Angiopoietin-Like Proteins in Angiogenesis, Inflammation and Cancer
Abstract
1. Introduction
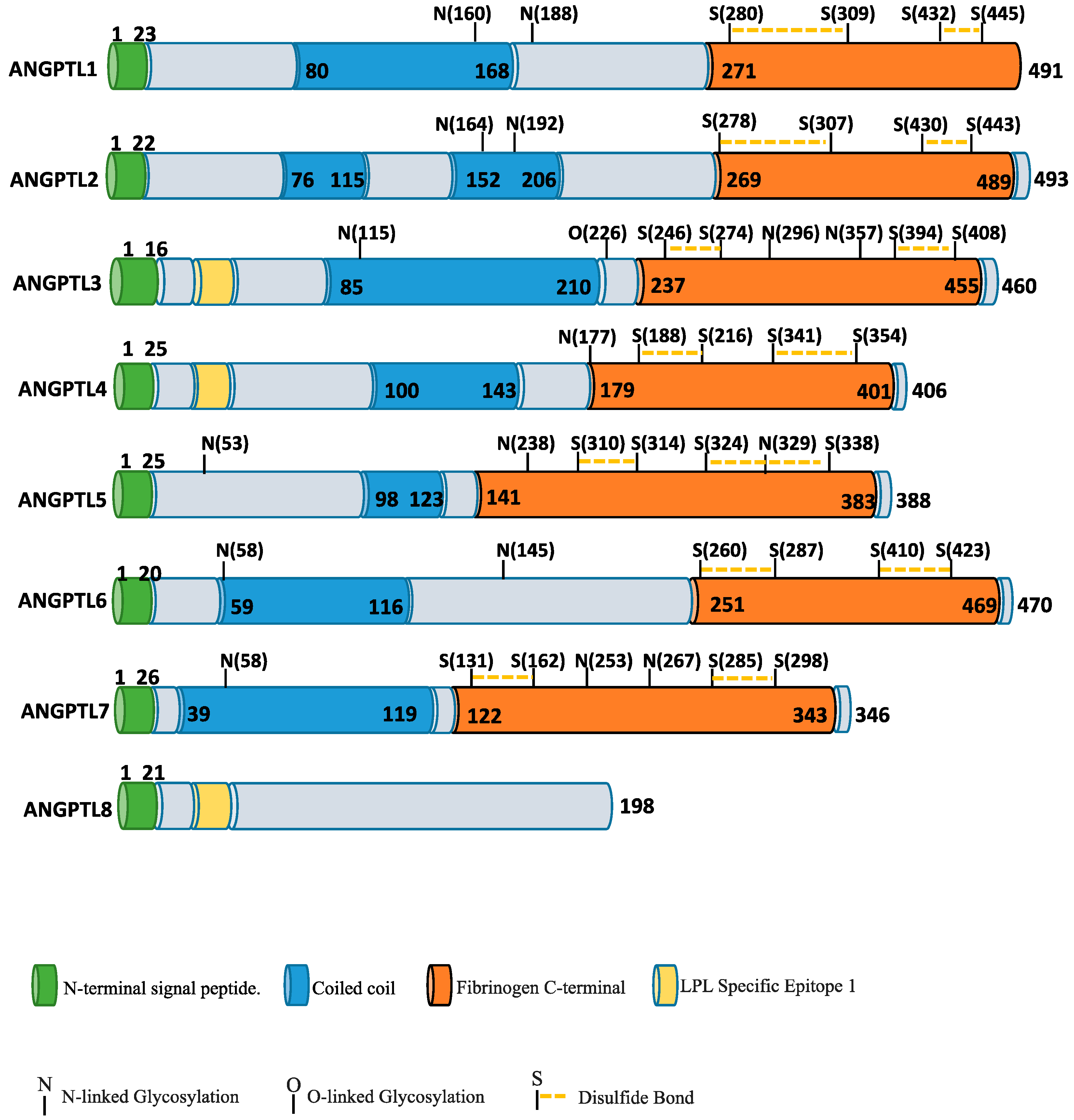
2. ANGPTL Protein Family and Angiogenesis
3. ANGPTL Proteins in Inflammation and Cancer
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jones, N.; Iljin, K.; Dumont, D.J.; Alitalo, K. Tie receptors: New modulators of angiogenic and lymphangiogenic responses. Nat. Rev. Mol. Cell Biol. 2001, 2, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Oike, Y.; Yasunaga, K.; Ito, Y.; Matsumoto, S.; Maekawa, H.; Morisada, T.; Arai, F.; Nakagata, N.; Takeya, M.; Masuho, Y.; et al. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 9494–9499. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Oike, Y.; Yasunaga, K.; Hamada, K.; Miyata, K.; Matsumoto, S.; Sugano, S.; Tanihara, H.; Masuho, Y.; Suda, T. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003, 63, 6651–6657. [Google Scholar] [PubMed]
- Deng, M.; Lu, Z.; Zheng, J.; Wan, X.; Chen, X.; Hirayasu, K.; Sun, H.; Lam, Y.; Chen, L.; Wang, Q.; et al. A motif in LILRB2 critical for Angptl2 binding and activation. Blood 2014, 124, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G. Angiopoietin-like proteins: A comprehensive look. Front. Endocrinol. (Lausanne) 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Umikawa, M.; Cui, C.; Li, J.; Chen, X.; Zhang, C.; Huynh, H.; Kang, X.; Silvany, R.; Wan, X.; et al. Inhibitory receptors bind angptls and support blood stem cells and leukaemia development. Nature 2012, 485, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Hato, T.; Tabata, M.; Oike, Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc. Med. 2008, 18, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Dhanabal, M.; LaRochelle, W.J.; Jeffers, M.; Herrmann, J.; Rastelli, L.; McDonald, W.F.; Chillakuru, R.A.; Yang, M.; Boldog, F.L.; Padigaru, M.; et al. Angioarrestin: An antiangiogenic protein with tumor-inhibiting properties. Cancer Res. 2002, 62, 3834–3841. [Google Scholar] [PubMed]
- Kim, I.; Moon, S.O.; Koh, K.N.; Kim, H.; Uhm, C.S.; Kwak, H.J.; Kim, N.G.; Koh, G.Y. Molecular cloning, expression, and characterization of angiopoietin-related protein. Angiopoietin-related protein induces endothelial cell sprouting. J. Biol. Chem. 1999, 274, 26523–26528. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Yamamoto, Y.; Nakano, M.; Masuda, T.; Odagiri, H.; Horiguchi, H.; Miyata, K.; Kadomatsu, T.; Motokawa, I.; Okada, S.; et al. Serum angptl2 levels reflect clinical features of breast cancer patients: Implications for the pathogenesis of breast cancer metastasis. Int. J. Biol. Markers 2014, 29, e239–e245. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Endo, M.; Yamamoto, Y.; Odagiri, H.; Kadomatsu, T.; Nakamura, T.; Tanoue, H.; Ito, H.; Yugami, M.; Miyata, K.; et al. Angptl2 increases bone metastasis of breast cancer cells through enhancing cxcr4 signaling. Sci. Rep. 2015, 5, 9170. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shu, T.; Liang, Y.; Gu, W.; Wang, C.; Song, X.; Fan, C.; Wang, W. GDC-0152 attenuates the malignant progression of osteosarcoma promoted by Angptl2 via PI3k/AKT but not p38MAPK signaling pathway. Int. J. Oncol. 2015, 46, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Nakano, M.; Kadomatsu, T.; Fukuhara, S.; Kuroda, H.; Mikami, S.; Hato, T.; Aoi, J.; Horiguchi, H.; Miyata, K.; et al. Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical driver of metastasis. Cancer Res. 2012, 72, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Piro, G.; Fassan, M.; Tamburrino, A.; Mina, M.M.; Zanotto, M.; Chiao, P.J.; Bassi, C.; Scarpa, A.; Tortora, G.; et al. An angiopoietin-like protein 2 autocrine signaling promotes EMT during pancreatic ductal carcinogenesis. Oncotarget 2015, 6, 13822–13834. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.K.; Aronow, B.J.; Tong, W.S.; Manka, D.; Tang, Y.; Bogdanov, V.Y.; Unruh, D.; Blomkalns, A.L.; Piegore, M.G., Jr.; Weintraub, D.S.; et al. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol. Genom. 2013, 45, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.A.; Kuo, T.C.; Tseng, C.F.; Ma, J.T.; Yang, S.T.; Yen, C.J.; Yang, C.Y.; Sung, S.Y.; Su, J.L. Angiopoietin-like protein 1 antagonizes met receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatology 2016, 64, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Dhanabal, M.; Jeffers, M.; LaRochelle, W.J.; Lichenstein, H.S. Angioarrestin: A unique angiopoietin-related protein with anti-angiogenic properties. Biochem. Biophys. Res. Commun. 2005, 333, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.M.; Singh, H.; Tahir, T.A.; Brindle, N.P. Effects of angiopoietins-1 and -2 on the receptor tyrosine kinase tie2 are differentially regulated at the endothelial cell surface. Cell Signal. 2010, 22, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.; Kadomatsu, T.; Fukuhara, S.; Miyata, K.; Ito, Y.; Endo, M.; Urano, T.; Zhu, H.J.; Tsukano, H.; Tazume, H.; et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009, 10, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Oike, Y.; Yasunaga, K.; Suda, T. Angiopoietin-related/angiopoietin-like proteins regulate angiogenesis. Int. J. Hematol. 2004, 80, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.H.; Kim, J.H.; Martinus, R.D.; Park, S.H. Angiopoietin-like protein 2, a chronic inflammatory mediator, is a new target induced by TGF-β1 through a Smad3-dependent mechanism. Biochem. Biophys. Res. Commun. 2013, 430, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.R.; Robbins, E.P.; Vemula, S.; Critser, P.J.; Whittington, C.; Voytik-Harbin, S.L.; Yoder, M.C. Angiopoietin-like protein 2 regulates endothelial colony forming cell vasculogenesis. Angiogenesis 2014, 17, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Oike, Y.; Tabata, M. Angiopoietin-like proteins—Potential therapeutic targets for metabolic syndrome and cardiovascular disease. Circ. J. 2009, 73, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Farhat, N.; Thorin-Trescases, N.; Mamarbachi, M.; Villeneuve, L.; Yu, C.; Martel, C.; Duquette, N.; Gayda, M.; Nigam, A.; Juneau, M.; et al. Angiopoietin-like 2 promotes atherogenesis in mice. J. Am. Heart Assoc. 2013, 2, e000201. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Miyata, K.; Tazume, H.; Sakaguchi, H.; Kadomatsu, T.; Horio, E.; Takahashi, O.; Komohara, Y.; Araki, K.; Hirata, Y.; et al. Perivascular adipose tissue-secreted angiopoietin-like protein 2 (Angptl2) accelerates neointimal hyperplasia after endovascular injury. J. Mol. Cell. Cardiol. 2013, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Toiyama, Y.; Shimura, T.; Kawamura, M.; Yasuda, H.; Saigusa, S.; Ohi, M.; Tanaka, K.; Mohri, Y.; Kusunoki, M. Angiopoietin-like protein 2 acts as a novel biomarker for diagnosis and prognosis in patients with esophageal cancer. Ann. Surg. Oncol. 2015, 22, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Camenisch, G.; Pisabarro, M.T.; Sherman, D.; Kowalski, J.; Nagel, M.; Hass, P.; Xie, M.H.; Gurney, A.; Bodary, S.; Liang, X.H.; et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J. Biol. Chem. 2002, 277, 17281–17290. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Teo, Z.; Sng, M.K.; Zhu, P.; Tan, N.S. Emerging roles of angiopoietin-like 4 in human cancer. Mol. Cancer Res. MCR 2012, 10, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Cazes, A.; Galaup, A.; Chomel, C.; Bignon, M.; Brechot, N.; Le Jan, S.; Weber, H.; Corvol, P.; Muller, L.; Germain, S.; et al. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ. Res. 2006, 99, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Galaup, A.; Cazes, A.; Le Jan, S.; Philippe, J.; Connault, E.; Le Coz, E.; Mekid, H.; Mir, L.M.; Opolon, P.; Corvol, P.; et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc. Natl. Acad. Sci. USA 2006, 103, 18721–18726. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Nakayama, T.; Hirakawa, H.; Hidaka, S.; Nagayasu, T. Clinicopathological significance of angiopoietin-like protein 4 expression in oesophageal squamous cell carcinoma. J. Clin. Pathol. 2010, 63, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.Y.; Pal, M.; Chong, H.C.; Zhu, P.; Tan, M.J.; Punugu, L.; Lam, C.R.; Yau, Y.H.; Tan, C.K.; Huang, R.L.; et al. Angiopoietin-Like 4 Interacts with Integrins β1 and β5 to Modulate Keratinocyte Migration. Am. J. Pathol. 2010, 177, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.; Zhang, X.H.; Wang, Q.; Nadal, C.; Gerald, W.L.; Gomis, R.R.; Massague, J. Tgfbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 2008, 133, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Tan, M.J.; Huang, R.L.; Tan, C.K.; Chong, H.C.; Pal, M.; Lam, C.R.; Boukamp, P.; Pan, J.Y.; Tan, S.H.; et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2−:H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell 2011, 19, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Hermann, L.M.; Pinkerton, M.; Jennings, K.; Yang, L.; Grom, A.; Sowders, D.; Kersten, S.; Witte, D.P.; Hirsch, R.; Thornton, S. Angiopoietin-like-4 is a potential angiogenic mediator in arthritis. Clin. Immunol. 2005, 115, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.W.; Teo, Z.; Tan, C.; Choo, C.C.; Loo, W.S.; Song, Y.; Tam, Z.Y.; Ng, S.P.; Koh, H.Z.; Ng, Y.S.; et al. Angptl4 t266m variant is associated with reduced cancer invasiveness. Biochim. Biophys. Acta 2017, 1864, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Ifon, E.T.; Pang, A.L.; Johnson, W.; Cashman, K.; Zimmerman, S.; Muralidhar, S.; Chan, W.Y.; Casey, J.; Rosenthal, L.J. U94 alters FN1 and ANGPTL4 gene expression and inhibits tumorigenesis of prostate cancer cell line PC3. Cancer Cell Int. 2005, 5, 19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, R.A.; Cardoso, J.C.; Power, D.M. Evolution of the angiopoietin-like gene family in teleosts and their role in skin regeneration. BMC Evol. Biol. 2017, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Dai, J.; Ying, K.; Zhao, E.; Jin, W.; Ye, Y.; Dai, J.; Xu, J.; Xie, Y.; Mao, Y. Identification of a novel human angiopoietin-like gene expressed mainly in heart. J. Hum. Genet. 2003, 48, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Oike, Y.; Akao, M.; Yasunaga, K.; Yamauchi, T.; Morisada, T.; Ito, Y.; Urano, T.; Kimura, Y.; Kubota, Y.; Maekawa, H.; et al. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat. Med. 2005, 11, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, H.; Miyata, K.; Tian, Z.; Aoi, J.; Kadomatsu, T.; Fukushima, S.; Ogata, A.; Takeda, N.; Zhao, J.; Zhu, S.; et al. Upregulation of ANGPTL6 in mouse keratinocytes enhances susceptibility to psoriasis. Sci. Rep. 2016, 6, 34690. [Google Scholar] [CrossRef] [PubMed]
- Parri, M.; Pietrovito, L.; Grandi, A.; Campagnoli, S.; De Camilli, E.; Bianchini, F.; Marchio, S.; Bussolino, F.; Jin, B.; Sarmientos, P.; et al. Angiopoietin-like 7, a novel pro-angiogenetic factor over-expressed in cancer. Angiogenesis 2014, 17, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Yeh, Y.H.; Chen, W.J.; Lin, K.H. Emerging regulation and function of betatrophin. Int. J. Mol. Sci. 2014, 15, 23640–23657. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Sriraman, D.; Cherian, P.; AlKhairi, I.; Elkum, N.; Behbehani, K.; Abubaker, J. Circulating angptl8/betatrophin is increased in obesity and reduced after exercise training. PLoS ONE 2016, 11, e0147367. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Pang, X.W.; Yu, S.T.; Su, Y.R.; Wang, H.C.; Yin, Y.H.; Wang, Y.D.; Chen, W.F. Identification of genes differentially expressed in human hepatocellular carcinoma by a modified suppression subtractive hybridization method. Int. J. Cancer 2004, 112, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.; Xu, A.; Cheng, Q.; Guo, D.Y.; Lim, Z.X.; Sun, C.K.; Fung, J.H.; Poon, R.T.; Fan, S.T.; Lo, C.M.; et al. Clinical relevance and therapeutic potential of angiopoietin-like protein 4 in hepatocellular carcinoma. Mol. Cancer 2014, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Oike, Y.; Satoh, S.; Tabata, Y.; Niikura, Y.; Morisada, T.; Akao, M.; Urano, T.; Ito, Y.; Miyamoto, T.; et al. Cooperative interaction of angiopoietin-like proteins 1 and 2 in zebrafish vascular development. Proc. Natl. Acad. Sci. USA 2005, 102, 13502–13507. [Google Scholar] [CrossRef] [PubMed]
- Arca, M.; Minicocci, I.; Maranghi, M. The angiopoietin-like protein 3: A hepatokine with expanding role in metabolism. Curr. Opin. Lipidol. 2013, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Angiopoietin-like 3 in lipoprotein metabolism. Nat. Rev. Endocrinol. 2017, 13, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M. Angplt3: A novel modulator of lipid metabolism. Glob. Cardiol. Sci. Pract. 2017, 2017, e201706. [Google Scholar] [CrossRef] [PubMed]
- Shimizugawa, T.; Ono, M.; Shimamura, M.; Yoshida, K.; Ando, Y.; Koishi, R.; Ueda, K.; Inaba, T.; Minekura, H.; Kohama, T.; et al. Angptl3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J. Biol. Chem. 2002, 277, 33742–33748. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Qamar, A.; Qu, L.; Qasim, A.N.; Mehta, N.N.; Reilly, M.P.; Rader, D.J. Differential association of plasma angiopoietin-like proteins 3 and 4 with lipid and metabolic traits. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Shimizugawa, T.; Shimamura, M.; Yoshida, K.; Noji-Sakikawa, C.; Ando, Y.; Koishi, R.; Furukawa, H. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): Angptl3 is cleaved and activated in vivo. J. Biol. Chem. 2003, 278, 41804–41809. [Google Scholar] [CrossRef] [PubMed]
- Farahbakhshian, E.; Verstegen, M.M.; Visser, T.P.; Kheradmandkia, S.; Geerts, D.; Arshad, S.; Riaz, N.; Grosveld, F.; van Til, N.P.; Meijerink, J.P. Angiopoietin-like protein 3 promotes preservation of stemness during ex vivo expansion of murine hematopoietic stem cells. PLoS ONE 2014, 9, e105642. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; So, J.H.; Kim, H.T.; Choi, J.H.; Lee, M.S.; Choi, S.Y.; Kim, C.H.; Kim, M.J. Angiopoietin-like 3 regulates hepatocyte proliferation and lipid metabolism in zebrafish. Biochem. Biophys. Res. Commun. 2014, 446, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Lee, R.G.; Brandt, T.A.; Tai, L.J.; Fu, W.; Peralta, R.; Yu, R.; Hurh, E.; Paz, E.; McEvoy, B.W.; et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N. Engl. J. Med. 2017, 377, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M.; et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef] [PubMed]
- McQueen, A.E.; Kanamaluru, D.; Yan, K.; Gray, N.E.; Wu, L.; Li, M.L.; Chang, A.; Hasan, A.; Stifler, D.; Koliwad, S.K.; et al. The c-terminal fibrinogen-like domain of angiopoietin-like 4 stimulates adipose tissue lipolysis and promotes energy expenditure. J. Biol. Chem. 2017, 292, 16122–16134. [Google Scholar] [CrossRef] [PubMed]
- Cushing, E.M.; Chi, X.; Sylvers, K.L.; Shetty, S.K.; Potthoff, M.J.; Davies, B.S.J. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol. Metab. 2017, 6, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Goh, Y.Y.; Chin, H.F.; Kersten, S.; Tan, N.S. Angiopoietin-like 4: A decade of research. Biosci. Rep. 2012, 32, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Mandard, S.; Zandbergen, F.; van Straten, E.; Wahli, W.; Kuipers, F.; Muller, M.; Kersten, S. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 2006, 281, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Ruge, T.; Sukonina, V.; Kroupa, O.; Makoveichuk, E.; Lundgren, M.; Svensson, M.K.; Olivecrona, G.; Eriksson, J.W. Effects of hyperinsulinemia on lipoprotein lipase, angiopoietin-like protein 4, and glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 in subjects with and without type 2 diabetes mellitus. Metabolism 2012, 61, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.T.; Smit, J.W.; Hammer, S.; Snel, M.; van der Meer, R.W.; Lamb, H.J.; Mattijssen, F.; Mudde, K.; Jazet, I.M.; Dekkers, O.M.; et al. Dietary modulation of plasma angiopoietin-like protein 4 concentrations in healthy volunteers and in patients with type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.C.; Chan, J.S.; Goh, C.Q.; Gounko, N.V.; Luo, B.; Wang, X.; Foo, S.; Wong, M.T.; Choong, C.; Kersten, S.; et al. Angiopoietin-like 4 stimulates stat3-mediated inos expression and enhances angiogenesis to accelerate wound healing in diabetic mice. Mol. Ther. 2014, 22, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Shi, F.; Basu, D.; Huq, A.; Routhier, S.; Day, R.; Jin, W. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J. Biol. Chem. 2011, 286, 15747–15756. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Han, J.; Hu, X.T.; He, C. Mechanisms involved in biological behavior changes associated with angptl4 expression in colon cancer cell lines. Oncol. Rep. 2012, 27, 1541–1547. [Google Scholar] [PubMed]
- Huang, R.L.; Teo, Z.; Chong, H.C.; Zhu, P.; Tan, M.J.; Tan, C.K.; Lam, C.R.; Sng, M.K.; Leong, D.T.; Tan, S.M.; et al. Angptl4 modulates vascular junction integrity by integrin signaling and disruption of intercellular ve-cadherin and claudin-5 clusters. Blood 2011, 118, 3990–4002. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, Z.N.; Yang, Y.; Xu, J.; Burchell, S.R.; Tang, J.; Zhang, J.; Xu, J.; Zhang, J.H. Angiopoietin-like 4: A double-edged sword in atherosclerosis and ischemic stroke? Exp. Neurol. 2015, 272, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Okochi-Takada, E.; Hattori, N.; Tsukamoto, T.; Miyamoto, K.; Ando, T.; Ito, S.; Yamamura, Y.; Wakabayashi, M.; Nobeyama, Y.; Ushijima, T. Angptl4 is a secreted tumor suppressor that inhibits angiogenesis. Oncogene 2014, 33, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Yang, S.; Kowalski, J.; Gerritsen, M.E. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J. Biol. Chem. 1999, 274, 9116–9121. [Google Scholar] [CrossRef] [PubMed]
- La Paglia, L.; Listi, A.; Caruso, S.; Amodeo, V.; Passiglia, F.; Bazan, V.; Fanale, D. Potential role of ANGPTL4 in the cross talk between metabolism and cancer through ppar signaling pathway. PPAR Res. 2017, 2017, 8187235. [Google Scholar] [CrossRef] [PubMed]
- Le Jan, S.; Amy, C.; Cazes, A.; Monnot, C.; Lamande, N.; Favier, J.; Philippe, J.; Sibony, M.; Gasc, J.M.; Corvol, P.; et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am. J. Pathol. 2003, 162, 1521–1528. [Google Scholar] [CrossRef]
- Perdiguero, E.G.; Galaup, A.; Durand, M.; Teillon, J.; Philippe, J.; Valenzuela, D.M.; Murphy, A.J.; Yancopoulos, G.D.; Thurston, G.; Germain, S. Alteration of developmental and pathological retinal angiogenesis in angptl4-deficient mice. J. Biol. Chem. 2011, 286, 36841–36851. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Hirakawa, S.; Shudou, M.; Nakaoka, Y.; Shirakata, Y.; Miyata, K.; Oike, Y.; Hashimoto, K.; Sayama, K. Targeted overexpression of Angptl6/angiopoietin-related growth factor in the skin promotes angiogenesis and lymphatic vessel enlargement in response to ultraviolet b. J. Dermatol. 2012, 39, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, X.; Tian, R.; Wei, W.; Hu, W.; Chen, X.; Han, W.; Chen, H.; Gong, Y. Angiopoietin-related growth factor (AGF) supports adhesion, spreading, and migration of keratinocytes, fibroblasts, and endothelial cells through interaction with rgd-binding integrins. Biochem. Biophys. Res. Commun. 2006, 347, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Pangtey, G.S.; Gupta, R.; Rehan, H.S.; Gupta, L.K. Correlation of long-term glycemic control as measured by glycated hemoglobin with serum angiopoietin-like 6 protein levels in type 2 diabetes mellitus patients. Indian J. Pharmacol. 2017, 49, 250–253. [Google Scholar] [PubMed]
- Namkung, J.; Koh, S.B.; Kong, I.D.; Choi, J.W.; Yeh, B.I. Serum levels of angiopoietin-related growth factor are increased in metabolic syndrome. Metabolism 2011, 60, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Katoh, M. Comparative integromics on angiopoietin family members. Int. J. Mol. Med. 2006, 17, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Peek, R.; Kammerer, R.A.; Frank, S.; Otte-Holler, I.; Westphal, J.R. The angiopoietin-like factor cornea-derived transcript 6 is a putative morphogen for human cornea. J. Biol. Chem. 2002, 277, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Toyono, T.; Usui, T.; Yokoo, S.; Taketani, Y.; Nakagawa, S.; Kuroda, M.; Yamagami, S.; Amano, S. Angiopoietin-like 7 is an anti-angiogenic protein required to prevent vascularization of the cornea. PLoS ONE 2015, 10, e0116838. [Google Scholar] [CrossRef] [PubMed]
- Comes, N.; Buie, L.K.; Borras, T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: Implications for glaucoma. Genes Cells Devoted Mol. Cell. Mech. 2011, 16, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wei, X.; Jiang, Z.; Wang, X.; Ye, W.; Liu, X.; Zhang, M.; Xu, Y.; Wu, D.; Lai, L.; et al. Loss of angiopoietin-like 7 diminishes the regeneration capacity of hematopoietic stem and progenitor cells. J. Hematol. Oncol. 2015, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Quagliarini, F.; Wang, Y.; Kozlitina, J.; Grishin, N.V.; Hyde, R.; Boerwinkle, E.; Valenzuela, D.M.; Murphy, A.J.; Cohen, J.C.; Hobbs, H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA 2012, 109, 19751–19756. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Park, J.S.; Melton, D.A. Betatrophin: A hormone that controls pancreatic beta cell proliferation. Cell 2013, 153, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.R.; Barrandon, O.; Cai, E.P.; Rios, J.S.; Chavez, J.; Bonnyman, C.W.; Lam, C.J.; Yi, P.; Melton, D.A.; Kushner, J.A. Resolving discrepant findings on ANGPTL8 in β-cell proliferation: A collaborative approach to resolving the betatrophin controversy. PLoS ONE 2016, 11, e0159276. [Google Scholar] [CrossRef] [PubMed]
- Aoi, J.; Endo, M.; Kadomatsu, T.; Miyata, K.; Ogata, A.; Horiguchi, H.; Odagiri, H.; Masuda, T.; Fukushima, S.; Jinnin, M.; et al. Angiopoietin-like protein 2 accelerates carcinogenesis by activating chronic inflammation and oxidative stress. Mol. Cancer Res. 2014, 12, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Aoi, J.; Endo, M.; Kadomatsu, T.; Miyata, K.; Nakano, M.; Horiguchi, H.; Ogata, A.; Odagiri, H.; Yano, M.; Araki, K.; et al. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011, 71, 7502–7512. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.C.; Tan, C.T.; Chang, Y.W.; Hong, C.C.; Lee, W.J.; Chen, M.W.; Jeng, Y.M.; Chiou, J.; Yu, P.; Chen, P.S.; et al. Angiopoietin-like protein 1 suppresses slug to inhibit cancer cell motility. J. Clin. Investig. 2013, 123, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Jiang, L.; Liu, M.; Yu, D.; Zhang, Y.; Li, Y.; Fang, S.; Li, Y.; Zhu, Y.H.; Yuan, Y.F.; et al. ANGPTL1 Interacts with integrin α1β1 to suppress HCC angiogenesis and metastasis by inhibiting JAK2/STAT3 signaling. Cancer Res. 2017, 77, 5831–5845. [Google Scholar] [CrossRef] [PubMed]
- Thorin-Trescases, N.; Thorin, E. Angiopoietin-like-2: A multifaceted protein with physiological and pathophysiological properties. Expert Rev. Mol. Med. 2014, 16, e17. [Google Scholar] [CrossRef] [PubMed]
- Kadomatsu, T.; Endo, M.; Miyata, K.; Oike, Y. Diverse roles of angptl2 in physiology and pathophysiology. Trends Endocrinol. Metab. 2014, 25, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Tsukano, H.; Endo, M.; Tabata, M.; Miyata, K.; Kadomatsu, T.; Miyashita, K.; Semba, K.; Nakamura, E.; Tsukano, M.; et al. Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am. J. Pathol. 2010, 176, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Horio, E.; Kadomatsu, T.; Miyata, K.; Arai, Y.; Hosokawa, K.; Doi, Y.; Ninomiya, T.; Horiguchi, H.; Endo, M.; Tabata, M.; et al. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.B.; Kost, T.A.; Hughes, S.H.; Davidson, N. Regulation of chicken alpha and beta actin genes and their hybrids inserted into myogenic mouse cells. Gene 1989, 80, 293–304. [Google Scholar] [CrossRef]
- Odagiri, H.; Kadomatsu, T.; Endo, M.; Masuda, T.; Morioka, M.S.; Fukuhara, S.; Miyamoto, T.; Kobayashi, E.; Miyata, K.; Aoi, J.; et al. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin α5β1, p38 MAPK, and matrix metalloproteinases. Sci. Signal. 2014, 7, ra7. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E.; Srour, E.F.; Cooper, S.; Wallace, C.T.; Hangoc, G.; Youn, B.S. Angiopoietin-like-2 and -3 act through their coiled-coil domains to enhance survival and replating capacity of human cord blood hematopoietic progenitors. Blood Cells Mol. Dis. 2012, 48, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, X.; Xie, J.; Zhan, M.; Yu, Z.; Xie, L.; Zeng, H.; Zhang, F.; Chen, G.; Yi, X.; et al. Angptl2/lilrb2 signaling promotes the propagation of lung cancer cells. Oncotarget 2015, 6, 21004–21015. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Piro, G.; Simionato, F.; Ligorio, F.; Cremolini, C.; Loupakis, F.; Ali, G.; Rossini, D.; Merz, V.; Santoro, R.; et al. Homeobox B9 mediates resistance to anti-VEGF therapy in colorectal cancer patients. Clin. Cancer Res. 2017, 23, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, T.; Shigemitsu, T.; Nishimata, H.; Takei, T.; Yoshida, M. Angiopoietin-like protein 2 is a potential biomarker for gastric cancer. Mol. Med. Rep. 2015, 11, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.Z.; Chen, Y.S.; Tu, C.T.; He, J.; Zhang, B.; Gao, W.D. ANGPTL2 expression in gastric cancer tissues and cells and its biological behavior. World J. Gastroenterol. 2016, 22, 10364–10370. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, T.; Shigemitsu, T.; Nishimata, H.; Kitazono, M.; Hori, E.; Tomiyoshi, A.; Takei, T.; Yoshida, M. Angiopoietin-like protein 2 as a potential biomarker for colorectal cancer. Mol. Clin. Oncol. 2015, 3, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Inoue, Y.; Shimura, T.; Fujikawa, H.; Saigusa, S.; Hiro, J.; Kobayashi, M.; Ohi, M.; Araki, T.; Tanaka, K.; et al. Serum angiopoietin-like protein 2 improves preoperative detection of lymph node metastasis in colorectal cancer. Anticancer Res. 2015, 35, 2849–2856. [Google Scholar] [PubMed]
- Horiguchi, H.; Endo, M.; Miyamoto, Y.; Sakamoto, Y.; Odagiri, H.; Masuda, T.; Kadomatsu, T.; Tanoue, H.; Motokawa, I.; Terada, K.; et al. Angiopoietin-like protein 2 renders colorectal cancer cells resistant to chemotherapy by activating spleen tyrosine kinase-phosphoinositide 3-kinase-dependent anti-apoptotic signaling. Cancer Sci. 2014, 105, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Calvetti, L.; Frizziero, M.; Tortora, G. Pancreatic cancer: Systemic combination therapies for a heterogeneous disease. Curr. Pharm. Des. 2014, 20, 6660–6669. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, V.; Melisi, D.; Bria, E.; Cuppone, F.; Ciuffreda, L.; Pino, M.S.; Gelibter, A.; Tortora, G.; Cognetti, F.; Milella, M. Emerging pathways and future targets for the molecular therapy of pancreatic cancer. Expert Opin. Ther. Targets 2011, 15, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, A.; Piro, G.; Carbone, C.; Tortora, G.; Melisi, D. Mechanisms of resistance to chemotherapeutic and anti-angiogenic drugs as novel targets for pancreatic cancer therapy. Front. Pharmacol. 2013, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Moccia, T.; Zhu, C.; Paradiso, G.; Budillon, A.; Chiao, P.J.; Abbruzzese, J.L.; Melisi, D. Anti-vegf treatment-resistant pancreatic cancers secrete proinflammatory factors that contribute to malignant progression by inducing an EMT cell phenotype. Clin. Cancer Res. 2011, 17, 5822–5832. [Google Scholar] [CrossRef] [PubMed]
- Gaianigo, N.; Melisi, D.; Carbone, C. Emt and treatment resistance in pancreatic cancer. Cancers 2017, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Piro, G.; Tamburrino, A.; Carbone, C.; Tortora, G. Rationale and clinical use of multitargeting anticancer agents. Curr. Opin. Pharmacol. 2013, 13, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ge, C.; Fang, T.; Zhao, F.; Chen, T.; Yao, M.; Li, J.; Li, H. ANGPTL2 promotes tumor metastasis in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2015, 30, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Suzuki, A.; Shitara, M.; Hikosaka, Y.; Okuda, K.; Moriyama, S.; Yano, M.; Fujii, Y. Angiopoietin-like protein ANGPTL2 gene expression is correlated with lymph node metastasis in lung cancer. Oncol. Lett. 2012, 4, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R.; Tsuda, H.; Kozaki, K.; Kanai, Y.; Kasamatsu, T.; Sengoku, K.; Hirohashi, S.; Inazawa, J.; Imoto, I. Frequent inactivation of a putative tumor suppressor, angiopoietin-like protein 2, in ovarian cancer. Cancer Res. 2008, 68, 5067–5075. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Yamasaki, M.; Hirai, K.; Matsubara, T.; Nomura, T.; Sato, F.; Mimata, H. Angiopoietin-like protein 2 induces androgen-independent and malignant behavior in human prostate cancer cells. Oncol. Rep. 2015, 33, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Wu, Y.W.; Wu, C.C.; Hwang, J.J.; Yang, W.S. Serum angiopoietin-like protein 2 concentrations are independently associated with heart failure. PLoS ONE 2015, 10, e0138678. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Ogawara, K.; Kasamatsu, A.; Okamoto, A.; Kasama, H.; Minakawa, Y.; Shimada, K.; Yokoe, H.; Shiiba, M.; Tanzawa, H.; et al. ANGPTL3 is a novel biomarker as it activates ERK/MAPK pathway in oral cancer. Cancer Med. 2015, 4, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Li, H.L.; Qi, X.; Yao, K.; Han, S.; Liu, N.; Yang, Y.K.; Li, S.W.; Yan, C.X. Clinical significance of angiopoietin-like protein 3 expression in patients with glioblastoma. Neoplasma 2016, 63, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Jiang, L.; Wang, W.; Jia, W.; Liu, F.; Jiao, X.; Zhu, X.; Bao, J.; Yu, H. Angiopoietin-like protein 3 is an indicator of prognosis in esophageal cancer patients. Int. J. Clin. Exp. Med. 2015, 8, 16101–16106. [Google Scholar] [PubMed]
- Yu, H.; Zhang, H.; Li, D.; Xue, H.; Pan, C.; Zhao, S.; Wang, L. Effects of angptl3 antisense oligodeoxynucleotides transfection on the cell growths and invasion of human hepatocellular carcinoma cells. Hepato-Gastroenterology 2011, 58, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Siamakpour-Reihani, S.; Owzar, K.; Jiang, C.; Turner, T.; Deng, Y.; Bean, S.M.; Horton, J.K.; Berchuck, A.; Marks, J.R.; Dewhirst, M.W.; et al. Prognostic significance of differential expression of angiogenic genes in women with high-grade serous ovarian carcinoma. Gynecol. Oncol. 2015, 139, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.Y.; Pal, M.; Chong, H.C.; Zhu, P.; Tan, M.J.; Punugu, L.; Tan, C.K.; Huang, R.L.; Sze, S.K.; Tang, M.B.; et al. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J. Biol. Chem. 2010, 285, 32999–33009. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Distinct roles of angiopoietin-like 4 in the regulation of central and peripheral lipid metabolism? Mol. Metab. 2015, 4, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Hirakawa, H.; Shibata, K.; Abe, K.; Nagayasu, T.; Taguchi, T. Expression of angiopoietin-like 4 in human gastric cancer: ANGPTL4 promotes venous invasion. Oncol. Rep. 2010, 24, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Hirakawa, H.; Shibata, K.; Nazneen, A.; Abe, K.; Nagayasu, T.; Taguchi, T. Expression of angiopoietin-like 4 (ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous invasion and distant metastasis. Oncol. Rep. 2011, 25, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.; Sng, M.K.; Chan, J.S.K.; Lim, M.M.K.; Li, Y.; Li, L.; Phua, T.; Lee, J.Y.H.; Tan, Z.W.; Zhu, P.; et al. Elevation of adenylate energy charge by angiopoietin-like 4 enhances epithelial-mesenchymal transition by inducing 14-3-3γ expression. Oncogene 2017, 36, 6408–6419. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, N.; Chang, L.S.; Koch, S.; Glinka, A.; Dolde, C.; Colozza, G.; Benitez, M.D.J.; De Robertis, E.M.; Niehrs, C. Angiopoietin-like 4 is a wnt signaling antagonist that promotes LRP6 turnover. Dev. Cell 2017, 43, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Wang, Y.; Lam, K.S.; Yau, M.H.; Cheng, K.K.; Zhang, J.; Zhu, W.; Wu, D.; Xu, A. Suppression of the raf/mek/erk signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Chomel, C.; Cazes, A.; Faye, C.; Bignon, M.; Gomez, E.; Ardidie-Robouant, C.; Barret, A.; Ricard-Blum, S.; Muller, L.; Germain, S.; et al. Interaction of the coiled-coil domain with glycosaminoglycans protects angiopoietin-like 4 from proteolysis and regulates its antiangiogenic activity. FASEB J. 2009, 23, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, Y.Y.; Kim, S.W.; Lee, J.S.; Wang, D.; DuBois, R.N. ANGPTL4 induction by prostaglandin e2 under hypoxic conditions promotes colorectal cancer progression. Cancer Res. 2011, 71, 7010–7020. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xie, J.; Lin, S.; Li, S.; Huang, Z.; Wang, Y.; Ye, J. The downregulation of angptl4 inhibits the migration and proliferation of tongue squamous cell carcinoma. Arch. Oral Biol. 2016, 71, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Irie, T.; Yamamoto, G.; Yasuhara, R.; Isobe, T.; Hokazono, C.; Tachikawa, T.; Kohno, Y.; Mishima, K. ANGPTL4 regulates the metastatic potential of oral squamous cell carcinoma. J. Oral Pathol. Med. 2015, 44, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Pan, B.Z.; Xiong, L.; Song, H.Z. Clinical significance of angiopoietin-like protein 4 expression in tissue and serum of esophageal squamous cell carcinoma patients. Med. Oncol. 2013, 30, 680. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.J.; Chan, S.H.; Lee, C.T.; Huang, W.C.; Tsai, J.P.; Chen, B.K. Oleic acid-induced ANGPTL4 enhances head and neck squamous cell carcinoma anoikis resistance and metastasis via up-regulation of fibronectin. Cancer Lett. 2017, 386, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, S.; Ning, S.; Jie, Y.; Ru, Y.; Gu, Y. Evaluation of TGFβ, XPO4, elF5A2 and ANGPTL4 as biomarkers in HCC. Exp. Ther. Med. 2013, 5, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ge, C.; Zhao, F.; Yan, M.; Hu, C.; Jia, D.; Tian, H.; Zhu, M.; Chen, T.; Jiang, G.; et al. Hypoxia-inducible factor 1 alpha-activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin β1 signaling in human hepatocellular carcinoma. Hepatology 2011, 54, 910–919. [Google Scholar] [CrossRef] [PubMed]
- El-Shal, A.S.; Zidan, H.E.; Rashad, N.M.; Wadea, F.M. Angiopoietin-like protein 3 and 4 expression 4 and their serum levels in hepatocellular carcinoma. Cytokine 2017, 96, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.L.; Pottala, J.V.; Egland, K.A. Classifying patients for breast cancer by detection of autoantibodies against a panel of conformation-carrying antigens. Cancer Prev. Res. 2014, 7, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.H.; Hu, P.; Fan, C.; Anders, C.K. Gene expression in “young adult type” breast cancer: A retrospective analysis. Oncotarget 2015, 6, 13688–13702. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, N.; Hu, P.; Bedard, P.; Clemons, M.; McCready, D.; Done, S.J. Identification of genomic signatures in circulating tumor cells from breast cancer. Int. J. Cancer 2015, 137, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, F.; Tokunaga, E.; Oki, E.; Maehara, Y.; Yamada, H.; Nakajima, K.; Nam, S.O.; Miyata, K.; Koyanagi, M.; Doi, K.; et al. Molecular hierarchy of heparin-binding EGF-like growth factor-regulated angiogenesis in triple-negative breast cancer. Mol. Cancer Res. MCR 2013, 11, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, T.; Brandt, D.T.; Kaddatz, K.; Stockert, J.; Naruhn, S.; Meissner, W.; Finkernagel, F.; Obert, J.; Lieber, S.; Scharfe, M.; et al. Inverse PPARβ/δ agonists suppress oncogenic signaling to the ANGPTL4 gene and inhibit cancer cell invasion. Oncogene 2013, 32, 5241–5252. [Google Scholar] [CrossRef] [PubMed]
- Verine, J.; Lehmann-Che, J.; Soliman, H.; Feugeas, J.P.; Vidal, J.S.; Mongiat-Artus, P.; Belhadj, S.; Philippe, J.; Lesage, M.; Wittmer, E.; et al. Determination of Angptl4 mRNA as a diagnostic marker of primary and metastatic clear cell renal-cell carcinoma. PLoS ONE 2010, 5, e10421. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Jia, L.; Zhou, Y.; Ren, L.; Li, J.; Zhang, J. Serum level of angptl4 as a potential biomarker in renal cell carcinoma. Urol. Oncol. 2017, 35, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.Y.; Jou, Y.C.; Tung, C.L.; Tsai, Y.S.; Wang, Y.H.; Chi, C.L.; Lin, R.I.; Hung, S.K.; Chuang, Y.M.; Wu, S.F.; et al. Epigenetic silencing of the dual-role signal mediator, ANGPTL4 in tumor tissues and its overexpression in the urothelial carcinoma microenvironment. Oncogene 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Geng, T.; Guo, X.; Liu, J.; Zhang, P.; Yang, D.; Li, J.; Yu, S.; Sun, Y. Co-expression of immunoglobulin-like transcript 4 and angiopoietin-like proteins in human non-small cell lung cancer. Mol. Med. Rep. 2015, 11, 2789–2796. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.G.; Zhao, Y.; Sethi, P.; Li, Y.Y.; Mahta, A.; Culicchia, F.; Lukiw, W.J. Micro-RNA-128 (miRNA-128) down-regulation in glioblastoma targets ARP5 (ANGPTL6), Bmi-1 and E2F-3a, key regulators of brain cell proliferation. J. Neuro-Oncol. 2010, 98, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Marchio, S.; Soster, M.; Cardaci, S.; Muratore, A.; Bartolini, A.; Barone, V.; Ribero, D.; Monti, M.; Bovino, P.; Sun, J.; et al. A complex of α6 integrin and E-cadherin drives liver metastasis of colorectal cancer cells through hepatic angiopoietin-like 6. EMBO Mol. Med. 2012, 4, 1156–1175. [Google Scholar] [CrossRef] [PubMed]
| Protein | Function | Tissue/Organ Expression | Receptor |
|---|---|---|---|
| ANGPTL1 | anti-angiogenic, permeability, anti-apoptotic [15,16,17] | Thyroid gland, liver, bladder, gallbladder, gastrointestinal tract (no esophagus), adipose tissue and skin | orphan nuclear receptor, site A apolipoprotein (AI) [18] |
| ANGPTL2 | Angiogenesis, development of cancer [19,20,21,22,23,24,25,26] | heart, adipose tissue, kidney, lung and skeletal muscle | integrins α5β1 and Toll-like receptor 4 (TLR4), LILRB2 [4,19,23] |
| ANGPTL3 | Angiogenesis and lipid metabolism | liver, kidney | alpha-5/beta-3, LILRB2 (weak) [27] |
| ANGPTL4 | Angiogenesis (pro- or anti- agiogenic factor), lipid metabolism, glucose metabolism, energy homeostasis, redox regulation, inflammation, endothelial cell integrity, development of cancer [3,28,29,30,31,32,33,34,35] | adipose tissue, liver, kidney, muscle and intestine, ovary, breast skin, testis, kidney urinary bladder, esophagus | fibronectin, vitronectin, integrin β1 and β5 [36,37] |
| ANGPTL5 | lipid and triglyceride metabolism [38,39] | adipose tissue and hearth, ovary, testis, skin, | LILRB2 [4] |
| ANGPTL6 | Angiogenesis, lipid metabolism, glucose metabolism [20,22,40,41] | Liver, gallbladder, placenta, bone marrow, placenta | orphan of receptor, |
| ANGPTL7 | angiogenesis [42] | eye | LILRB2 (weak) [4] |
| ANGPTL8 | lipid metabolism [43,44,45] | liver, adipose tissue | orphan of receptor |
| Name | Role in Cancer | Inflammation | Cancer Disease Association | Function |
|---|---|---|---|---|
| ANGPTL1 | Tumor suppressor | not reported | low expression in kidney, lung, prostate, bladder, thyroid, breast and lung cancers, melanoma and hepatocarcinoma | Reduces migratory and invasive abilities of different cancer cell lines in vitro and to suppress the epithelial to mesenchymal transition (EMT). |
| ANGPTL2 | Tumor promoting | proinflammatory | high expression in esophageal, colorectal, prostate, pancreatic lung, breast and skin cancers, hepatocarinoma | Pro-angiogenic and antiapoptotic abilities. Increase migratory and invasive ability. Driver of metastases was demonstrated in lung, breast and liver cancer and in osteosarcoma cell lines. |
| ANGPTL3 | Tumor promoting | proinflammatory | high expression in oral squamous cell carcinoma, hepatocarcinoma and ovarian cancer | Cancer growth, motility and invasion. |
| ANGPTL4 | Tumor-type dependent | proinflammatory | high expression in lung, colorectal, oral, breast cancers, hepatocarcinoma, oral squamous cell carcinoma, | A pro- and an anti-angiogenic protein, regulating vascular integrity and angiogenesis in a context-dependent manner suggesting that it might be tumor-type dependent. |
| ANGPTL5 | Tumor promoting | proinflammatory | high expression in non-small cell lung cancer | Among its related pathways are Hematopoietic Stem Cell Differentiation Pathways and Lineage-specific Markers. An important paralog of this gene is ANGPTL3. |
| ANGPTL6 | Tumor promoting | proinflammatory | high expression in glioma, glioblastoma multiforme and colorectal cancer | Tumor growth, and metastases driver. |
| ANGPTL7 | Tumor promoting | proinflammatory | high expression in colorectal, lung, breast and ovarian cancers | A pro-angiogenic factor. Stimulates proliferation, motility, invasiveness and capability to form capillary-like networks in human differentiated endothelial cells. |
| ANGPTL8 | not reported | not reported | Breast Angiosarcoma and Breast Sarcoma | Promote proliferation of pancreatic beta cells and increase insulin release in an insulin-deficient mouse model of insulin resistance. Among its related pathways are Metabolism and Lipoprotein metabolism. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, C.; Piro, G.; Merz, V.; Simionato, F.; Santoro, R.; Zecchetto, C.; Tortora, G.; Melisi, D. Angiopoietin-Like Proteins in Angiogenesis, Inflammation and Cancer. Int. J. Mol. Sci. 2018, 19, 431. https://doi.org/10.3390/ijms19020431
Carbone C, Piro G, Merz V, Simionato F, Santoro R, Zecchetto C, Tortora G, Melisi D. Angiopoietin-Like Proteins in Angiogenesis, Inflammation and Cancer. International Journal of Molecular Sciences. 2018; 19(2):431. https://doi.org/10.3390/ijms19020431
Chicago/Turabian StyleCarbone, Carmine, Geny Piro, Valeria Merz, Francesca Simionato, Raffaela Santoro, Camilla Zecchetto, Giampaolo Tortora, and Davide Melisi. 2018. "Angiopoietin-Like Proteins in Angiogenesis, Inflammation and Cancer" International Journal of Molecular Sciences 19, no. 2: 431. https://doi.org/10.3390/ijms19020431
APA StyleCarbone, C., Piro, G., Merz, V., Simionato, F., Santoro, R., Zecchetto, C., Tortora, G., & Melisi, D. (2018). Angiopoietin-Like Proteins in Angiogenesis, Inflammation and Cancer. International Journal of Molecular Sciences, 19(2), 431. https://doi.org/10.3390/ijms19020431






