Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment
Abstract
:1. Introduction
2. Results
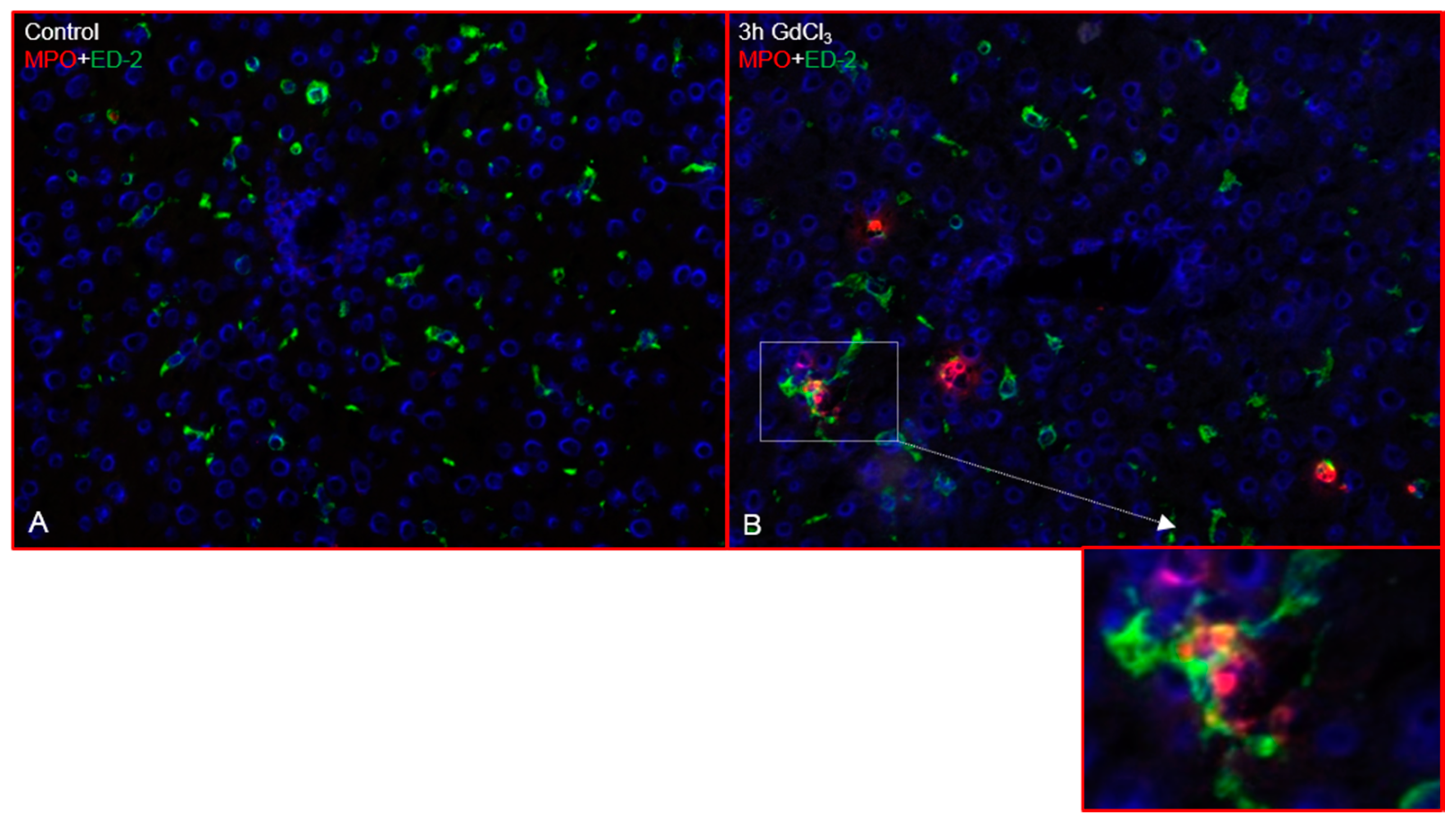
2.1. Immunofluorescence Analysis of the Rat Liver Following Intraperitoneal Injection of GdCl3 or Zymosan
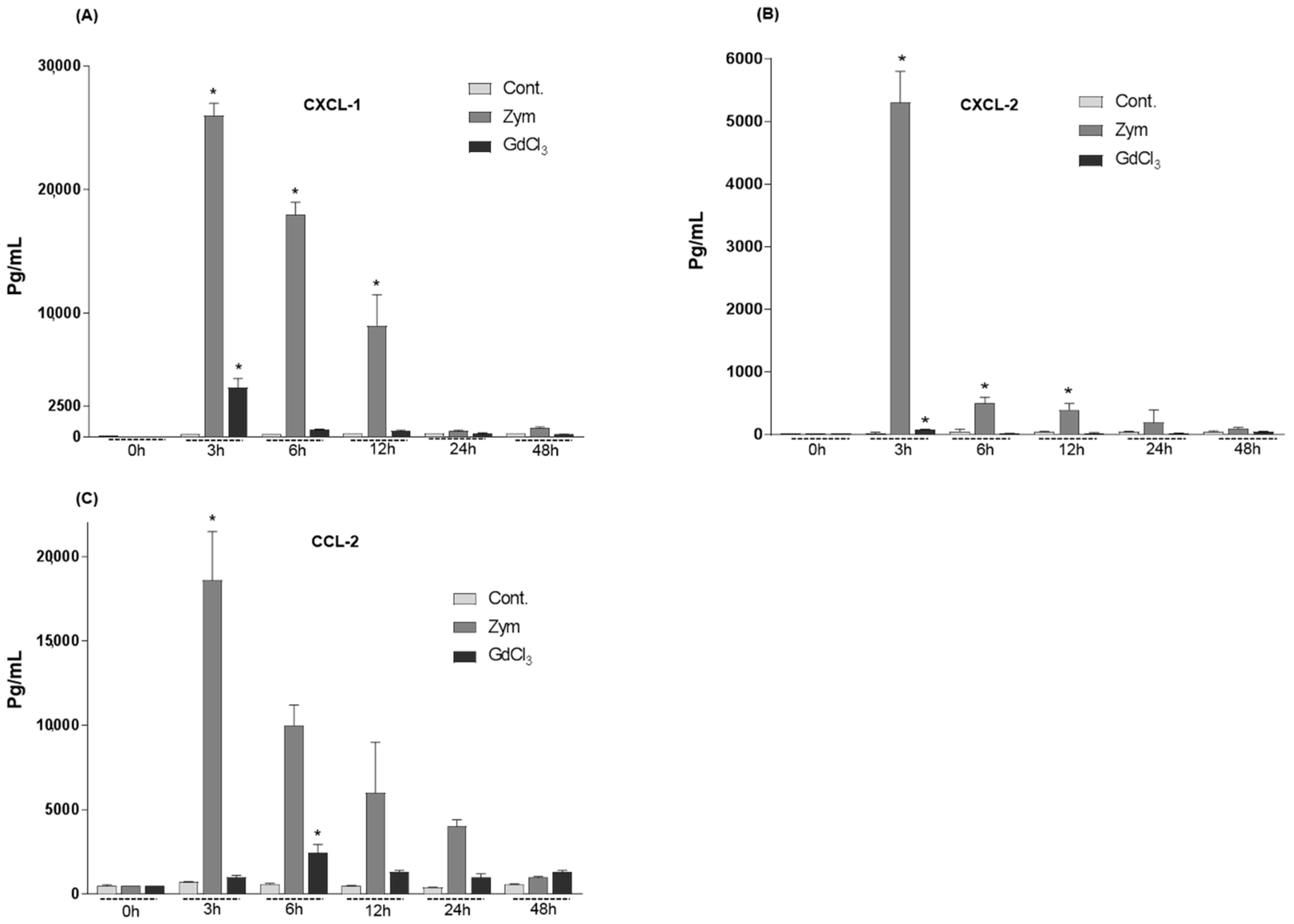
2.2. Changes in the Serum Levels of CXCL-1, CXCL-2 and CCL-2 of Rat after GdCl3 or Zymosan Administration
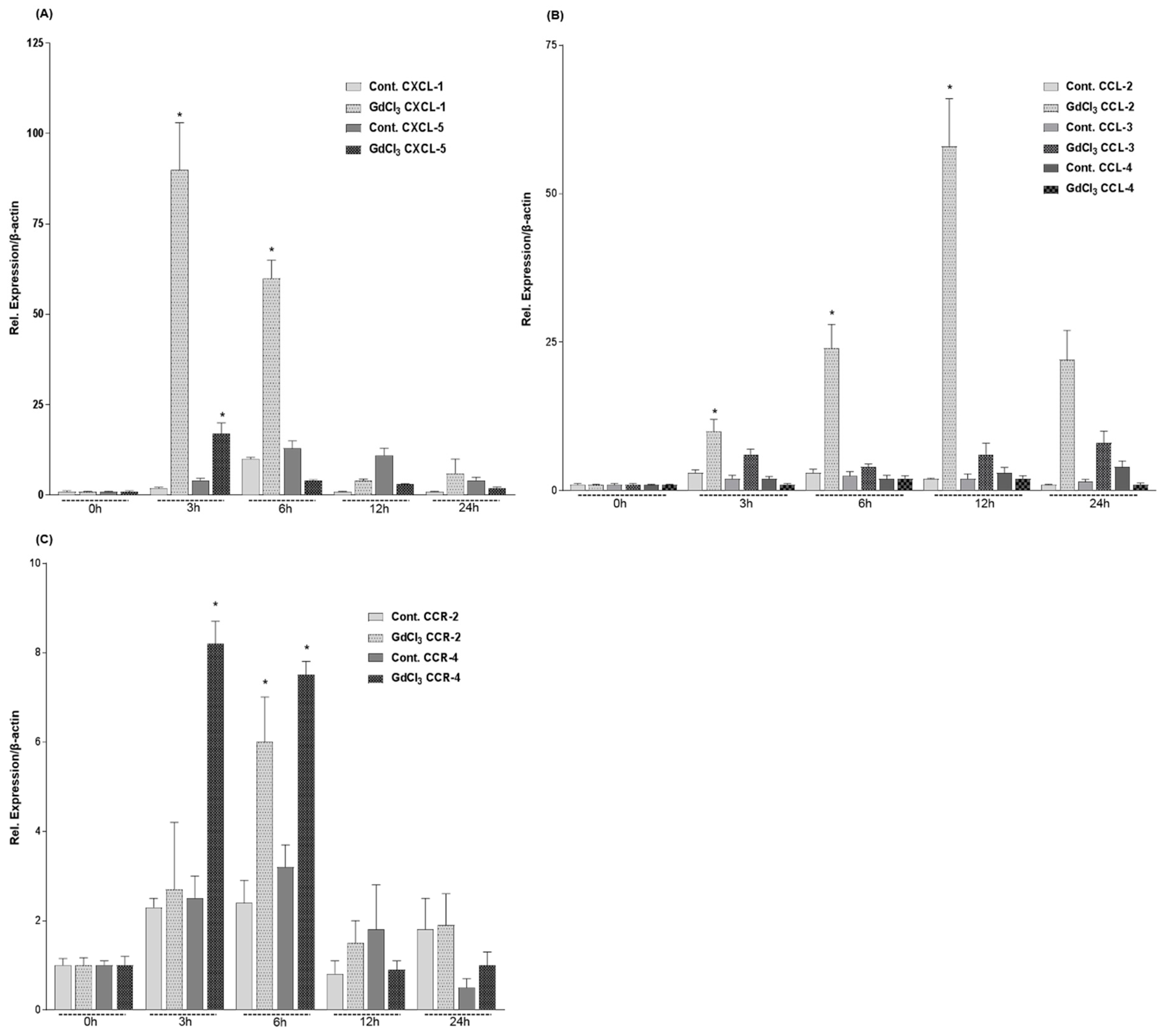
2.3. Expression of CXC- and CC-Chemokines in Rat Livers after GdCl3 Administration
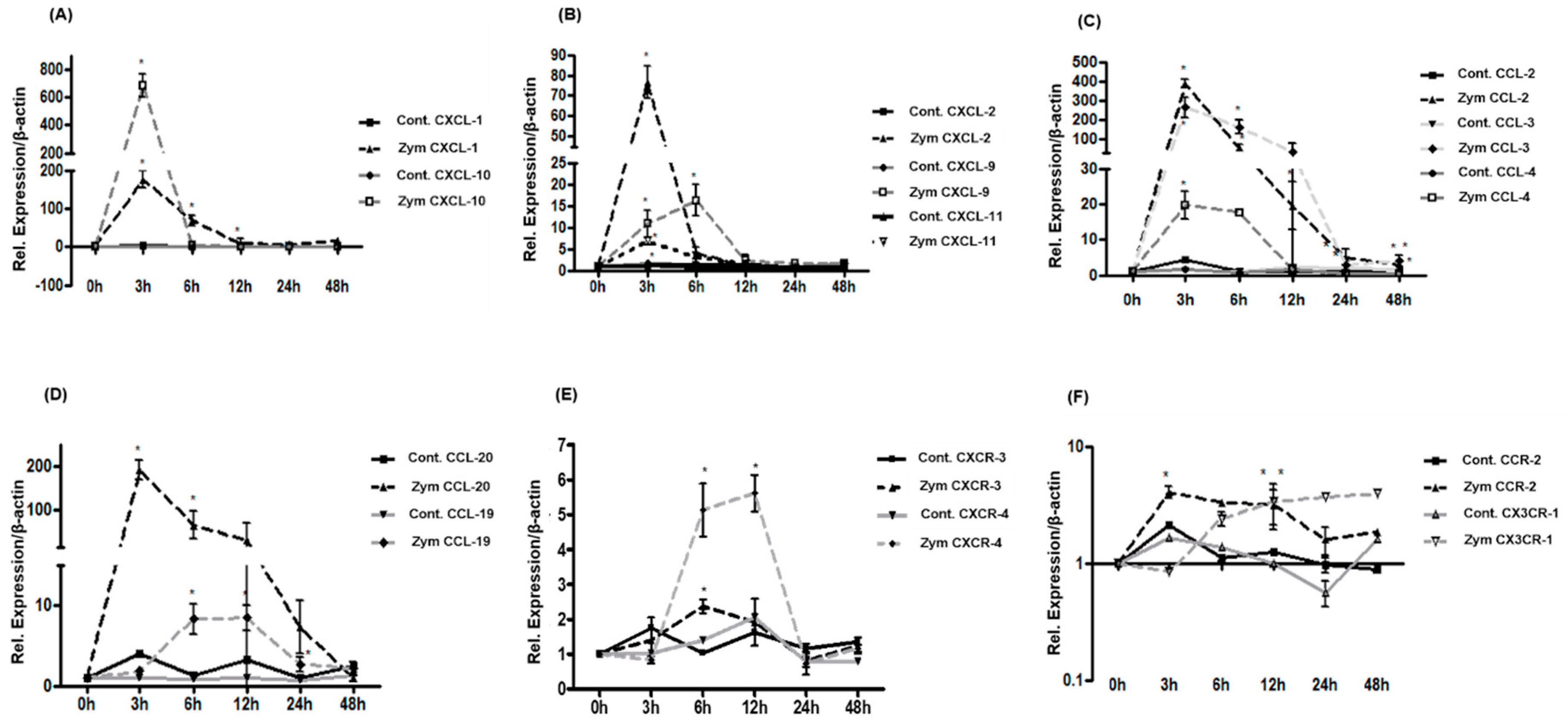
2.4. Expression of CXC-, CC-Chemokines and Chemokine Receptors in Rat Liver after Zymosan Administration
2.5. Expression of the Genes of Adhesion Molecules in Rat Livers after GdCl3 or Zymosan Administration
2.6. Expression of CC- and CXC-Chemokine Receptors in C3H/HeJ Mice after GdCl3 or Zymosan Administration
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
4.3. Antibodies
4.4. GdCl3 and Zymosan Administration to Rats and C3H/HeJ Endotoxin-Resistant Mice
4.5. Liver Immunohistochemistry
4.6. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (PCR)
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amanzada, A.; Malik, I.A.; Nischwitz, M.; Sultan, S.; Naz, N.; Ramadori, G. Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflamed liver. Histochem. Cell Biol. 2011, 135, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Amanzada, A.; Moriconi, F.; Mansuroglu, T.; Cameron, S.; Ramadori, G.; Malik, I.A. Induction of chemokines and cytokines before neutrophils and macrophage recruitment in different regions of rat liver after TAA administration. Lab. Investig. 2014, 94, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Moriconi, F.; Malik, I.; Dudas, J. Physiology and pathophysiology of liver inflammation, damage and repair. J. Physiol. Pharmacol. 2008, 59 (Suppl. 1), 107–117. [Google Scholar] [PubMed]
- Malik, I.A.; Moriconi, F.; Sheikh, N.; Naz, N.; Khan, S.; Dudas, J.; Mansuroglu, T.; Hess, C.F.; Rave-Frank, M.; Christiansen, H.; et al. Single-dose gamma-irradiation induces up-regulation of chemokine gene expression and recruitment of granulocytes into the portal area but not into other regions of rat hepatic tissue. Am. J. Pathol. 2010, 176, 1801–1815. [Google Scholar] [CrossRef] [PubMed]
- Bajt, M.L.; Farhood, A.; Jaeschke, H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1188–G1195. [Google Scholar] [CrossRef] [PubMed]
- Dorman, R.B.; Gujral, J.S.; Bajt, M.L.; Farhood, A.; Jaeschke, H. Generation and functional significance of CXC chemokines for neutrophil-induced liver injury during endotoxemia. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G880–G886. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Christ, B. Cytokines and the hepatic acute-phase response. Semin. Liver Dis. 1999, 19, 141–155. [Google Scholar] [CrossRef]
- Schlayer, H.J.; Laaff, H.; Peters, T.; Woort-Menker, M.; Estler, H.C.; Karck, U.; Schaefer, H.E.; Decker, K. Involvement of tumor necrosis factor in endotoxin-triggered neutrophil adherence to sinusoidal endothelial cells of mouse liver and its modulation in acute phase. J. Hepatol. 1988, 7, 239–249. [Google Scholar] [CrossRef]
- Simonet, W.S.; Hughes, T.M.; Nguyen, H.Q.; Trebasky, L.D.; Danilenko, D.M.; Medlock, E.S. Long-term impaired neutrophil migration in mice overexpressing human interleukin-8. J. Clin. Investig. 1994, 94, 1310–1319. [Google Scholar] [CrossRef]
- Ruttinger, D.; Vollmar, B.; Wanner, G.A.; Messmer, K. In vivo assessment of hepatic alterations following gadolinium chloride-induced Kupffer cell blockade. J. Hepatol. 1996, 25, 960–967. [Google Scholar] [CrossRef]
- Michael, S.L.; Pumford, N.R.; Mayeux, P.R.; Niesman, M.R.; Hinson, J.A. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology 1999, 30, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, H.; Kinoshita, M.; Nakashima, M.; Habu, Y.; Shono, S.; Uchida, T.; Shinomiya, N.; Seki, S. Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice. Hepatology 2008, 48, 1979–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, S.; Dickson, E.M.; San Martin, M.E.; van Rooijen, N.; Papa, E.F.; Harty, M.W.; Tracy, T.F., Jr.; Gregory, S.H. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology 2006, 130, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Blazka, M.E.; Germolec, D.R.; Simeonova, P.; Bruccoleri, A.; Pennypacker, K.R.; Luster, M.I. Acetaminophen-induced hepatotoxicity is associated with early changes in NF-kB and NF-IL6 DNA binding activity. J. Inflamm. 1995, 47, 138–150. [Google Scholar] [PubMed]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chevre, R.; Gonzalez, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005, 22, 285–294. [Google Scholar] [CrossRef]
- Bonder, C.S.; Ajuebor, M.N.; Zbytnuik, L.D.; Kubes, P.; Swain, M.G. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 2004, 172, 45–53. [Google Scholar] [CrossRef]
- Jaeschke, H. Chemokines, neutrophils, and inflammatory liver injury. Shock 1996, 6, 403–404. [Google Scholar] [CrossRef]
- Ohta, Y.; Imai, Y.; Matsura, T.; Kitagawa, A.; Yamada, K. Preventive effect of neutropenia on carbon tetrachloride-induced hepatotoxicity in rats. J. Appl. Toxicol. 2006, 26, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, F.; Ahmad, G.; Ramadori, P.; Malik, I.; Sheikh, N.; Merli, M.; Riggio, O.; Dudas, J.; Ramadori, G. Phagocytosis of gadolinium chloride or zymosan induces simultaneous upregulation of hepcidin- and downregulation of hemojuvelin- and Fpn-1-gene expression in murine liver. Lab. Investig. 2009, 89, 1252–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underhill, D.M. Macrophage recognition of zymosan particles. J. Endotoxin Res. 2003, 9, 176–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedland, J.S.; Remick, D.G.; Shattock, R.; Griffin, G.E. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur J. Immunol. 1992, 22, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, M.V.; Capo, C.; Bongrand, P.; Mege, J.L. Zymosan-stimulated tumor necrosis factor-alpha production by human monocytes. Down-modulation by phorbol ester. J. Immunol. 1992, 148, 2229–2236. [Google Scholar] [PubMed]
- Petit, F.; Bagby, G.J.; Lang, C.H. Tumor necrosis factor mediates zymosan-induced increase in glucose flux and insulin resistance. Am. J. Physiol. 1995, 268 Pt 1, E219–E228. [Google Scholar] [CrossRef]
- Hardonk, M.J.; Dijkhuis, F.W.; Hulstaert, C.E.; Koudstaal, J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J. Leukoc. Biol. 1992, 52, 296–302. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Trautwein, C.; Tacke, F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front. Physiol. 2012, 3, 56. [Google Scholar] [CrossRef]
- Zaldivar, M.M.; Berres, M.L.; Sahin, H.; Nellen, A.; Heinrichs, D.; Schmitz, P.; Gassler, N.; Streetz, K.L.; Trautwein, C.; Wasmuth, H.E. The chemokine receptor CXCR3 limits injury after acute toxic liver damage. Lab. Investig. 2012, 92, 724–734. [Google Scholar] [CrossRef] [Green Version]
- Moriconi, F.; Christiansen, H.; Raddatz, D.; Dudas, J.; Hermann, R.M.; Rave-Frank, M.; Sheikh, N.; Saile, B.; Hess, C.F.; Ramadori, G. Effect of radiation on gene expression of rat liver chemokines: In vivo and in vitro studies. Radiat. Res. 2008, 169, 162–169. [Google Scholar] [CrossRef]
- Christiansen, H.; Batusic, D.; Saile, B.; Hermann, R.M.; Dudas, J.; Rave-Frank, M.; Hess, C.F.; Schmidberger, H.; Ramadori, G. Identification of genes responsive to gamma radiation in rat hepatocytes and rat liver by cDNA array gene expression analysis. Radiat. Res. 2006, 165, 318–325. [Google Scholar] [CrossRef]
- Neubauer, K.; Lindhorst, A.; Tron, K.; Ramadori, G.; Saile, B. Decrease of PECAM-1-gene-expression induced by proinflammatory cytokines IFN-gamma and IFN-alpha is reversed by TGF-beta in sinusoidal endothelial cells and hepatic mononuclear phagocytes. BMC Physiol. 2008, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, F.; Malik, I.A.; Amanzada, A.; Blaschke, M.; Raddatz, D.; Khan, S.; Ramadori, G. The anti-TNF-alpha antibody infliximab indirectly regulates PECAM-1 gene expression in two models of in vitro blood cell activation. Lab. Investig. 2012, 92, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Morishima, T.; Kimura, H.; Nishikawa, K.; Tsurumi, T.; Kuzushima, K. Antigen-driven expansion and contraction of CD8+-activated T cells in primary EBV infection. J. Immunol. 1999, 163, 5735–5740. [Google Scholar] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van, H.C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018. [Google Scholar] [CrossRef]
- Saile, B.; Ramadori, G. Inflammation, damage repair and liver fibrosis—Role of cytokines and different cell types. Z. Gastroenterol. 2007, 45, 77–86. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111–116. [Google Scholar] [CrossRef]
- Sultan, S.; Ahmad, S.; Rave-Frank, M.; Malik, I.A.; Hess, C.F.; Christiansen, H.; Cameron, S. Induction of Lipocalin2 in a Rat Model of Lung Irradiation. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Ramadori, P.; Ahmad, G.; Ramadori, G. Cellular and molecular mechanisms regulating the hepatic erythropoietin expression during acute-phase response: A role for IL-6. Lab. Investig. 2010, 90, 1306–1324. [Google Scholar] [CrossRef]




| Primer | Forward 5′ → 3′ | Reverse 5′ → 3′ |
|---|---|---|
| CXCL-1 | GGC AGG GAT TCA CTT CAA GA | GCC ATC GGT GCA ATC TAT CT |
| CXCL-2 | ATC CAG AGC TTG ACG GTG AC | AGG TAC GAT CCA GGC TTC CT |
| CXCL-5 | CTC AAG CTG CTC CTT TCT CG | GCG ATC ATT TTG GGG TTA AT |
| CXCL-9 | GCC TTG ACT CCA GCA CGG T | GAC TTC ATG GCA GAG CCG AG |
| CXCL-10 | CTG TCG TTC TCT GCC TCG TG | GGA TCC CTT CTT GAG TCC CAC TCA |
| CXCL-11 | AGA ACA TGT GAT GGG CCC TC | GGG TCA GCT TCT TGG CAC AG |
| CCL-2 | AGG CAG ATG CAG TTA ATG CCC | ACA CCT GCT GCT GGT GAT TCT C |
| CCL-3 | TTT TGA GAC CAG CAG CAG CCT TT | CTC AAG CCC CTG CTC TAC AC |
| CCL-4 | TCC CGG AAG ATT CAT CGG | GCA CAG ATT TGC CTG CCT TTT |
| CCL-19 | AGA ACG CAT CAT CCG AAG AC | TGC TCA CAC TCA CGT TCA CA |
| CCL-20 | CAA CTT TGA CTG CTG CCT CA | TTC CAT CCC AGA AAA GCA TC |
| CCR-2 | CTT GTG GCC CTT ATT TTC CA | AGA TGA GCC TCA CAG CCC TA |
| CCR-4 | GAA TGC CAC AGA TGT CAC AG | GCA CAA ACA GTA AAT CCG AG |
| CXCR-3 | TAC CTT GAG GTC AGT GAA CG | AAA GAG GAG GCT GTA GAG GA |
| CXCR-4 | GCT GAG GAG CAT GAC AGA CA | GAT GAA GGC CAG GAT GAG AA |
| CX3CR-1 | TGA CTG GCA GAT CCA GAG GTT | GTA GAA TAT GGA CAG GAA CAC |
| ICAM-1 | TGC ACG TCC CTG GTG ATA CTC | TGT CAA ACG GGA GAT GAA TGG |
| ICAM-2 | AGC AGC AGG CAG AGA GTT TC | TCT GCC ACA GAG CAG AGA GA |
| PECAM-1 | TCA GCT GCC AGT CAG TAA ATG G | TCT GGA AGT TGC TCT TTG CTC TT |
| VCAM-1 | ACA TGT GCT GCT GTT GGC TGT | GCT CAG CGT CAG TGT GGA TGT A |
| β-actin | ACC ACC ATG TAG CCA GGC ATT | CCA CAC AGA GTA CTT GCC CTC A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Ramadori, G.; Moriconi, F. Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment. Int. J. Mol. Sci. 2018, 19, 3891. https://doi.org/10.3390/ijms19123891
Ahmad S, Ramadori G, Moriconi F. Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment. International Journal of Molecular Sciences. 2018; 19(12):3891. https://doi.org/10.3390/ijms19123891
Chicago/Turabian StyleAhmad, Shakil, Giuliano Ramadori, and Federico Moriconi. 2018. "Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment" International Journal of Molecular Sciences 19, no. 12: 3891. https://doi.org/10.3390/ijms19123891




