Inhibition of Phosphodiesterase 4 by FCPR03 Alleviates Lipopolysaccharide-Induced Depressive-Like Behaviors in Mice: Involvement of p38 and JNK Signaling Pathways
Abstract
:1. Introduction
2. Results
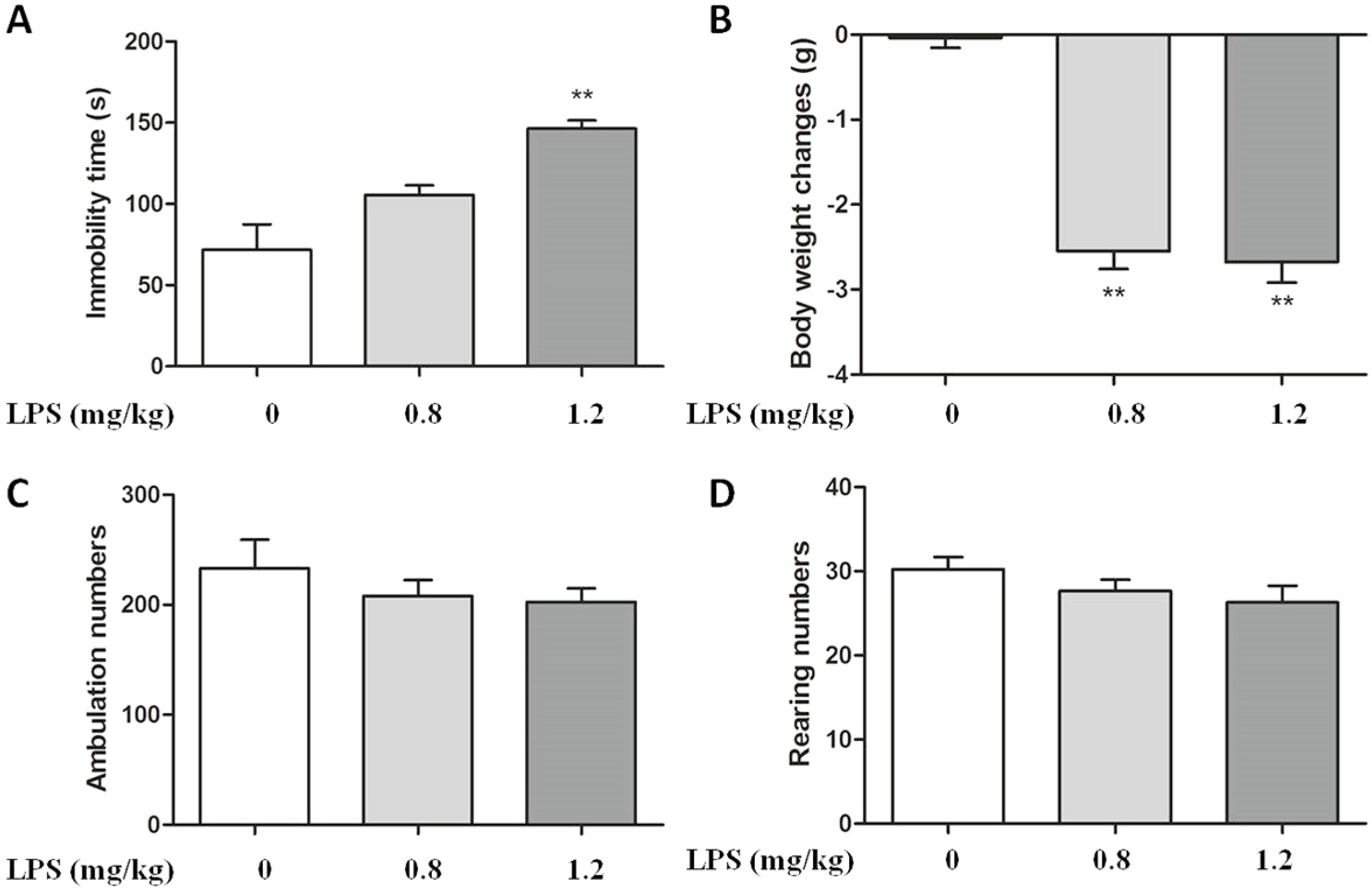
2.1. Effect of LPS on Body Weight and Immobility Time in C57BL/6 Mice
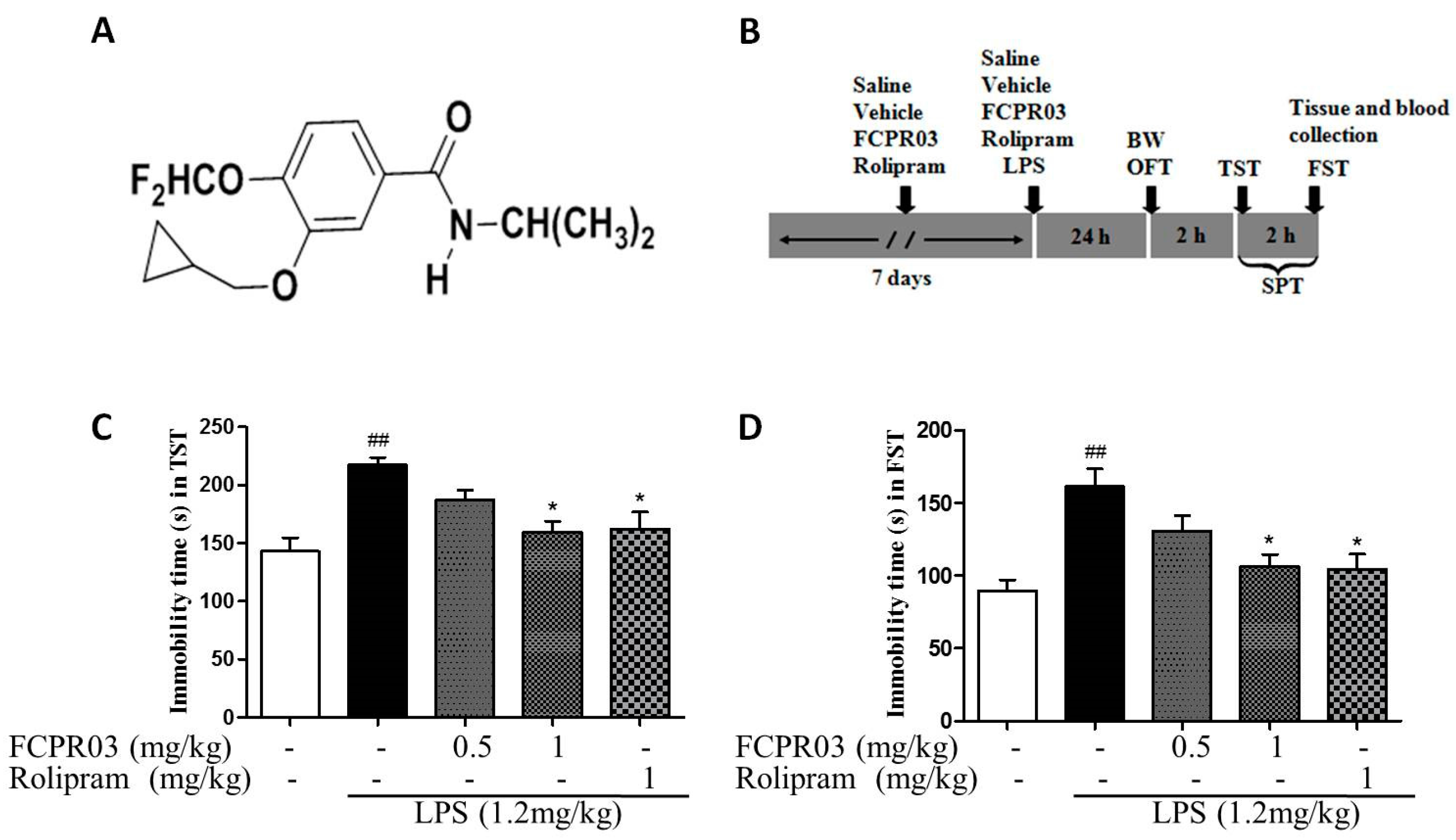
2.2. The Antidepressant Effect of FCPR03 in LPS-Treated Mice
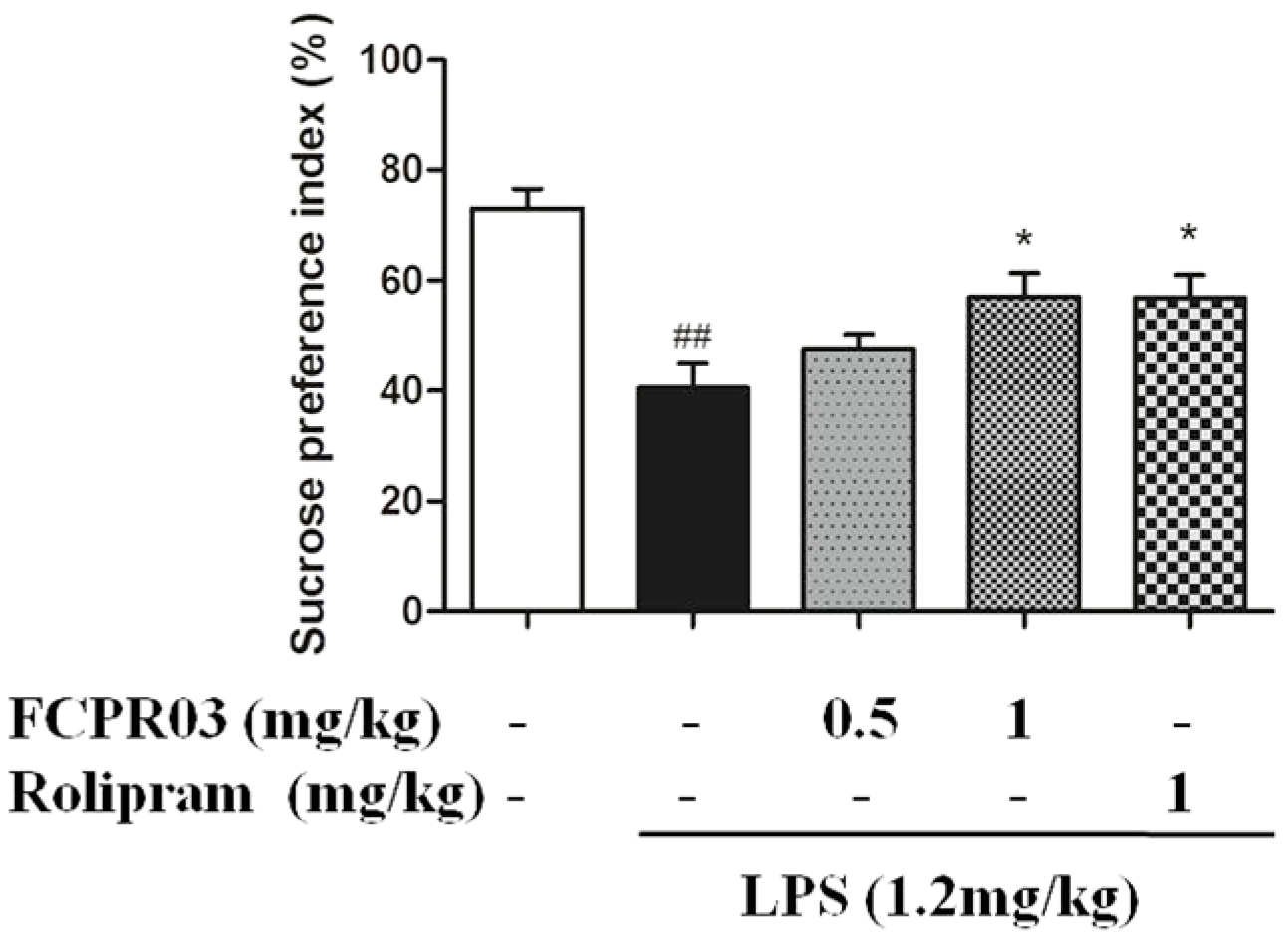
2.3. FCPR03 Increased Sucrose Preference in Mice Challenged with LPS
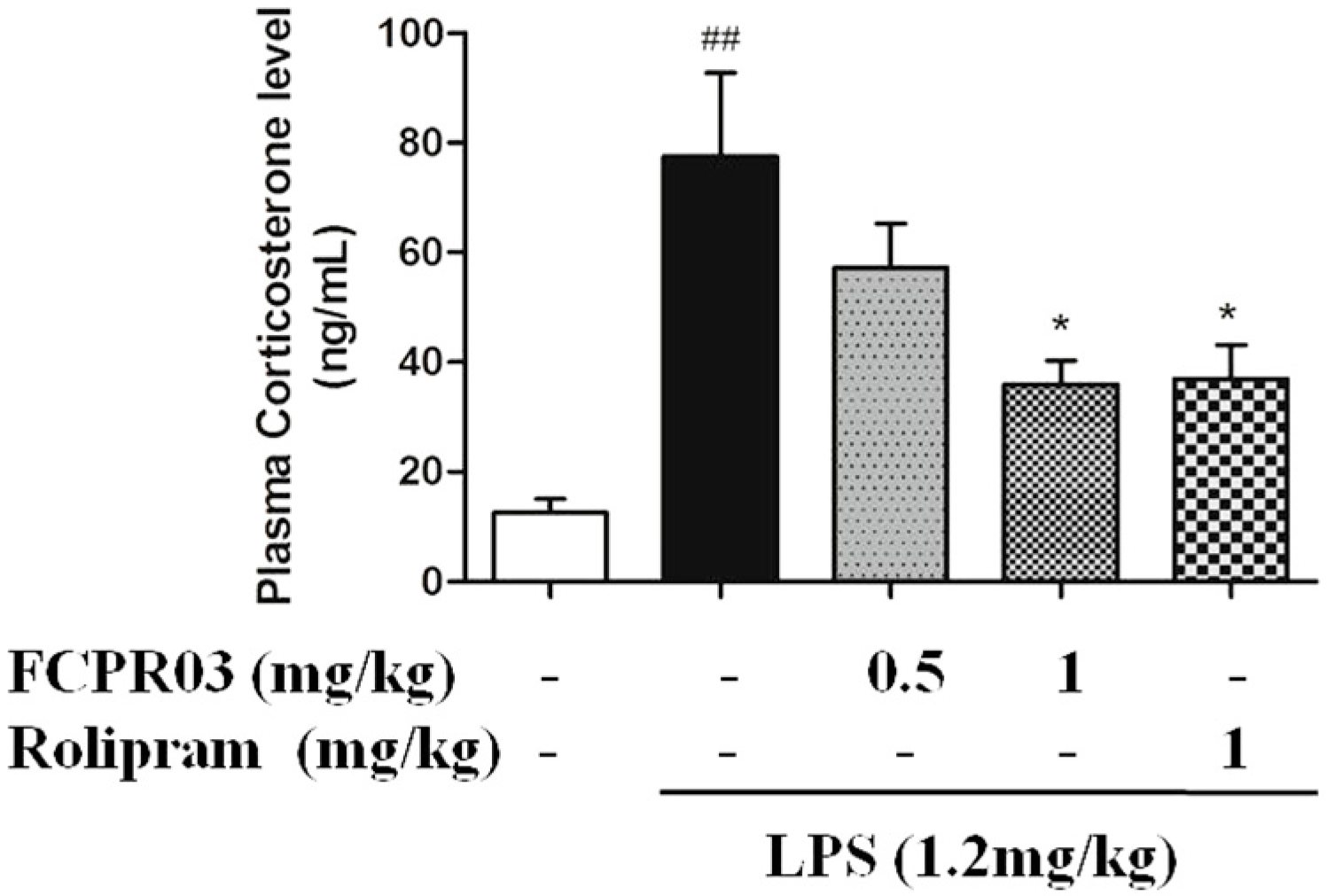
2.4. FCPR03 Decreased the Level of Plasma Corticosterone in LPS-Treated Mice
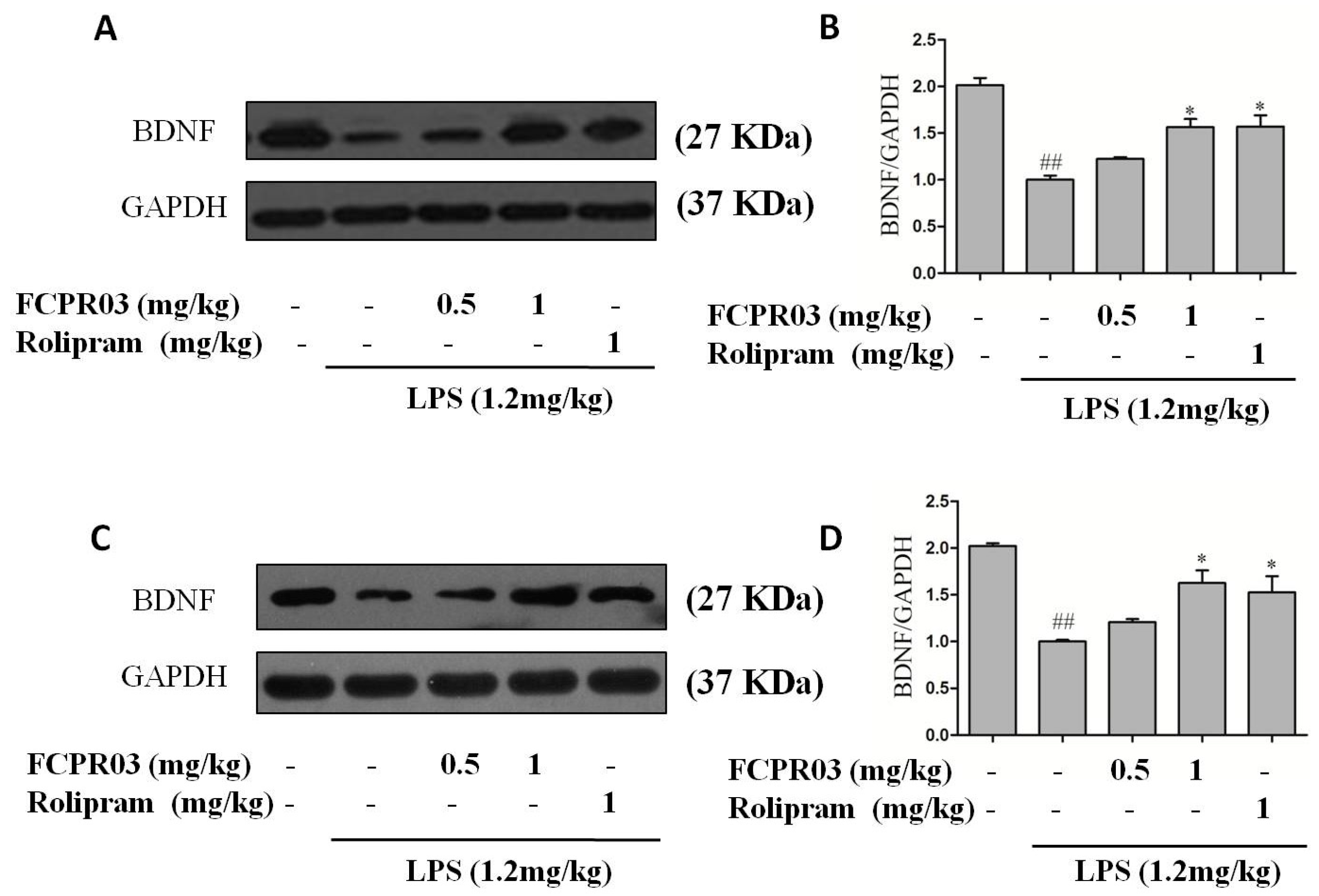
2.5. FCPR03 Stimulated the Expression of BDNF in LPS-Treated Mice
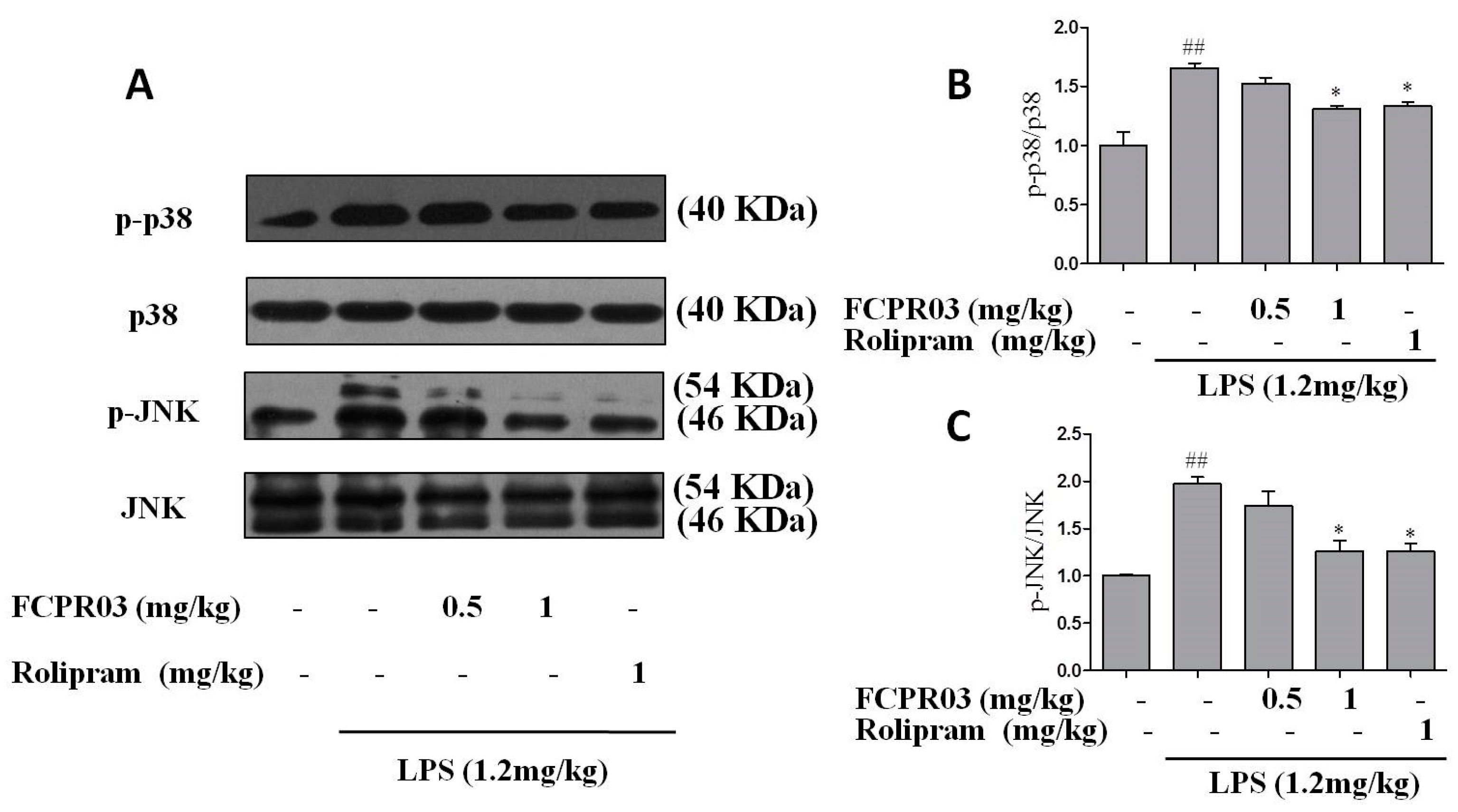
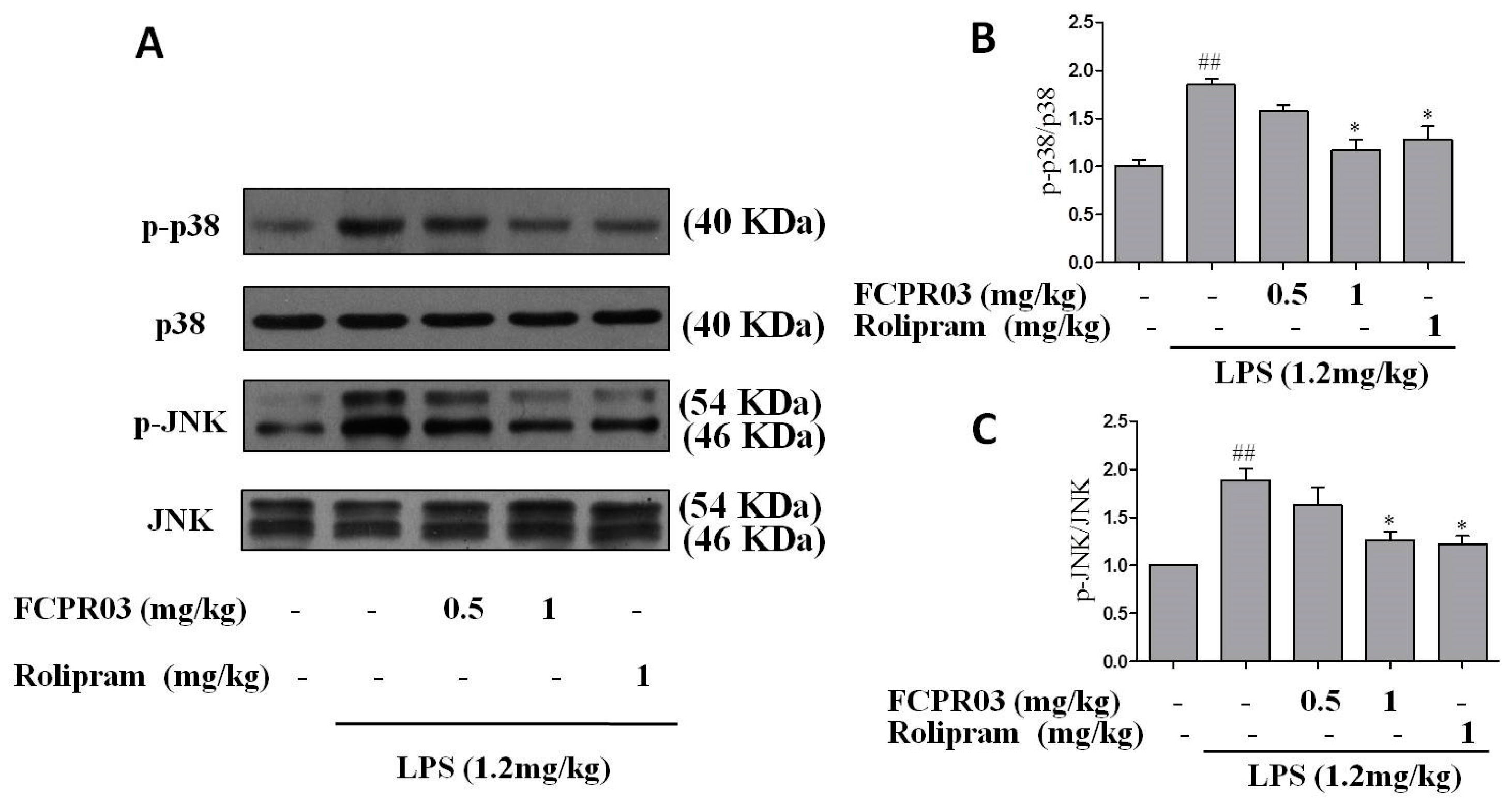
2.6. FCPR03 Increased the Phosphorylation of p38 and JNK in LPS-Treated Mice
3. Discussion
4. Materials and Animals
4.1. Animals
4.2. Drug Preparation and Treatment
4.3. Mouse Model of Depression
4.4. Sucrose Preference Test
4.5. Open Field Test
4.6. Forced Swim Test and Tail Suspension Test
4.7. Assessment of Corticosterone Level in Plasma
4.8. Western Blot Analysis
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hegerl, U.; Rummel-Kluge, C.; Varnik, A.; Arensman, E.; Koburger, N. Alliances against depression—A community based approach to target depression and to prevent suicidal behaviour. Neurosci. Biobehav. Rev. 2013, 37, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Villa, R.F. The Neurobiology of Depression: An Integrated Overview from Biological Theories to Clinical Evidence. Mol. Neurobiol. 2017, 54, 4847–4865. [Google Scholar] [CrossRef] [PubMed]
- Lanni, C.; Govoni, S.; Lucchelli, A.; Boselli, C. Depression and antidepressants: Molecular and cellular aspects. Cell. Mol. Life Sci. 2009, 66, 2985–3008. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Quirion, R.; Little, P.J.; Cheng, Y.; Feng, Z.P.; Sun, H.S.; Xu, J.; Zheng, W. Forkhead Box O Transcription Factors as Possible Mediators in the Development of Major Depression. Neuropharmacology 2015, 99, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Whiskey, E.; Taylor, D. A review of the adverse effects and safety of noradrenergic antidepressants. J. Psychopharmacol. 2013, 27, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I. The efficacy, tolerability, and safety of contemporary antidepressants. J. Clin. Psychiatry 2010, 71, e03. [Google Scholar] [CrossRef] [PubMed]
- Harmer, C.J.; Duman, R.S.; Cowen, P.J. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017, 4, 409–418. [Google Scholar] [CrossRef]
- Jeon, S.W.; Kim, Y.K. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J. Psychiatry 2016, 6, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xie, G.; Zhang, Z.; Wang, C.; Li, W.; Zhou, W.; Tang, Y. Levels of serum interleukin (IL)-6, IL-1beta, tumour necrosis factor-alpha and leptin and their correlation in depression. Aust. N. Z. J. Psychiatry 2007, 41, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.J.; Karelina, K.; Zhang, N.; Walton, J.C.; Morris, J.S.; Devries, A.C. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol. Psychiatry 2010, 15, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, C.; Yu, H.; Cai, X.; Shen, X.; Sun, X.; Wang, J.; Zhang, Y.; Wang, C. Lentivirus-mediated interleukin-1beta (IL-1beta) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J. Neuroinflamm. 2017, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Domingues, M.; Fronza, M.; Vieira, B.; Begnini, K.; Lenardao, E.J.; Seixas, F.K.; Collares, T.; Nogueira, C.W.; Savegnago, L. Antidepressant-like effect of a new selenium-containing compound is accompanied by a reduction of neuroinflammation and oxidative stress in lipopolysaccharide-challenged mice. J. Psychopharmacol. 2017, 31, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Trofimov, A.; Markova, N.; Nikolenko, V.; Steinbusch, H.W.; Chekhonin, V.; Schroeter, C.; Lesch, K.P.; Anthony, D.C.; Strekalova, T. Low-dose lipopolysaccharide (LPS) inhibits aggressive and augments depressive behaviours in a chronic mild stress model in mice. J. Neuroinflamm. 2016, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Wang, H.; Su, Q.; Chen, Y.; Xue, W.; Xia, B.; Duan, J.; Chen, G. Paeonol attenuates lipopolysaccharide-induced depressive-like behavior in mice. Psychiatry Res. 2016, 238, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O. Kolaviron, a natural flavonoid from the seeds of Garcinia kola, reduces LPS-induced inflammation in macrophages by combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-kappaB, p38, Akt, p-c-JUN and JNK. Biochim. Biophys. Acta 2014, 1840, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, S.I.; Lee, H.J.; Kim, S.S.; Kwon, Y.S.; Chun, W. Isorhamnetin-3-O-Glucuronide Suppresses JNK and p38 Activation and Increases Heme-Oxygenase-1 in Lipopolysaccharide-Challenged RAW264.7 Cells. Drug Dev. Res. 2016, 77, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Su, W.J.; Zhang, Y.; Chen, Y.; Gong, H.; Lian, Y.J.; Peng, W.; Liu, Y.Z.; Wang, Y.X.; You, Z.L.; Feng, S.J.; et al. NLRP3 gene knockout blocks NF-kappaB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav. Brain Res. 2017, 322, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; Da Pozzo, E.; Zappelli, E.; Martini, C. Trazodone treatment protects neuronal-like cells from inflammatory insult by inhibiting NF-kappaB, p38 and JNK. Cell. Signal. 2015, 27, 1609–1629. [Google Scholar] [CrossRef] [PubMed]
- Spina, D. PDE4 inhibitors: Current status. Br. J. Pharmacol. 2008, 155, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Giembycz, M.A. Can the anti-inflammatory potential of PDE4 inhibitors be realized: Guarded optimism or wishful thinking? Br. J. Pharmacol. 2008, 155, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Rogliani, P.; Matera, M.G. The discovery of roflumilast for the treatment of chronic obstructive pulmonary disease. Expert Opin. Drug Discov. 2016, 11, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Cheng, Y.; Wang, C.; Wu, J.; Zou, Z.; Niu, B.; Yu, H.; Wang, H.; Xu, J. FFPM, a PDE4 inhibitor, reverses learning and memory deficits in APP/PS1 transgenic mice via cAMP/PKA/CREB signaling and anti-inflammatory effects. Neuropharmacology 2017, 116, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Sloan, V.S.; Stevens, R.M.; Schafer, P. Apremilast: A novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Ther. Adv. Musculoskelet. Dis. 2010, 2, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Prickaerts, J.; Heckman, P.R.A.; Blokland, A. Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin. Investig. Drugs 2017, 26, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Mahesh, R.; Bhatt, S. Etazolate, a phosphodiesterase-4 enzyme inhibitor produces antidepressant-like effects by blocking the behavioral, biochemical, neurobiological deficits and histological abnormalities in hippocampus region caused by olfactory bulbectomy. Psychopharmacology 2015, 232, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Schwarz, T.; Gob, E.; Heydenreich, N.; Brede, M.; Meuth, S.G.; Kleinschnitz, C. The phosphodiesterase-4 inhibitor rolipram protects from ischemic stroke in mice by reducing blood-brain-barrier damage, inflammation and thrombosis. Exp. Neurol. 2013, 247, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Bletsch, M.; Stanley, J.; Wheeler, D.; Scott, R.; Tully, T. The PDE4 inhibitor HT-0712 improves hippocampus-dependent memory in aged mice. Neuropsychopharmacology 2014, 39, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Ryter, S.W.; Kyung, S.Y.; Lee, S.P.; Jeong, S.H. The phosphodiesterase 4 inhibitor rolipram protects against cigarette smoke extract-induced apoptosis in human lung fibroblasts. Eur. J. Pharmacol. 2013, 706, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, A.; Camara, S.; Ilieva, M.; Riederer, P.; Michel, T.M. Brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3) levels in post-mortem brain tissue from patients with depression compared to healthy individuals—A proof of concept study. Eur. Psychiatry 2017, 46, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.; et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Cheng, Y.F.; Zou, Z.Q.; Ge, B.C.; Yu, H.; Huang, C.; Wang, H.T.; Yang, X.M.; Xu, J.P. Discovery of N-Alkyl Catecholamides as Selective Phosphodiesterase-4 Inhibitors with Anti-neuroinflammation Potential Exhibiting Antidepressant-like Effects at Non-emetic Doses. ACS Chem. Neurosci. 2016, 8, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.Q.; Chen, J.J.; Feng, H.F.; Cheng, Y.F.; Wang, H.T.; Zhou, Z.Z.; Guo, H.B.; Zheng, W.; Xu, J.P. Novel Phosphodiesterase 4 Inhibitor FCPR03 Alleviates Lipopolysaccharide-Induced Neuroinflammation by Regulation of cAMP/PKA/CREB Signaling Pathway and NF-kappaB Inhibition. J. Pharmacol. Exp. Ther. 2017, 362, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.F.; Huang, F.; Wang, H.T.; Wang, G.H.; Yuan, X.; Zhang, M.Z.; Guo, H.B.; Cheng, Y.F.; Xu, J.P. Involvement of norepinephrine and serotonin system in antidepressant-like effects of hederagenin in the rat model of unpredictable chronic mild stress-induced depression. Pharm. Biol. 2015, 53, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, P.O.; Goodyer, I.M. Childhood adversity and allostatic overload of the hypothalamic-pituitary-adrenal axis: A vulnerability model for depressive disorders. Dev. Psychopathol. 2011, 23, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Brovedani, P. The hippocampus, neurotrophic factors and depression: Possible implications for the pharmacotherapy of depression. CNS Drugs 2011, 25, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhao, Y.H.; Zeng, M.J.; Fang, F.; Li, M.; Qin, T.T.; Ye, L.Y.; Li, H.W.; Qu, R.; Ma, S.P. Saikosaponin D relieves unpredictable chronic mild stress induced depressive-like behavior in rats: Involvement of HPA axis and hippocampal neurogenesis. Psychopharmacology 2017, 234, 3385–3394. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef]
- Sawamoto, A.; Okuyama, S.; Amakura, Y.; Yoshimura, M.; Yamada, T.; Yokogoshi, H.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone Ameliorates Depressive-Like Behavior and Hippocampal Neurochemical Changes in Chronic Unpredictable Mild Stressed Mice by Regulating the Brain-Derived Neurotrophic Factor: Requirement for ERK Activation. Int. J. Mol. Sci. 2017, 18, 2133. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Budni, J.; Freitas, A.E.; Neis, V.B.; Ribeiro, C.M.; de Oliveira Balen, G.; Rieger, D.K.; Leal, R.B.; Rodrigues, A.L. TNF-alpha-induced depressive-like phenotype and p38 (MAPK) activation are abolished by ascorbic acid treatment. Eur. Neuropsychopharmacol. 2015, 25, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Tamano, H.; Nishio, R.; Murakami, T. Behavioral Abnormality Induced by Enhanced Hypothalamo-Pituitary-Adrenocortical Axis Activity under Dietary Zinc Deficiency and Its Usefulness as a Model. Int. J. Mol. Sci. 2016, 17, 1149. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.L.; Abella, C.; Hernandez, J. Diazepam increases the hypothalamic-pituitary-adrenocortical (HPA) axis activity by a cyclic AMP-dependent mechanism. Br. J. Pharmacol. 2001, 133, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Cover, P.O.; Poyser, R.H.; Buckingham, J.C. Stimulation of the hypothalamo-pituitary-adrenal axis in the rat by three selective type-4 phosphodiesterase inhibitors: In vitro and in vivo studies. Br. J. Pharmacol. 1997, 121, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Hadley, A.J.; Kumari, M.; Cover, P.O.; Osborne, J.; Poyser, R.; Flack, J.D.; Buckingham, J.C. Stimulation of the hypothalamo-pituitary-adrenal axis in the rat by the type 4 phosphodiesterase (PDE-4) inhibitor, denbufylline. Br. J. Pharmacol. 1996, 119, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Pettipher, E.R.; Eskra, J.D.; Labasi, J.M. The inhibitory effect of rolipram on TNF-alpha production in mouse blood ex vivo is dependent upon the release of corticosterone and adrenaline. Cytokine 1997, 9, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Mahesh, R.; Bhatt, S.; Pandey, D. Molecular modifications by regulating cAMP signaling and oxidant-antioxidant defence mechanisms, produce antidepressant-like effect: A possible mechanism of etazolate aftermaths of impact accelerated traumatic brain injury in rat model. Neurochem. Int. 2017, 111, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Yang, W.X.; Zhang, Y.; Zhao, N.; Zhang, Y.Z.; Liu, Y.Q.; Xu, Y.; Wilson, S.P.; O’Donnell, J.M.; Zhang, H.T.; et al. Phosphodiesterase-4D Knock-down in the Prefrontal Cortex Alleviates Chronic Unpredictable Stress-Induced Depressive-Like Behaviors and Memory Deficits in Mice. Sci. Rep. 2015, 5, 11332. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Mahesh, R.; Bhatt, S. Type 4 phosphodiesterase enzyme inhibitor, rolipram rescues behavioral deficits in olfactory bulbectomy models of depression: Involvement of hypothalamic-pituitary-adrenal axis, cAMP signaling aspects and antioxidant defense system. Pharmacol. Biochem. Behav. 2015, 132, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Kvarta, M.D.; Bradbrook, K.E.; Dantrassy, H.M.; Bailey, A.M.; Thompson, S.M. Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. J. Neurophysiol. 2015, 114, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Popova, D.; Castren, E.; Taira, T. Chronic fluoxetine administration enhances synaptic plasticity and increases functional dynamics in hippocampal CA3-CA1 synapses. Neuropharmacology 2017, 126, 250–256. [Google Scholar] [CrossRef] [PubMed]
- McAvoy, K.; Russo, C.; Kim, S.; Rankin, G.; Sahay, A. Fluoxetine induces input-specific hippocampal dendritic spine remodeling along the septotemporal axis in adulthood and middle age. Hippocampus 2015, 25, 1429–1446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cheng, Y.; Wang, H.; Wang, C.; Wilson, S.P.; Xu, J.; Zhang, H.T. RNA interference-mediated knockdown of long-form phosphodiesterase-4D (PDE4D) enzyme reverses amyloid-beta42-induced memory deficits in mice. J. Alzheimers Dis. 2014, 38, 269–280. [Google Scholar] [PubMed]
- Hong, E.J.; McCord, A.E.; Greenberg, M.E. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron 2008, 60, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Castren, E.; Kojima, M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol. Dis. 2017, 97, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Tu, Y.F.; Huang, C.C.; Ho, C.J. JNK signaling is the shared pathway linking neuroinflammation, blood-brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J. Neuroinflamm. 2012, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005, 15, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Poddar, R.; Paul, S. Novel crosstalk between ERK MAPK and p38 MAPK leads to homocysteine-NMDA receptor-mediated neuronal cell death. J. Neurochem. 2013, 124, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Budni, J.; Ribeiro, C.M.; Rieger, D.K.; Leal, R.B.; Rodrigues, A.L.S. Subchronic administration of ascorbic acid elicits antidepressant-like effect and modulates cell survival signaling pathways in mice. J. Nutr. Biochem. 2016, 38, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Du, J.K.; Hu, X.; Yu, Q.; Li, D.X.; Wang, C.N.; Zhu, X.Y.; Liu, Y.J. Protective effects of resveratrol on mitochondrial function in the hippocampus improves inflammation-induced depressive-like behavior. Physiol. Behav. 2017, 182, 54–61. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Zou, Z.; Zhang, X.; Peng, W.; Chen, C.; Ye, Y.; Xu, J.; Wang, H. Inhibition of Phosphodiesterase 4 by FCPR03 Alleviates Lipopolysaccharide-Induced Depressive-Like Behaviors in Mice: Involvement of p38 and JNK Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 513. https://doi.org/10.3390/ijms19020513
Yu H, Zou Z, Zhang X, Peng W, Chen C, Ye Y, Xu J, Wang H. Inhibition of Phosphodiesterase 4 by FCPR03 Alleviates Lipopolysaccharide-Induced Depressive-Like Behaviors in Mice: Involvement of p38 and JNK Signaling Pathways. International Journal of Molecular Sciences. 2018; 19(2):513. https://doi.org/10.3390/ijms19020513
Chicago/Turabian StyleYu, Hui, Zhengqiang Zou, Xiaolin Zhang, Wanli Peng, Chen Chen, Yicheng Ye, Jiangping Xu, and Haitao Wang. 2018. "Inhibition of Phosphodiesterase 4 by FCPR03 Alleviates Lipopolysaccharide-Induced Depressive-Like Behaviors in Mice: Involvement of p38 and JNK Signaling Pathways" International Journal of Molecular Sciences 19, no. 2: 513. https://doi.org/10.3390/ijms19020513



