Effect of Ball Mill Treatment on the Physicochemical Properties and Digestibility of Protein Extracts Generated from Scallops (Chlamys farreri)
Abstract
:1. Introduction
2. Results and Discussion
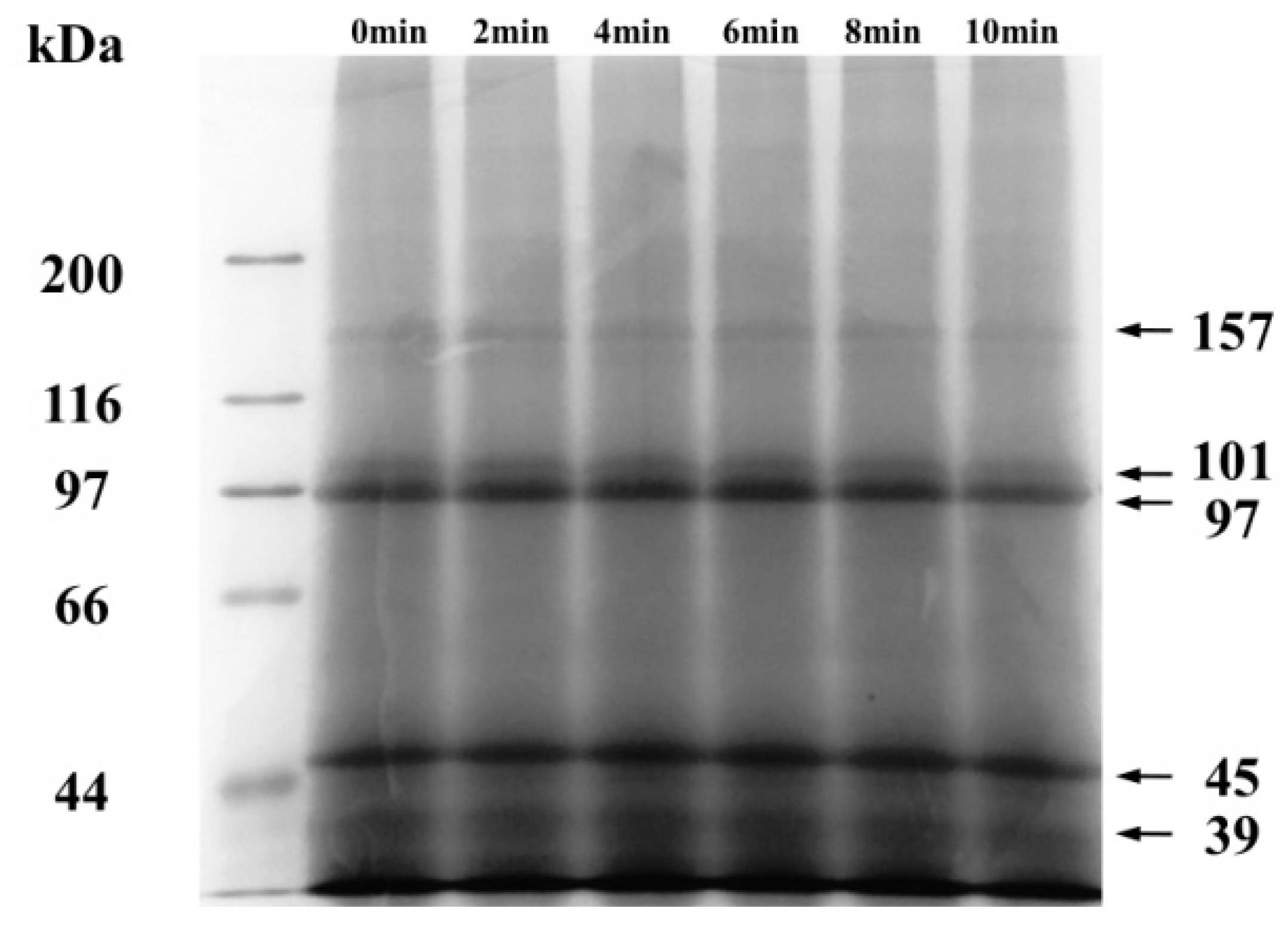
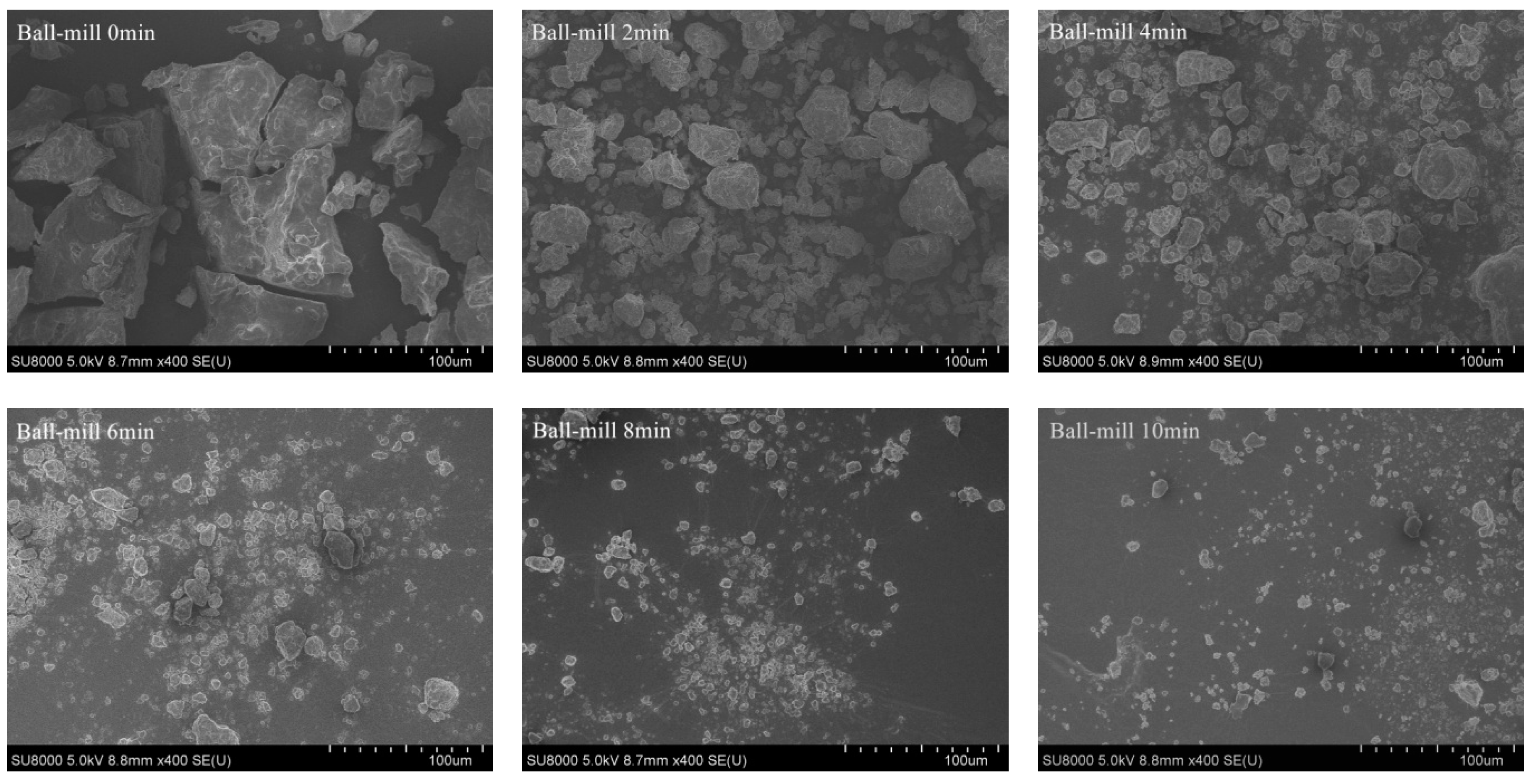
2.1. Effect of Ball-Milling of Protein Composition and Microstructure of CFP
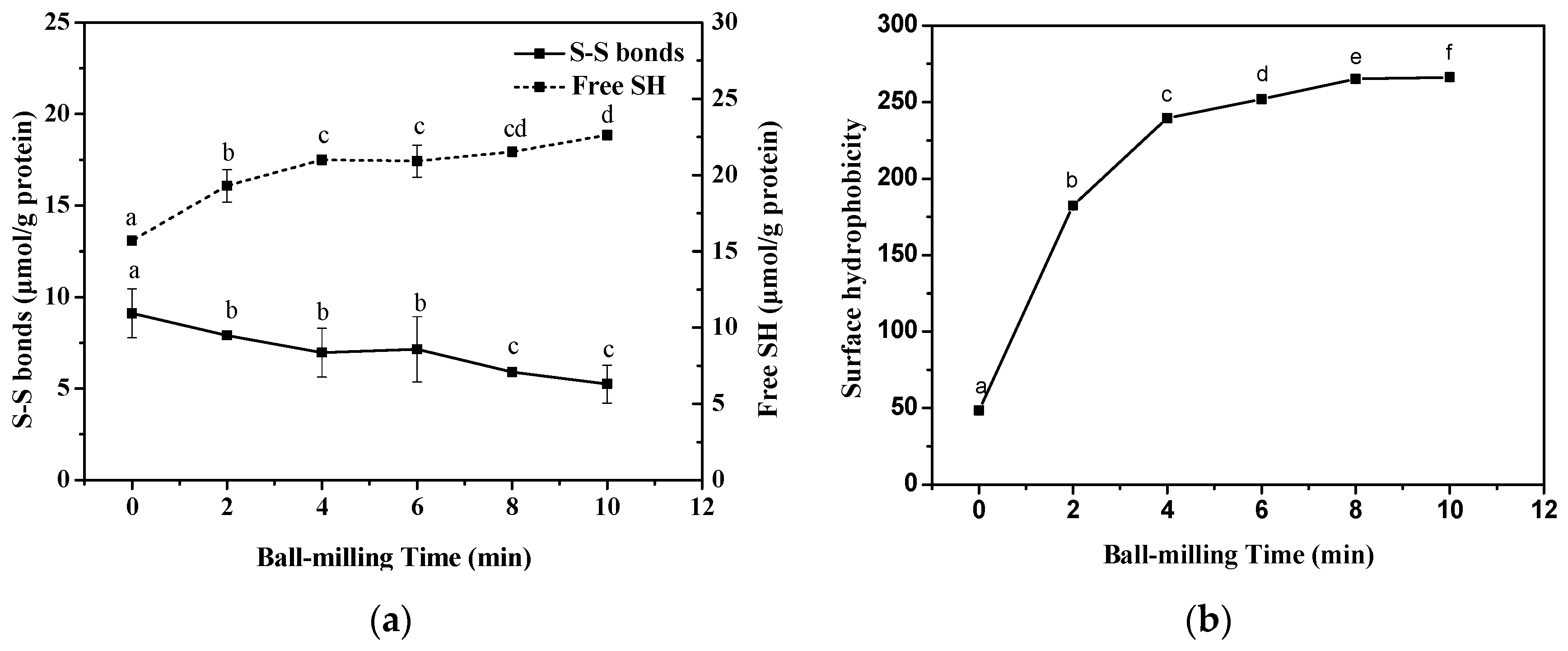
2.2. Effect of Ball-Milling on Sulfhydryl (SH) Groups
2.3. Effect of Ball-Milling on Surface Hydrophobicity (H0) Index
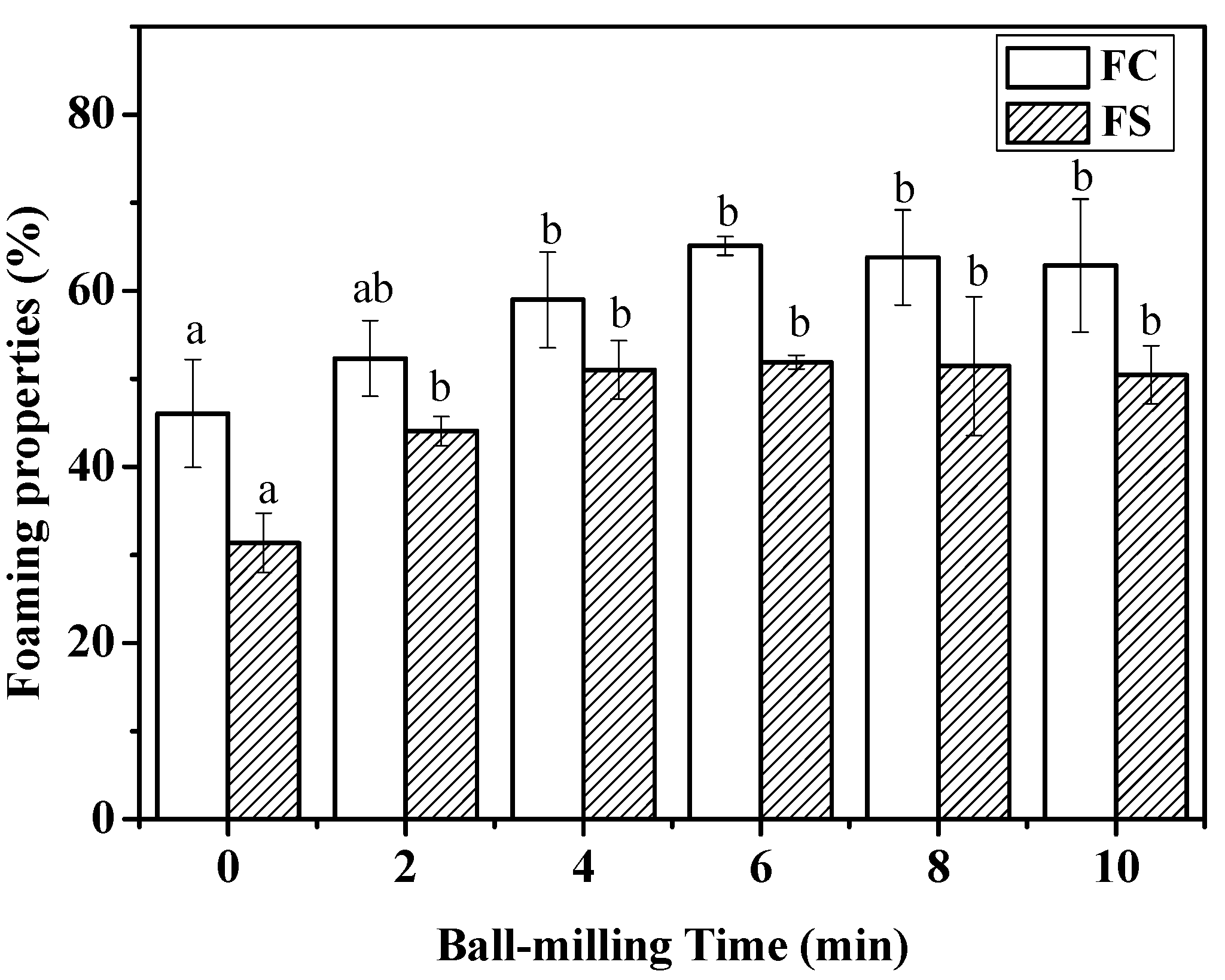
2.4. Effect of Ball-Milling on Foaming Capacity and Foaming Stability
2.5. Effect of Ball-Milling on Digestibility during the Digestions
2.6. Effect of Ball-Milling on the Number of Identified Peptides
3. Materials and Methods
3.1. Materials
3.2. Generation of CFP Extract
3.3. Ball Mill Treatment of CFP
3.4.Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
3.5. Scanning Electron Microscopy (SEM)
3.6. Determination of Sulfhydryl (SH) Groups
3.7. Determination of Protein Surface Hydrophobicity (H0) Index
3.8. Foaming Properties
3.9. Digestibility Determination
3.10. UPLC-Q-TOF Analysis of Hydrolysate
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, G.; Jiang, Z.; Ning, Y.; Zhu, D.; Zhang, M. Identification and characterization of a novel calreticulin involved in the immune response of the Zhikong scallop, Chlamys farreri. Fish Shellfish Immunol. 2017, 64, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Xie, J.; Zhang, D.; Han, Y.; Wang, C. Polypeptide from Chlamys farreri, suppresses ultraviolet-B irradiation-induced apoptosis through restoring ER redox homeostasis, scavenging ROS generation, and suppressing the PERK-eIF2a-CHOP pathway in HaCaT cells. J. Photochem. Photobiol. B Biol. 2015, 151, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Chen, M.; Yang, H.; Delaporte, M.; Zhao, S. Immune condition of Chlamys farreri, in response to acute temperature challenge. Aquaculture 2007, 27, 479–487. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Byun, H.G.; Kim, S.K. Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process Biochem. 2014, 35, 471–478. [Google Scholar] [CrossRef]
- Zhou, Z.; Ni, D.; Wang, M.; Wang, L.; Shi, X. The phenoloxidase activity and antibacterial function of a tyrosinase from scallop Chlamys farreri. Fish Shellfish Immunol. 2012, 33, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.X.; Wang, C.B. Inhibitory effect of polypeptides from Chlamys farreri on UVB-induced apoptosis and DNA damage in normal human dermal fibroblasts in vitro. Acta Pharmacol. Sin. 2003, 24, 1006–1010. [Google Scholar] [PubMed]
- Han, Y.; Zhao, H.; Jiang, Q.; Gao, H.; Wang, C. Chemopreventive mechanism of polypeptides from Chlamy Farreri (PCF) against UVB-induced malignant transformation of HaCaT cells. Mutagenesis 2015, 30, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Skomedal, H.; Skjanes, K.; Mazur-Marzec, H. Microalgal Proteins for Feed, Food and Health. In Microalgal-Based Biofuels and Bioproducts. From Feedstock Cultivation to End Products; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Garcia-Vaquero, M.; Hayes, M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev. Int. 2016, 32, 15–45. [Google Scholar] [CrossRef]
- Chen, H.; Weiss, J.; Shahidi, F. Nanotechnology in nutraceuticals and functional foods. Food Technol. 2006, 60, 277–308. [Google Scholar]
- Zhao, X.; Yang, Z.; Gai, G.; Yang, Y. Effect of superfine grinding on properties of ginger powder. J. Food Eng. 2009, 91, 217–222. [Google Scholar] [CrossRef]
- Chen, Q.M.; Fu, M.R.; Yue, F.L.; Cheng, Y.Y. Effect of superfine grinding on physicochemical properties, antioxidant activity and phenolic content of red rice (Oryza sativa L.). Food Nutr. Sci. 2015, 6, 1277–1284. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.H.; Zhu, K.X.; Peng, W.; Zhang, S.K.; Wang, B. Effect of superfine green tea powder on the thermodynamic, rheological and fresh noodle making properties of wheat flour. LWT Food Sci. Technol. 2012, 46, 23–28. [Google Scholar] [CrossRef]
- Shahrbabak, M.S.; Rozbahan, Y.; Shahrbabak, M.M.; Moradi, M. Influence of different levels of digestible undegradable protein on the carcass characteristic of Kermani male lambs in Iran. Int. J. Agric. Biol. 2009, 11, 643–646. [Google Scholar]
- Liu, B.; Wang, H.; Hu, T.; Zhang, P.; Zhang, Z.; Pan, S. Ball-milling changed the physicochemical properties of SPI and its cold-set gels. J. Food Eng. 2017, 195, 158–165. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Wu, T.; Liang, B.; Shi, C.; Zhang, M. Effect of superfine grinding on the structural and physicochemical properties of whey protein and applications for microparticulated proteins. Food Sci. Biotechnol. 2015, 24, 1637–1643. [Google Scholar] [CrossRef]
- Zhao, X.; Du, F.; Zhu, Q.; Qiu, D.; Yin, W.; Ao, Q. Effect of superfine pulverization on properties of Astragalus membranaceus powder. Powder Technol. 2010, 203, 620–625. [Google Scholar] [CrossRef]
- Wang, C.C.R.; Jhihying, C.; Poyuan, C. Effect of micronization on functional properties of the water caltrop (Trapa taiwanensis Nakai) pericarp. Food Chem. 2009, 113, 970–974. [Google Scholar] [CrossRef]
- He, H.L.; Liu, D.; Ma, C.B. Review on the angiotensin-I-converting enzyme (ACE) inhibitor peptides from marine proteins. Appl. Biochem. Biotechnol. 2013, 169, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Benedé, S.; López-Expósito, I.; Giménez, G.; Grishina, G.; Bardina, L.; Sampson, H.A. In vitro digestibility of bovine β-casein with simulated and human oral and gastrointestinal fluids. Identification and IgE-reactivity of the resultant peptides. Food Chem. 2014, 143, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Jiao, W.; Zhang, K.; Bao, Z.; Wang, S.; Liu, W. Proteomic analysis of scallop hepatopancreatic extract provides insights into marine polysaccharide digestion. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Muttakin, S.; Kim, M.S.; Lee, D.U. Tailoring physicochemical and sensorial properties of defatted soybean flour using jet-milling technology. Food Chem. 2015, 187, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Meng, N.; Zhang, B.; Zhao, S.; Xiong, S.; Xie, F. Effects of concurrent ball milling and octenyl succinylation on structure and physicochemical properties of starch. Carbohydr. Polym. 2017, 155, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Chen, Y.; Kong, X.; Hua, Y. Comparative studies on sulfhydryl determination of soy protein using two aromatic disulfide reagents and two fluorescent reagents. J. Agric. Food Chem. 2013, 61, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ecy, L.C.; Wan, L.; Tian, M.; Pan, S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013, 32, 303–311. [Google Scholar] [CrossRef]
- Zayas, J.F. Foaming Properties of Proteins Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 260–309. [Google Scholar]
- Makri, E.; Papalamprou, E.; Doxastakis, G. Study of functional properties of seed storage proteins from indigenous European legume crops (lupin, pea, broad bean) in admixture with polysaccharides. Food Hydrocoll. 2005, 19, 583–594. [Google Scholar] [CrossRef]
- Campbell, L.J.; Gu, X.; Dewar, S.J.; Euston, S.R. Effects of heat treatment and glucono -δ-lactone-induced acidification on characteristics of soy protein isolate. Food Hydrocoll. 2009, 23, 344–351. [Google Scholar] [CrossRef]
- Iametti, S.; Donnizzelli, E.; Vecchio, G.; Rovere, P.P.; Gola, S.; Bonomi, F. Macroscopic and structural consequences of high-pressure treatment of ovalbumin solutions. J. Agric. Food Chem. 1998, 46, 3521–3527. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Garcia-vaquero, M.; Lopezalonso, M.; Hayes, M. Assessment of the functional properties of protein extracted from the brown seaweed Himanthalia elongata (Linnaeus) S.F. Gray. Food Res. Int. 2016, 99, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.E.H.; Schöps, R.; Reynell, L.; Ulbrich-Hofmann, R.; Neubert, R.H.H.; Raith, K. Peptic digestion of beta-casein Time course and fate of possible bioactive peptides. J. Chromatogr. A 2007, 1166, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.J.; Rivera, M.; Hawsawi, O.; Jin, Z.; Hudson, T.; Wang, G. Muscadine grape skin extract induces an unfolded protein response-mediated autophagy in prostate cancer cells: A TMT-based quantitative proteomic analysis. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Feng, L.; Wang, Z.; Qiao, M.; Shahidi, F.; Lu, W. Sequence analysis and molecular docking of antithrombotic peptides from casein hydrolysate by trypsin digestion. J. Funct. Foods 2017, 32, 313–323. [Google Scholar] [CrossRef]


| Amino Acid Number of Peptides from Hydrolysis Fragment | 7–10 AA | 11–15 AA | 16–20 AA | 21–30 AA | Total Peptides |
|---|---|---|---|---|---|
| P60 0 min | 24 | 10 | 1 | 1 | 36 |
| P60 2 min | 28 | 23 | 2 | 2 | 55 |
| P60 4 min | 33 | 16 | 2 | 1 | 52 |
| PC2 0 min | 80 | 21 | 6 | 1 | 108 |
| PC2 2 min | 120 | 38 | 5 | 1 | 164 |
| PC2 4 min | 138 | 45 | 4 | 1 | 188 |
| PC4 0 min | 354 | 109 | 12 | 2 | 477 |
| PC4 2 min | 385 | 87 | 13 | 0 | 485 |
| PC4 4 min | 392 | 85 | 14 | 0 | 491 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Wu, C.; Chen, H.; Wang, Z.; Yu, C.; Du, M. Effect of Ball Mill Treatment on the Physicochemical Properties and Digestibility of Protein Extracts Generated from Scallops (Chlamys farreri). Int. J. Mol. Sci. 2018, 19, 531. https://doi.org/10.3390/ijms19020531
Wu D, Wu C, Chen H, Wang Z, Yu C, Du M. Effect of Ball Mill Treatment on the Physicochemical Properties and Digestibility of Protein Extracts Generated from Scallops (Chlamys farreri). International Journal of Molecular Sciences. 2018; 19(2):531. https://doi.org/10.3390/ijms19020531
Chicago/Turabian StyleWu, Di, Chao Wu, Hui Chen, Zhenyu Wang, Cuiping Yu, and Ming Du. 2018. "Effect of Ball Mill Treatment on the Physicochemical Properties and Digestibility of Protein Extracts Generated from Scallops (Chlamys farreri)" International Journal of Molecular Sciences 19, no. 2: 531. https://doi.org/10.3390/ijms19020531