Hypoxia Enhances Differentiation of Adipose Tissue-Derived Stem Cells toward the Smooth Muscle Phenotype
Abstract
:1. Introduction
2. Results
2.1. Changes in Gene Expression of SMC Marker Genes during Differentiation
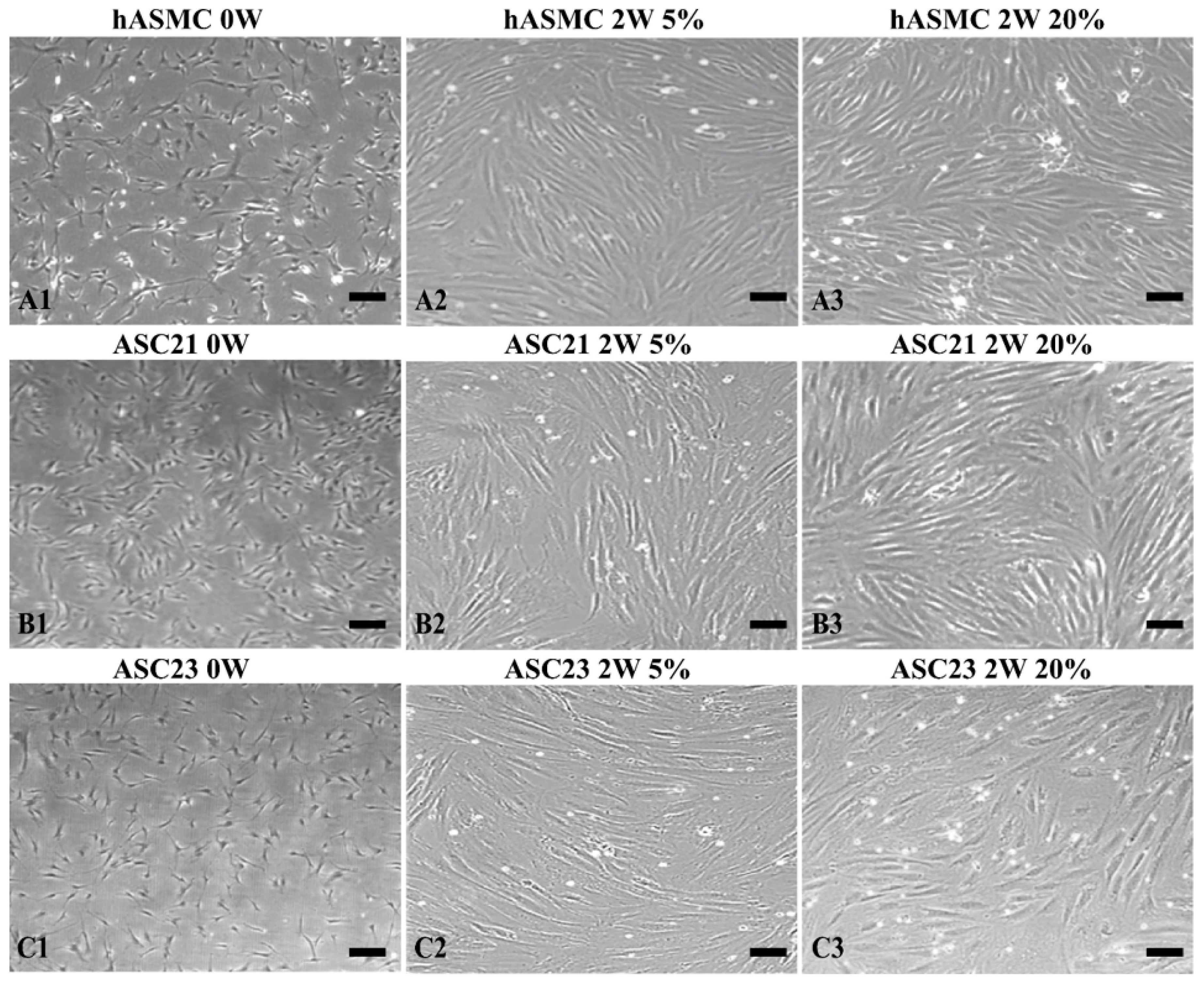
2.2. Morphological Changes of SMCs and ASCs
2.3. Effect of Hypoxia on Differentiation of Cells at the Protein Level
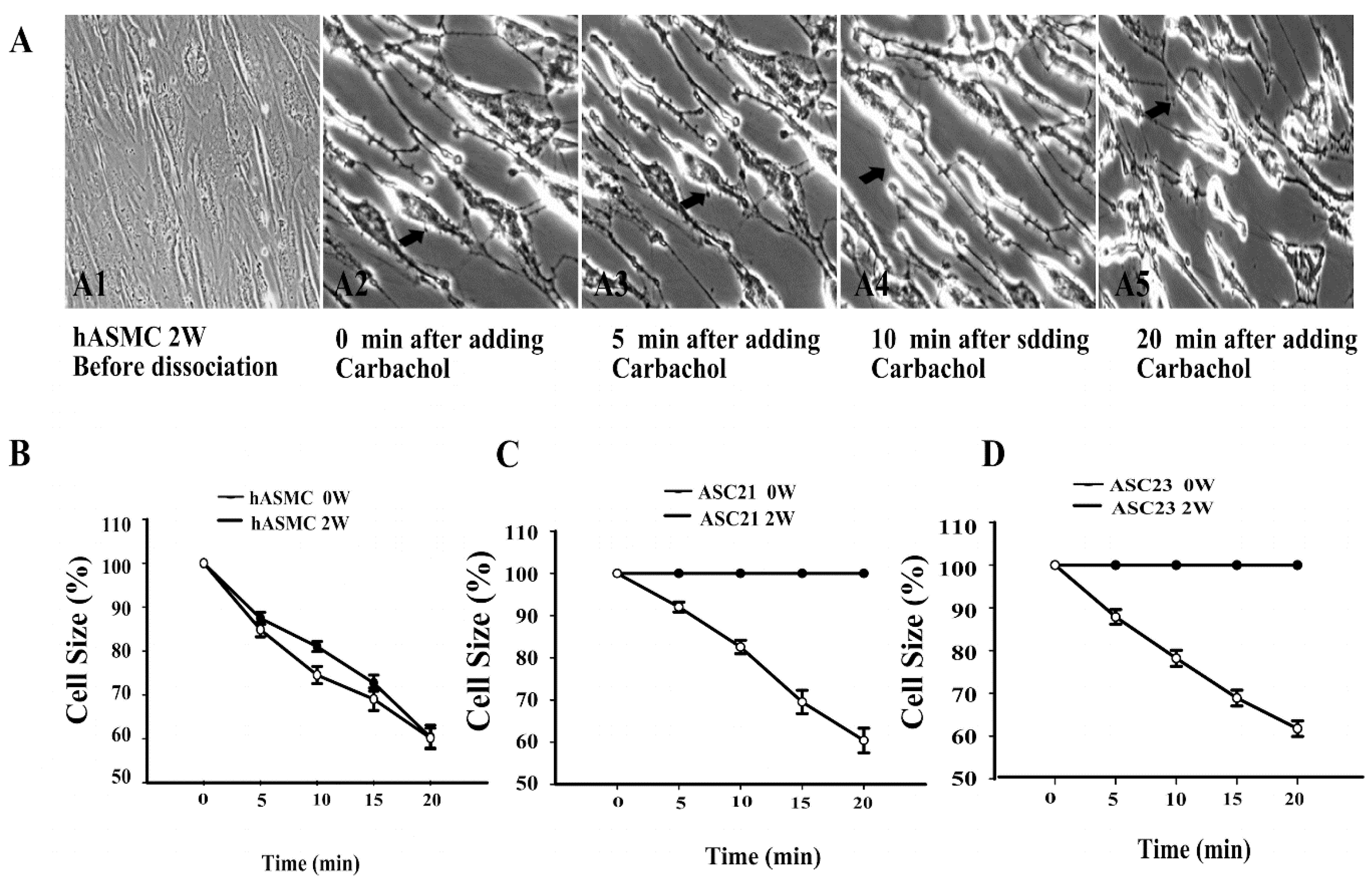
2.4. Dynamic Contraction Process of Differentiated Stem Cells
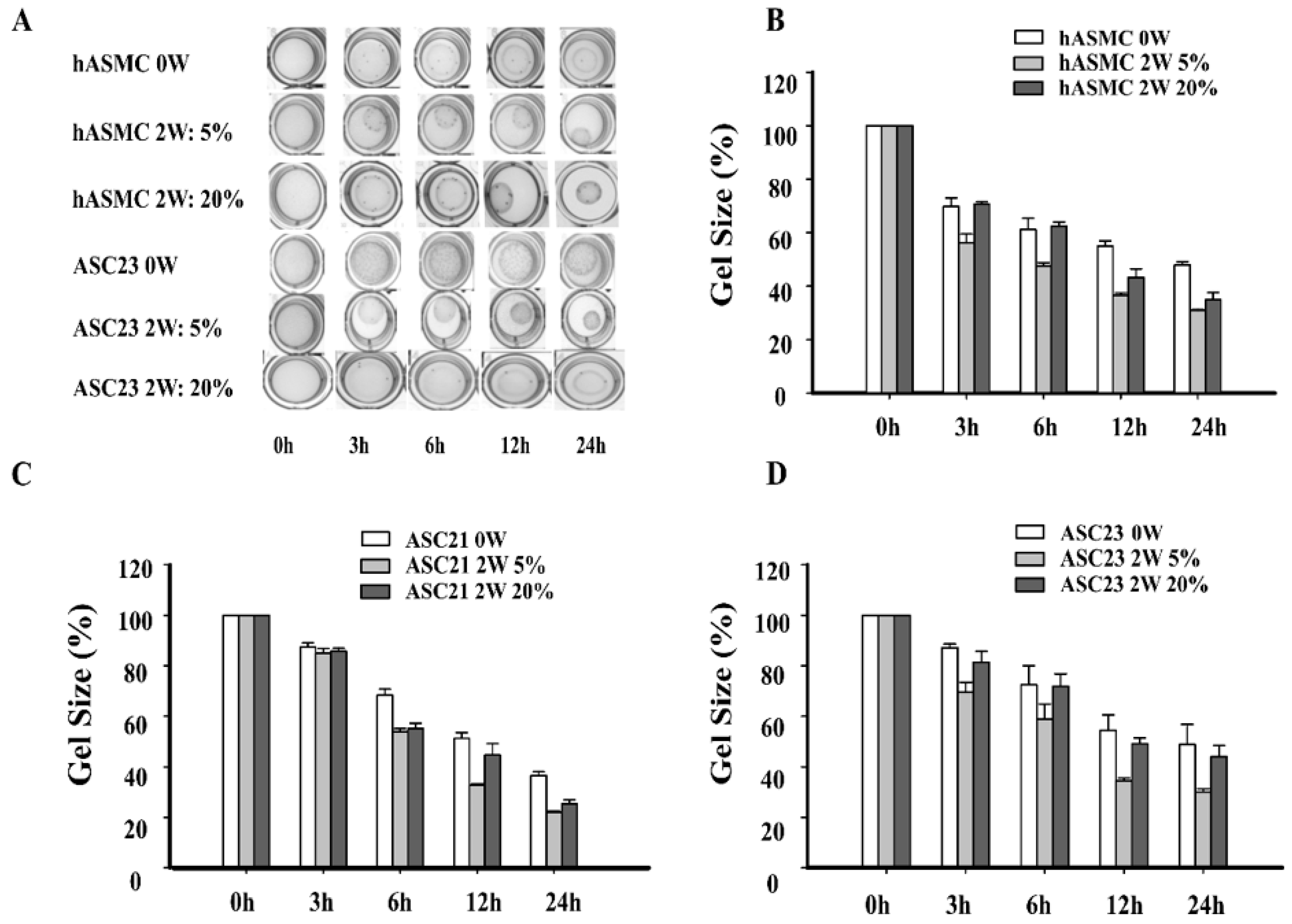
2.5. Effect of Hypoxia on Contractile Ability of SMCs Differentiated Form ASCs by Collagen Gel Lattice Assay
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Human Adipose Tissue-Derived Stem Cells
4.2. The Effect of Hypoxia on the Differentiation of SMCs and ASCs
4.3. Real Time Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.4. Immunofluorescence Staining
4.5. Quantitative Carbachol Contraction Assay
4.6. Effect of Hypoxia on Contractile Function
4.7. Statistical Analysis
4.8. Ethics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SMC | Smooth muscle cell |
| ASC | Adipose tissue-derived stem cell |
| α-SMA | Alpha-smooth muscle actin |
| MHC | Myosin heavy chain |
| PBS | Phosphate-buffered saline |
| SVF | Stromal vascular fraction |
| FBS | Fetal bovine serum |
| SMC | Smooth muscle cell |
| TGF-β1 | Transforming growth factor beta 1 |
| BMP | Bone morphogenetic protein |
| BMP4 | Bone morphogenetic protein 4 |
| VEGF | Vascular endothelial growth factor |
| RT-PCR | Real time reverse transcription polymerase chain reaction |
| OD | Optical density |
| cDNA | Complementary DNA |
| SEM | Standard error of the mean |
References
- Bitar, K.N.; Raghavan, S.; Zakhem, E. Tissue engineering in the gut: Developments in neuromusculature. Gastroenterology 2014, 146, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Leroi, A.M.; Kamm, M.A.; Weber, J.; Denis, P.; Hawley, P.R. Internal anal sphincter repair. Int. J. Colorectal Dis. 1997, 12, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Patel, B.; Beynon, J.; Carr, N.D. Surgical management of anorectal incontinence due to internal anal sphincter deficiency. Br. J. Surg. 1997, 84, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 2011, 20, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Jeon, E.S.; Kim, M.R.; Jho, S.K.; Ryu, S.W.; Kim, J.H. Angiotensin II-induced differentiation of adipose tissue-derived mesenchymal stem cells to smooth muscle-like cells. Int. J. Biochem. Cell Biol. 2008, 40, 2482–2491. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.V.; Alfonso, Z.; Zhang, R.; Leung, J.; Wu, B.; Ignarro, L.J. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc. Natl. Acad. Sci. USA 2006, 103, 12167–12172. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, S.; Cen, L.; Liu, Q.; Liu, W.; Cao, Y.; Cui, L. Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-beta1 and bone morphogenetic protein-4. Tissue Eng. Part A 2010, 16, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Lagna, G.; Ku, M.M.; Nguyen, P.H.; Neuman, N.A.; Davis, B.N.; Hata, A. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J. Biol. Chem. 2007, 282, 37244–37255. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, H.; Harris, L.J.; Zhang, P.; McIlhenny, S.; Srinivas, V.; Tulenko, T.; DiMuzio, P.J. The role of hypoxia in stem cell differentiation and therapeutics. J. Surg. Res. 2011, 165, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci. World J. 2013, 2013, 632972. [Google Scholar] [CrossRef] [PubMed]
- Zachar, V.; Duroux, M.; Emmersen, J.; Rasmussen, J.G.; Pennisi, C.P.; Yang, S.; Fink, T. Hypoxia and adipose-derived stem cell-based tissue regeneration and engineering. Expert Opin. Biol. Ther. 2011, 11, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Malladi, P.; Xu, Y.; Chiou, M.; Giaccia, A.J.; Longaker, M.T. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1139–C1146. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Lee, Y.J.; Yun, Z. Differentiation arrest by hypoxia. J. Biol. Chem. 2006, 281, 30678–30683. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.G.; Frobert, O.; Pilgaard, L.; Kastrup, J.; Simonsen, U.; Zachar, V.; Fink, T. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy 2011, 13, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.S.; Adesida, A.B.; Hardingham, T.E. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res. Ther. 2007, 9, R55. [Google Scholar] [CrossRef] [PubMed]
- Merceron, C.; Vinatier, C.; Portron, S.; Masson, M.; Amiaud, J.; Guigand, L.; Cherel, Y.; Weiss, P.; Guicheux, J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am. J. Physiol. Cell Physiol. 2010, 298, C355–C364. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.; Georgi, N.; Moreira Teixeira, L.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc. Natl. Acad. Sci. USA 2014, 111, 13954–13959. [Google Scholar] [CrossRef] [PubMed]
- Gultice, A.D.; Kulkarni-Datar, K.; Brown, T.L. Hypoxia-inducible factor 1alpha (HIF1A) mediates distinct steps of rat trophoblast differentiation in gradient oxygen. Biol. Reprod. 2009, 80, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Rubin, J.P.; Marra, K.G. Regulation of alpha-smooth muscle actin protein expression in adipose-derived stem cells. Cells Tissues Organs 2006, 183, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Q.; Moe, M.C.; Zhu, T. Regulation of cell-mediated collagen gel contraction in human retinal pigment epithelium cells by vascular endothelial growth factor compared with transforming growth factor-beta2. Clin. Exp. Ophthalmol. 2012, 40, e76–e86. [Google Scholar] [CrossRef] [PubMed]
- Sheares, K.K.; Jeffery, T.K.; Long, L.; Yang, X.; Morrell, N.W. Differential effects of TGF-beta1 and BMP-4 on the hypoxic induction of cyclooxygenase-2 in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L919–L927. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.; Rasmussen, J.G.; Lund, P.; Pilgaard, L.; Soballe, K.; Zachar, V. Isolation and expansion of adipose-derived stem cells for tissue engineering. Front. Biosci. 2011, 3, 256–263. [Google Scholar] [CrossRef]
- Pilgaard, L.; Lund, P.; Duroux, M.; Lockstone, H.; Taylor, J.; Emmersen, J.; Fink, T.; Ragoussis, J.; Zachar, V. Transcriptional signature of human adipose tissue-derived stem cells (hASCs) preconditioned for chondrogenesis in hypoxic conditions. Exp. Cell Res. 2009, 315, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Zachar, V.; Fink, T.; Pennisi, C.P. Moderate Hypoxia Influences Potassium Outward Currents in Adipose-Derived Stem Cells. PLoS ONE 2014, 9, e104912. [Google Scholar] [CrossRef] [PubMed]
- Riis, S.; Stensballe, A.; Emmersen, J.; Pennisi, C.P.; Birkelund, S.; Zachar, V.; Fink, T. Mass spectrometry analysis of adipose-derived stem cells reveals a significant effect of hypoxia on pathways regulating extracellular matrix. Stem Cell Res. Ther. 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.H.; Shade, D.L.; Tamm, E.; DeSantis, L. Single-cell contraction assay for human ciliary muscle cells. Effect of carbachol. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1876–1879. [Google Scholar]
- Ngo, P.; Ramalingam, P.; Phillips, J.A.; Furuta, G.T. Collagen gel contraction assay. Methods Mol. Biol. 2006, 341, 103–109. [Google Scholar] [PubMed]


| Gene | Forward Primer Sequence | Reverse Primer Sequence | Annealing Temperature (°C) |
|---|---|---|---|
| β-actin | 5′-ATC ATG TTT GAG ACC TTC AA-3′ | 5′-AAA GCC CTG GAA CTT GAC C-3′ | 58 |
| α-SMA | 5′-AGC AGC CCA GCC AAG CAC TG-3′ | 5′-AGC CGG CCT TAC AGA GCC CA-3′ | 60 |
| Calponin | 5′-CTG GCT GCA GCT TAT TGA TG-3′ | 5′-CTG AGA GAG TGG ATC GAG GG-3′ | 60 |
| Caldesmon | 5′-TCT GAG CCT TCT GGT TGG TC-3′ | 5′-CCT CGG GAA GAA GTT TCA GA-3′ | 60 |
| SM-MHC | 5′-AAA GCC CTG GAA CTT GAC C-3′ | 5′-AGA TTT TGC TCT GCC CTA TCC-3′ | 60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zachar, V.; Pennisi, C.P.; Fink, T.; Maeda, Y.; Emmersen, J. Hypoxia Enhances Differentiation of Adipose Tissue-Derived Stem Cells toward the Smooth Muscle Phenotype. Int. J. Mol. Sci. 2018, 19, 517. https://doi.org/10.3390/ijms19020517
Wang F, Zachar V, Pennisi CP, Fink T, Maeda Y, Emmersen J. Hypoxia Enhances Differentiation of Adipose Tissue-Derived Stem Cells toward the Smooth Muscle Phenotype. International Journal of Molecular Sciences. 2018; 19(2):517. https://doi.org/10.3390/ijms19020517
Chicago/Turabian StyleWang, Fang, Vladimir Zachar, Cristian Pablo Pennisi, Trine Fink, Yasuko Maeda, and Jeppe Emmersen. 2018. "Hypoxia Enhances Differentiation of Adipose Tissue-Derived Stem Cells toward the Smooth Muscle Phenotype" International Journal of Molecular Sciences 19, no. 2: 517. https://doi.org/10.3390/ijms19020517
APA StyleWang, F., Zachar, V., Pennisi, C. P., Fink, T., Maeda, Y., & Emmersen, J. (2018). Hypoxia Enhances Differentiation of Adipose Tissue-Derived Stem Cells toward the Smooth Muscle Phenotype. International Journal of Molecular Sciences, 19(2), 517. https://doi.org/10.3390/ijms19020517






