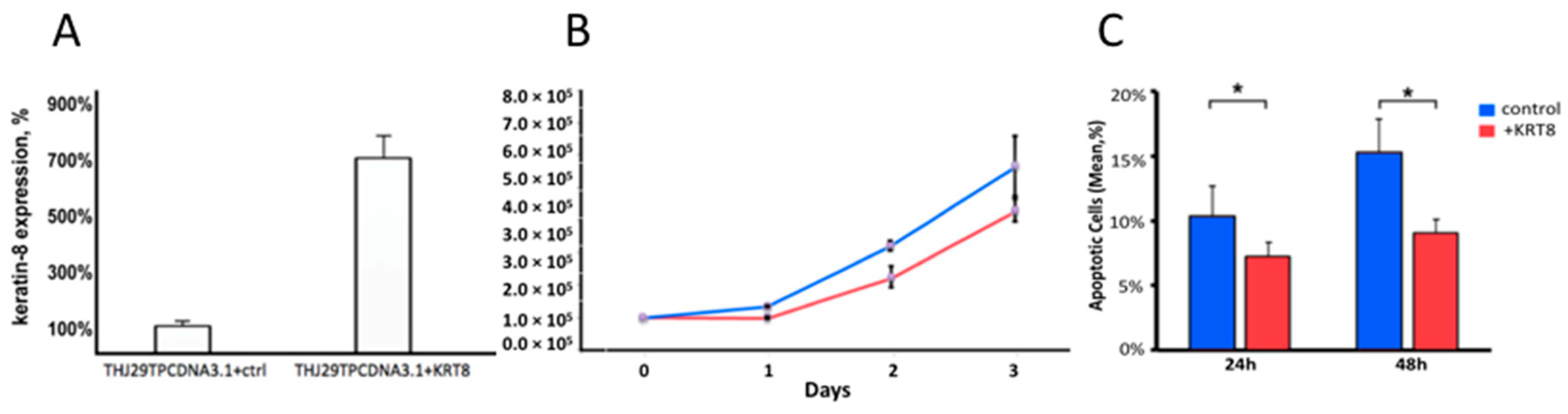
2.6. KRT8 Overexpression
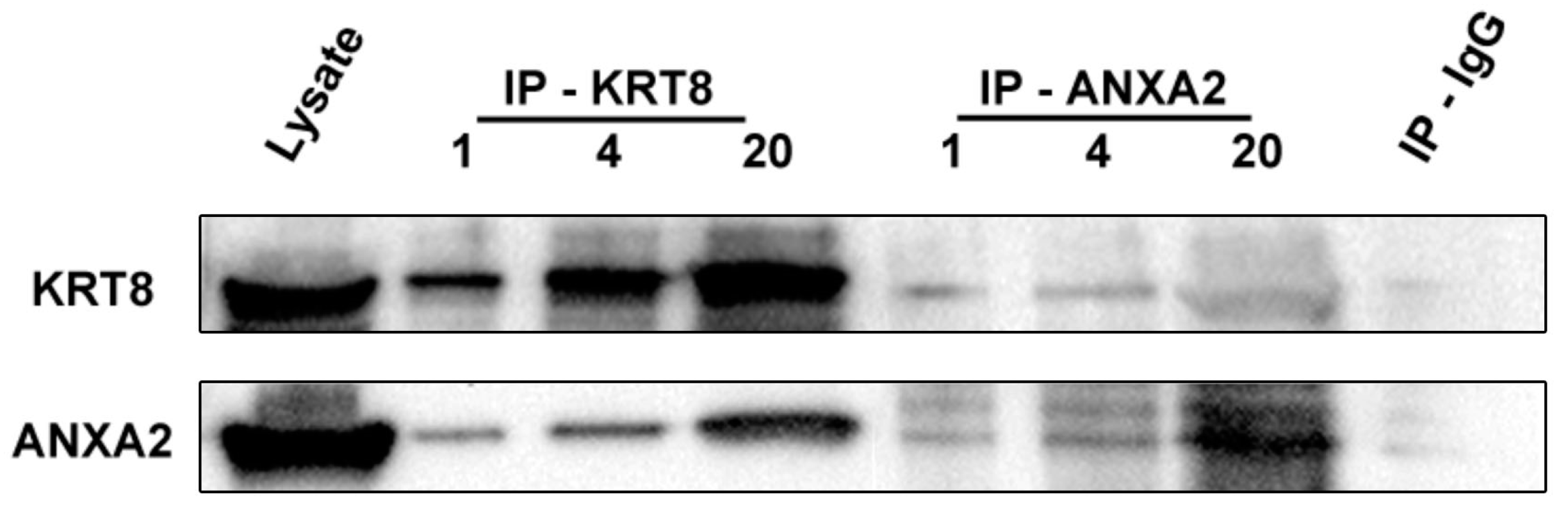
2.7. Identification of Annexin-A2 as a KRT8 Binding Protein
3. Discussion
4. Material and Methods
4.1. Human Patient Cohort Selection
4.2. General Laboratory Reagents
4.3. Genomic Data Analysis
4.4. Statistical Analysis
4.5. Immunohistochemistry
4.6. Quantitative In-Situ Protein Expression Measurement
4.7. shRNA Lentivirus Knockdown
4.8. Tetracycline-Inducible shRNA Knockdown
4.9. Flow-Cytometry-Based Cell Viability and Apoptosis Assays
4.10. Western Blot
4.11. KRT8 Plasmid Transduction
4.12. Mass Spectrometry
4.13. Protein Identification
4.14. Co-Immunoprecipitation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E. Anaplastic thyroid cancer: Rare, fatal, and neglected. Surgery 2012, 152, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Ain, K.B.; Asa, S.L.; Bible, K.C.; Brierley, J.D.; Burman, K.D.; Kebebew, E.; Lee, N.Y.; Nikiforov, Y.E.; Rosenthal, M.S.; et al. American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012, 22, 1104–1139. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Odejide, O.O.; Wirth, L.J.; Chan, A.W. Survival of a patient with anaplastic thyroid cancer following intensity-modulated radiotherapy and sunitinib—A case report. Anticancer Res. 2012, 32, 1743–1746. [Google Scholar] [PubMed]
- Dey, P.; Togra, J.; Mitra, S. Intermediate filament: Structure, function, and applications in cytology. Diagn. Cytopathol. 2014, 42, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Kovatich, A.J.; Kärkkäinen, P. Keratin subsets in papillary and follicular thyroid lesions. A paraffin section analysis with diagnostic implications. Virchows Arch. 1997, 431, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Chesire, D.R.; Ewing, C.M.; Gage, W.R.; Isaacs, W.B. In vitro evidence for complex modes of nuclear β-catenin signaling during prostate growth and tumorigenesis. Oncogene 2002, 21, 2679–2694. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.J.; Lingle, W.L.; Salisbury, J.L.; Maihle, N.J. A transformation-associated complex involving tyrosine kinase signal adapter proteins and caldesmon links v-ErbB signaling to actin stress fiber disassembly. Proc. Natl. Acad. Sci. USA 1997, 94, 11351–11356. [Google Scholar] [CrossRef] [PubMed]
- Bellaye, P.S.; Wettstein, G.; Burgy, O.; Besnard, V.; Joannes, A.; Colas, J.; Causse, S.; Marchal-Somme, J.; Fabre, A.; Crestani, B.; et al. The small heat-shock protein αB-crystallin is essential for the nuclear localization of Smad4: Impact on pulmonary fibrosis. J. Pathol. 2014, 232, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Popova, J.S.; Garrison, J.C.; Rhee, S.G.; Rasenick, M.M. Tubulin, Gq, and phosphatidylinositol 4,5-bisphosphate interact to regulate phospholipase cβ1 signaling. J. Biol. Chem. 1997, 272, 6760–6765. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. Moonlighting proteins: Old proteins learning new tricks. Trends Genet. 2003, 19, 415–417. [Google Scholar] [CrossRef]
- Escobar-Hoyos, L.F.; Shah, R.; Roa-Peña, L.; Vanner, E.A.; Najafian, N.; Banach, A.; Nielsen, E.; Al-Khalil, R.; Akalin, A.; Talmage, D.; et al. Keratin-17 promotes p27KIP1 nuclear export and degradation and offers potential prognostic utility. Cancer Res. 2015, 75, 3650–3662. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.M.; Arutyunov, A.; Ilagan, E.; Yao, N.; Wills-Karp, M.; Coulombe, P.A. Regulation of c-x-c chemokine gene expression by keratin 17 and hnRNP K in skin tumor keratinocytes. J. Cell Biol. 2015, 208, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, Z.; Wang, J.; Shao, X.; Cui, Z.; Yang, C.; Zhu, Z.; Xiong, D. Overexpression of cell surface cytokeratin 8 in multidrug-resistant MCF-7/MX cells enhances cell adhesion to the extracellular matrix. Neoplasia 2008, 10, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Q.Y.; Tsao, S.W.; Cheung, Y.H.; Wong, A.; Chiu, J.F. Cytokeratin 8 silencing in human nasopharyngeal carcinoma cells leads to cisplatin sensitization. Cancer Lett. 2008, 265, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Araujo, T.G.; Marangoni, K.; Rocha, R.M.; Maia, Y.C.; Araujo, G.R.; Alcântar, T.M.; Alves, P.T.; Calábria, L.; Neves, A.F.; Soares, F.A.; et al. Dynamic dialog between cytokeratin 18 and Annexin A1 in breast cancer: A transcriptional disequilibrium. Acta Histochem. 2014, 116, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.M.; Murray, C.I.; Van Eyk, J.E.; Coulombe, P.A. Identification of novel interaction between Annexin A2 and Keratin 17: Evidence for reciprocal regulation. J. Biol. Chem. 2012, 287, 7573–7581. [Google Scholar] [CrossRef] [PubMed]
- Boczonadi, V.; Määttä, A. Annexin A9 is a periplakin interacting partner in membrane-targeted cytoskeletal linker protein complexes. FEBS Lett. 2012, 586, 3090–3096. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.; Loranger, A.; Lavoie, J.N.; Marceau, N. Cytoskeleton keratin regulation of FASR signaling through modulation of actin/ezrin interplay at lipid rafts in hepatocytes. Apoptosis 2012, 17, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Fortier, A.M.; Asselin, E.; Cadrin, M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J. Biol. Chem. 2013, 288, 11555–11571. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Li, C.H.; Ruppert, J.G.; Chang, C.C. Cytokeratin 8-MHC class I interactions: A potential novel immune escape phenotype by a lymph node metastatic carcinoma cell line. Biochem. Biophys. Res. Commun. 2013, 441, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Depianto, D.; Kerns, M.L.; Dlugosz, A.A.; Coulombe, P.A. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat. Genet. 2010, 42, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Arthur, J.S.; Kuma, Y.; Peggie, M.; Carr, J.; Murray-Tait, V.; Centeno, F.; Goedert, M.; Morrice, N.A.; Cuenda, A. P38γ regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005, 24, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Perdiz, D.; Mackeh, R.; Poüs, C.; Baillet, A. The ins and outs of tubulin acetylation: More than just a post-translational modification? Cell Signal. 2011, 23, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, G.; Helfman, D.M. Cytoskeletal changes in cell transformation and tumorigenesis. Curr. Opin. Genet. Dev. 2001, 11, 41–47. [Google Scholar] [CrossRef]
- Yuan, K.; Liu, Y.; Chen, H.N.; Zhang, L.; Lan, J.; Gao, W.; Dou, Q.; Nice, E.C.; Huang, C. Thiol-based redox proteomics in cancer research. Proteomics 2015, 15, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Waters, K.M.; Stenoien, D.L.; Sowa, M.B.; von Neubeck, C.; Chrisler, W.B.; Tan, R.; Sontag, R.L.; Weber, T.J. Annexin A2 modulates radiation-sensitive transcriptional programming and cell fate. Radiat. Res. 2013, 179, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Lin, C.F. Annexin A2: Its molecular regulation and cellular expression in cancer development. Dis. Mark. 2014, 2014, 308976. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, S.; Takano, S.; Yoshitomi, H.; Kimura, F.; Satoh, M.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Furukawa, K.; et al. Akt/mTOR signaling pathway is crucial for gemcitabine resistance induced by Annexin II in pancreatic cancer cells. J. Surg. Res. 2012, 178, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Marlow, L.A.; D'Innocenzi, J.; Zhang, Y.; Rohl, S.D.; Cooper, S.J.; Sebo, T.; Grant, C.; McIver, B.; Kasperbauer, J.L.; Wadsworth, J.T.; et al. Detailed molecular fingerprinting of four new anaplastic thyroid carcinoma cell lines and their use for verification of RhoB as a molecular therapeutic target. J. Clin. Endocrinol. Metab. 2010, 95, 5338–5347. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Onoda, N.; Ishikawa, T.; Ogisawa, K.; Takenaka, C.; Yano, Y.; Hato, F.; Hirakawa, K. Peroxisome proliferator-activated receptor γ activation induces cell cycle arrest via the p53-independent pathway in human anaplastic thyroid cancer cells. Jpn. J. Cancer Res. 2002, 93, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. Rsem: Accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qiu, P.; Ji, Y. Tcga-assembler: Open-source software for retrieving and processing TCGA data. Nat. Methods 2014, 11, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, P.M.; Merkley, M.; Lee, J.R.; Adam, B.L.; Gourin, C.G.; Podolsky, R.H.; Haffty, B.G.; Papadavid, E.; Sasaki, C.; Psyrri, A.; et al. Use of combination proteomic analysis to demonstrate molecular similarity of head and neck squamous cell carcinoma arising from different subsites. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Zrazhevskiy, P.; True, L.D.; Gao, X. Multicolor multicycle molecular profiling with quantum dots for single-cell analysis. Nat. Protoc. 2013, 8, 1852–1869. [Google Scholar] [CrossRef] [PubMed]
- Levenson, R.; Beechem, J.; McNamara, G. Spectral imaging in preclinical research and clinical pathology. Anal. Cell Pathol. 2012, 35, 339–361. [Google Scholar] [CrossRef]
- Fang, B.; Hoffman, M.A.; Mirza, A.S.; Mishall, K.M.; Li, J.; Peterman, S.M.; Smalley, K.S.; Shain, K.H.; Weinberger, P.M.; Wu, J.; et al. Evaluating kinase ATP uptake and tyrosine phosphorylation using multiplexed quantification of chemically labeled and post-translationally modified peptides. Methods 2015, 81, 41–49. [Google Scholar] [CrossRef] [PubMed]
| All Patients | ATC | PTC | BND | |
|---|---|---|---|---|
| Number (n) | 17 | 8 | 5 | 4 |
| Age, range (mean), years | 37–82 (60) | 37–82 (65) | 38–78 (56) | 48–59 (54) |
| Gender (male/female) | 5/12 | 3/5 | 1/4 | 1/3 |
| Ethnicity | ||||
| African-American | 7 | 1 | 3 | 3 |
| Caucasian | 10 | 7 | 2 | 1 |
| Asian or other | 0 | 0 | 0 | 0 |
| Keratin-8 Expression (Mean ± SD) | p Value (Compared to Benign) | |
|---|---|---|
| Cancer Tissue | 331.3 ± 266 | 0.017 * |
| ATC | 268.2 ± 223 | 0.100 |
| PTC | 425.9 ± 332 | 0.131 |
| Benign Thyroid | 83.8 ± 58 | – |
| BND | 93.5 ± 43 | 0.82 |
| Adjacent Normal | 80.0 ± 67 | 0.92 |
| Stroma | 9.4 ± 8 | 0.013 * |
| ACT1 cell line | 490.3 ± 178 | – |
| THJ11T cell line | 138.2 ± 46 | – |
| THJ16T cell line | 162.7 ± 51 | – |
| THJ29T cell line | 1.40 ± 1.2 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Xu, Q.; Pabla, S.; Koomen, J.; Biddinger, P.; Sharma, A.; Pabla, S.; Pacholczyk, R.; Chang, C.-C.; Friedrich, K.; et al. Cytokeratin-8 in Anaplastic Thyroid Carcinoma: More Than a Simple Structural Cytoskeletal Protein. Int. J. Mol. Sci. 2018, 19, 577. https://doi.org/10.3390/ijms19020577
Guo D, Xu Q, Pabla S, Koomen J, Biddinger P, Sharma A, Pabla S, Pacholczyk R, Chang C-C, Friedrich K, et al. Cytokeratin-8 in Anaplastic Thyroid Carcinoma: More Than a Simple Structural Cytoskeletal Protein. International Journal of Molecular Sciences. 2018; 19(2):577. https://doi.org/10.3390/ijms19020577
Chicago/Turabian StyleGuo, Dehuang, Qinqin Xu, Sarabjot Pabla, John Koomen, Paul Biddinger, Ashok Sharma, Simarjot Pabla, Rafal Pacholczyk, Chien-Chung Chang, Kevin Friedrich, and et al. 2018. "Cytokeratin-8 in Anaplastic Thyroid Carcinoma: More Than a Simple Structural Cytoskeletal Protein" International Journal of Molecular Sciences 19, no. 2: 577. https://doi.org/10.3390/ijms19020577
APA StyleGuo, D., Xu, Q., Pabla, S., Koomen, J., Biddinger, P., Sharma, A., Pabla, S., Pacholczyk, R., Chang, C.-C., Friedrich, K., Mohammed, K., Smallridge, R. C., Copland, J. A., She, J.-X., & Weinberger, P. M. (2018). Cytokeratin-8 in Anaplastic Thyroid Carcinoma: More Than a Simple Structural Cytoskeletal Protein. International Journal of Molecular Sciences, 19(2), 577. https://doi.org/10.3390/ijms19020577





