Bioreducible Polymer Micelles Based on Acid-Degradable Poly(ethylene glycol)-poly(amino ketal) Enhance the Stromal Cell-Derived Factor-1α Gene Transfection Efficacy and Therapeutic Angiogenesis of Human Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Results
2.1. Characterization and Gene Transfection Efficiency of PEG-PAK Micelles
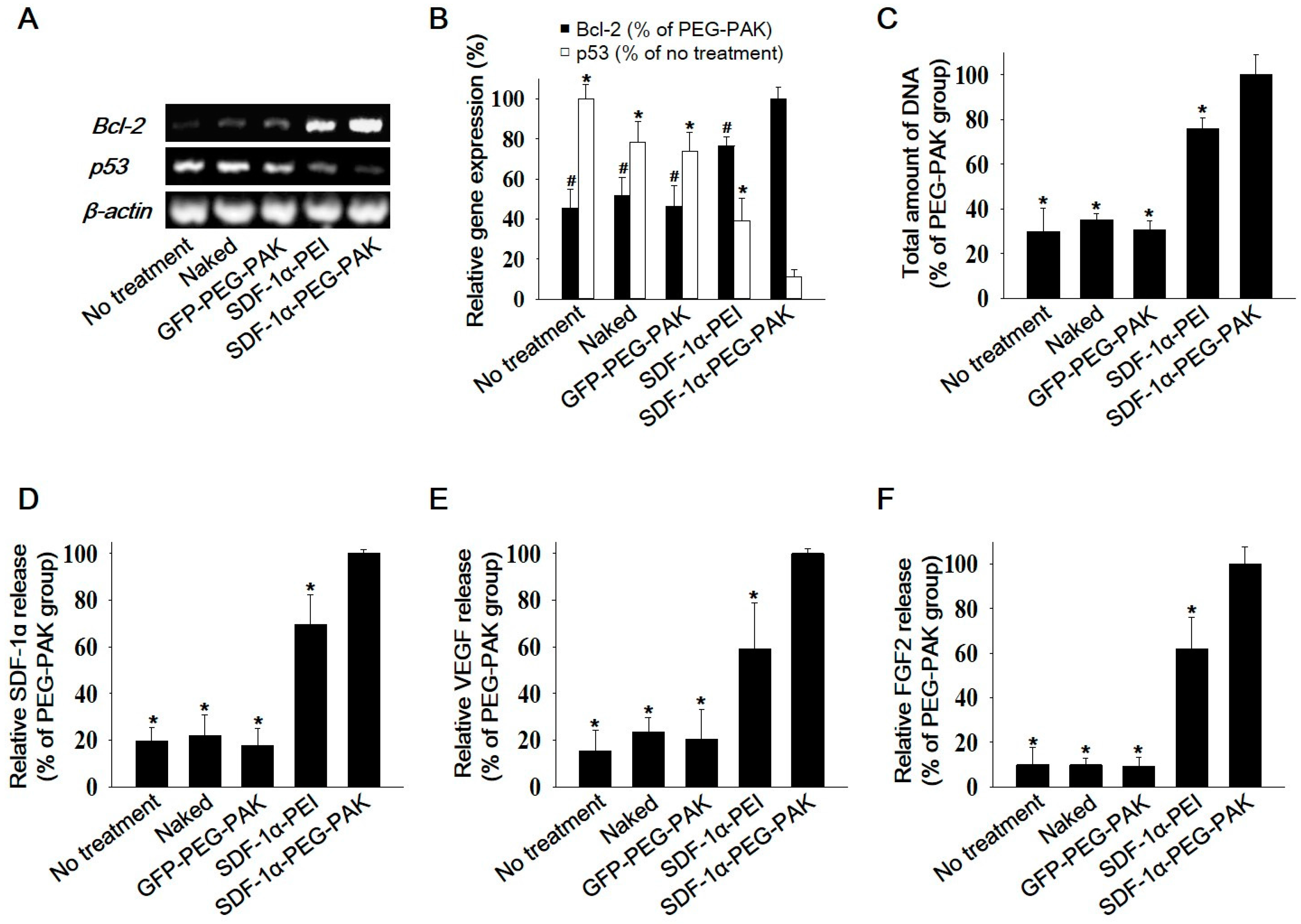
2.2. Decreased Apoptosis and Enhanced Secretion of Pro-Angiogenic Factors in hADSCs Overexpressing SDF-1α
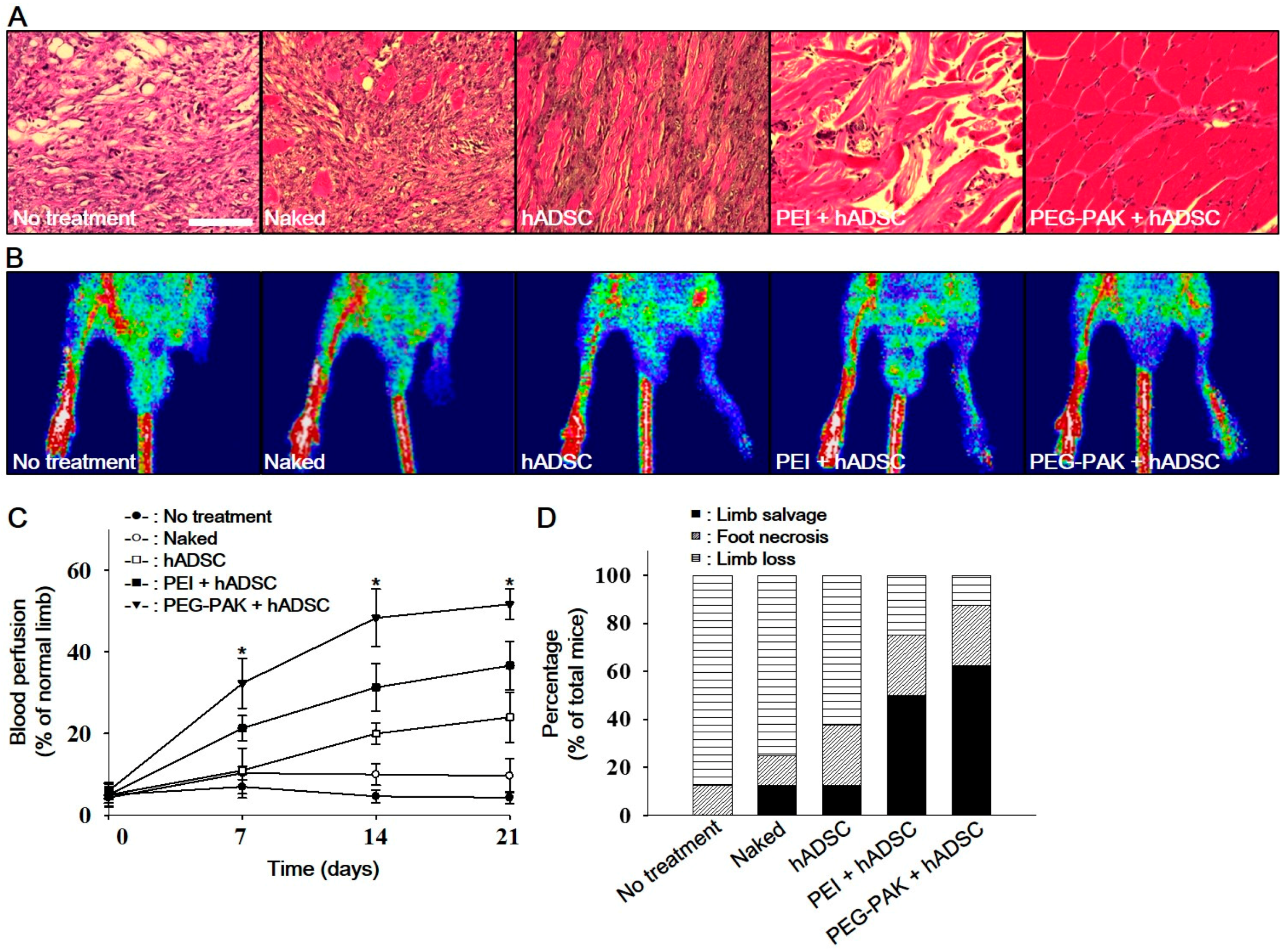
2.3. Effect of hADSCs Transfected with SDF-1α pDNA/PEG-PAK Micelles in Ischemic Limbs
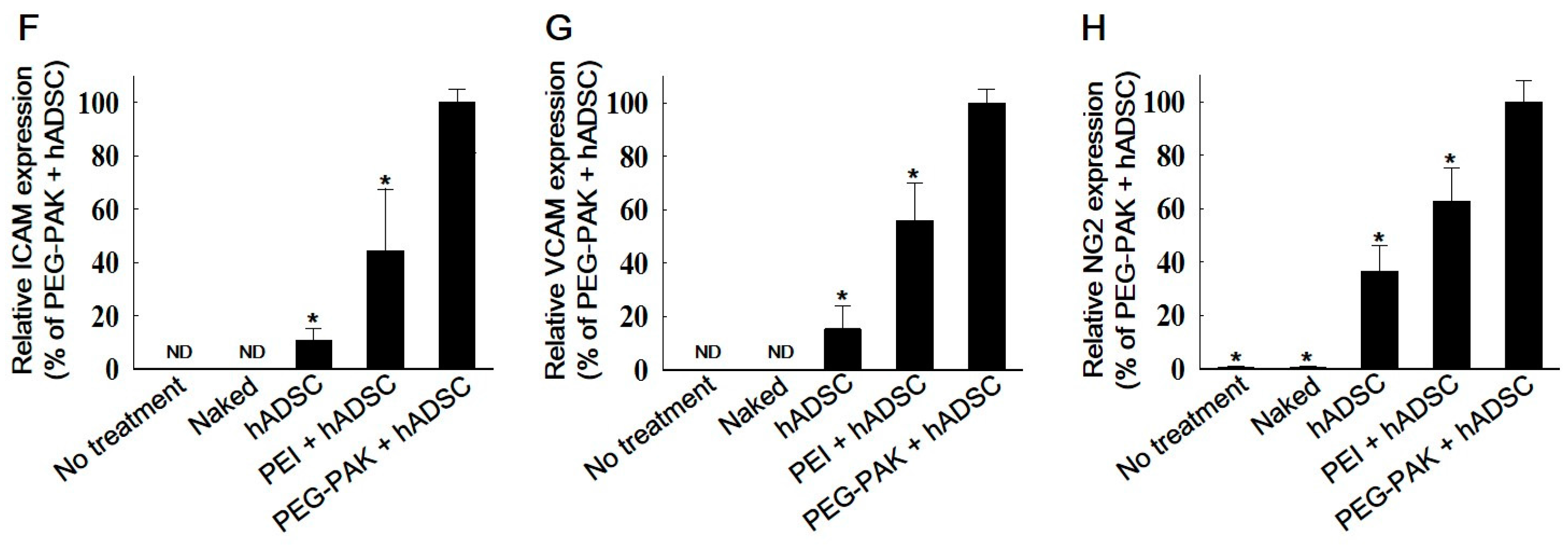
2.4. In Vivo Pro-Angiogenic Effect of hADSCs Transfected with SDF-1α pDNA/PEG-PAK Micelles
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Acid-Degradable (PEG-PAK)
4.2.1. Synthesis of Acid-Degradable Ketal Crosslinker (Compound 2)
4.2.2. Synthesis of Compound 3
4.2.3. Synthesis of Acid-Degradable PEG-PAK
4.3. Transmission Electron Microscopy (TEM)
4.4. hADSCs Culture
4.5. In Vitro SDF-1α Transfection
4.6. Confocal Laser Scanning Microscopy
4.7. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.8. Western Blot Analysis
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Model of Mouse Hindlimb Ischemia
4.11. Transplantation of SDF-1α Transfected hADSC into Ischemic Mouse Hindlimbs
4.12. Histology and Laser Doppler Imaging Analysis
4.13. Immunohistochemistry
4.14. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, A.; Tao, C.; Li, X.; Jin, P. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2013, 441, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, B.; Cheng, Z.; Jia, X.; Mao, L.; Fu, H.; Che, Y.; Ou, L.; Liu, L.; Kong, D. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell 2011, 2, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Lataillade, J.J.; Clay, D.; Bourin, P.; Hérodin, F.; Dupuy, C.; Jasmin, C.; le Bousse-Kerdilès, M.C. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G0/G1 transition in CD34+ cells: Evidence for an autocrine/paracrine mechanism. Blood 2002, 99, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mal, N.; Kiedrowski, M.; Chacko, M.; Askari, A.T.; Popovic, Z.B.; Koc, O.N.; Penn, M.S. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007, 21, 3197–3207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Ou, L.; Zhou, X.; Li, F.; Jia, X.; Zhang, Y.; Liu, X.; Li, Y.; Ward, C.A.; Melo, L.G.; et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther. 2008, 16, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Li, P.; Sheng, N.; Liu, L.; Pan, H.; Wang, C.; Cai, L.; Ma, Y. Sialic acid-targeted nanovectors with phenylboronic acid-grafted polyethylenimine robustly enhance siRNA-based cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 9565–9576. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Bieber, T.; Li, Y.; Elsässer, H.P.; Kissel, T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999, 16, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Jiang, G.; Birrell, L.K.; El-Sayed, M.E. Degradable, pH-sensitive, membrane-destabilizing, comb-like polymers for intracellular delivery of nucleic acids. Biomaterials 2010, 31, 7150–7166. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Song, B.W.; Cha, M.J.; Choi, I.G.; Hwang, K.C. Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin. Biol. Ther. 2010, 10, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P. Stem cells for clinical use in cardiovascular medicine: Current limitations and future perspectives. Thromb. Haemost. 2005, 94, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Sekine, H.; Shimizu, T.; Dobashi, I.; Matsuura, K.; Hagiwara, N.; Takahashi, M.; Kobayashi, E.; Yamato, M.; Okano, T. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng. Part A 2011, 17, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Shibata, R.; Numaguchi, Y.; Kito, T.; Suzuki, H.; Shimizu, K.; Ito, A.; Honda, H.; Murohara, T. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Cho, S.W.; La, W.G.; Lee, T.J.; Yang, H.S.; Sun, A.Y.; Baek, S.H.; Rhie, J.W.; Kim, B.S. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 2011, 32, 2734–2747. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Lee, S.; Shin, J.Y.; Lee, T.J.; Kim, B.S. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng. Part A 2012, 18, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Mangi, A.A.; Noiseux, N.; Kong, D.; He, H.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003, 9, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.M.; Cottage, C.T.; Wu, W.; Din, S.; Gude, N.A.; Avitabile, D.; Quijada, P.; Collins, B.L.; Fransioli, J.; Sussman, M.A. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation 2009, 120, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Tang, Y.; Zhang, Y.C.; Qian, K.; Shen, L.; Phillips, M.I. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J. Am. Coll. Cardiol. 2005, 46, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, R.; Oppenheim, J.J. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation 2003, 10, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.T.; Wang, D.A. Stromal cell-derived factor-1 (SDF-1): Homing factor for engineered regenerative medicine. Expert Opin. Biol. Ther. 2011, 11, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Kusano, K.F.; Masuo, O.; Kawamoto, A.; Silver, M.; Murasawa, S.; Bosch-Marce, M.; Masuda, H.; Losordo, D.W.; Isner, J.M.; et al. Stromal cell–derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003, 107, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Cheng, L.; Sun, X.; Wu, Y.; Hu, L.; Wang, J.; Zhao, H.; Lu, G. LFA-1 and VLA-4 involved in human high proliferative potential endothelial progenitor cells homing to ischemic tissue. Thromb. Haemost. 2006, 96, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Grabovsky, V.; Habler, L.; Sandbank, J.; Arenzana-Seisdedos, F.; Petit, I.; Ben-Hur, H.; Lapidot, T.; Alon, R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J. Clin. Investig. 1999, 104, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Kwon, Y.J. Controlled delivery of plasmid DNA and siRNA to intracellular targets using ketalized polyethylenimine. Biomacromolecules 2008, 9, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Riis, S.; Newman, R.; Ipek, H.; Andersen, J.I.; Kuninger, D.; Boucher, S.; Vemuri, M.C.; Pennisi, C.P.; Zachar, V.; Fink, T. Hypoxia enhances the wound-healing potential of adipose-derived stem cells in a novel human primary keratinocyte-based scratch assay. Int. J. Mol. Med. 2017, 39, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Moon, S.H.; Lee, S.H.; Kang, S.W.; Kim, J.; Lim, J.M.; Kim, H.S.; Kim, B.S.; Chung, H.M. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation 2007, 116, 2409–2419. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-J.; Shim, M.S.; Yu, T.; Choi, K.; Kim, D.-I.; Lee, S.-H.; Bhang, S.H. Bioreducible Polymer Micelles Based on Acid-Degradable Poly(ethylene glycol)-poly(amino ketal) Enhance the Stromal Cell-Derived Factor-1α Gene Transfection Efficacy and Therapeutic Angiogenesis of Human Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2018, 19, 529. https://doi.org/10.3390/ijms19020529
Lee T-J, Shim MS, Yu T, Choi K, Kim D-I, Lee S-H, Bhang SH. Bioreducible Polymer Micelles Based on Acid-Degradable Poly(ethylene glycol)-poly(amino ketal) Enhance the Stromal Cell-Derived Factor-1α Gene Transfection Efficacy and Therapeutic Angiogenesis of Human Adipose-Derived Stem Cells. International Journal of Molecular Sciences. 2018; 19(2):529. https://doi.org/10.3390/ijms19020529
Chicago/Turabian StyleLee, Tae-Jin, Min Suk Shim, Taekyung Yu, Kyunghee Choi, Dong-Ik Kim, Soo-Hong Lee, and Suk Ho Bhang. 2018. "Bioreducible Polymer Micelles Based on Acid-Degradable Poly(ethylene glycol)-poly(amino ketal) Enhance the Stromal Cell-Derived Factor-1α Gene Transfection Efficacy and Therapeutic Angiogenesis of Human Adipose-Derived Stem Cells" International Journal of Molecular Sciences 19, no. 2: 529. https://doi.org/10.3390/ijms19020529