Relevance of Breast Cancer Resistance Protein to Pharmacokinetics of Florfenicol in Chickens: A Perspective from In Vivo and In Vitro Studies
Abstract
:1. Introduction
2. Results
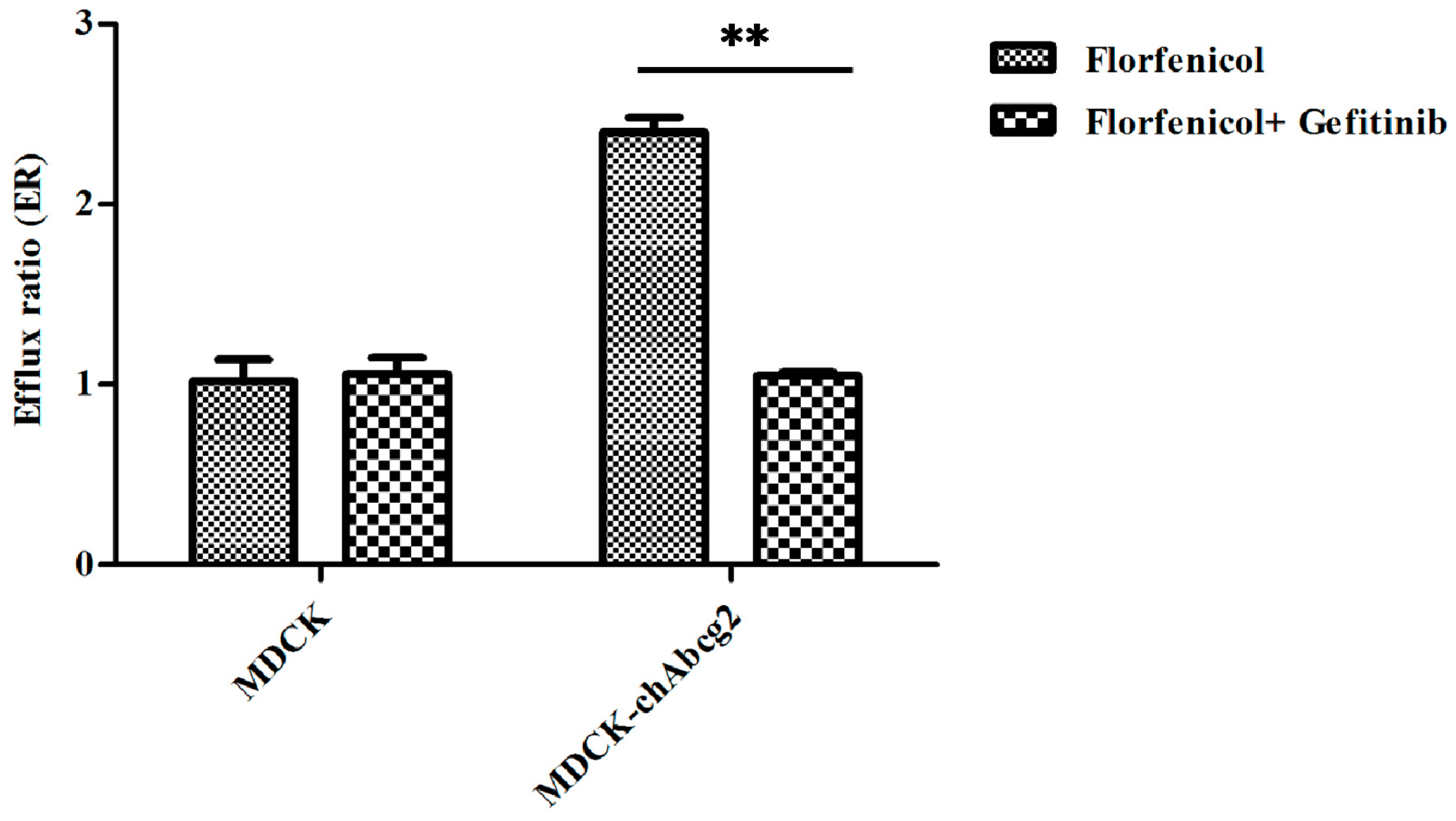
2.1. Florfenicol is a Substrate of Chicken BCRP Indicated by the Bidirectional Transport Assay in MDCK-chAbcg2 Cells
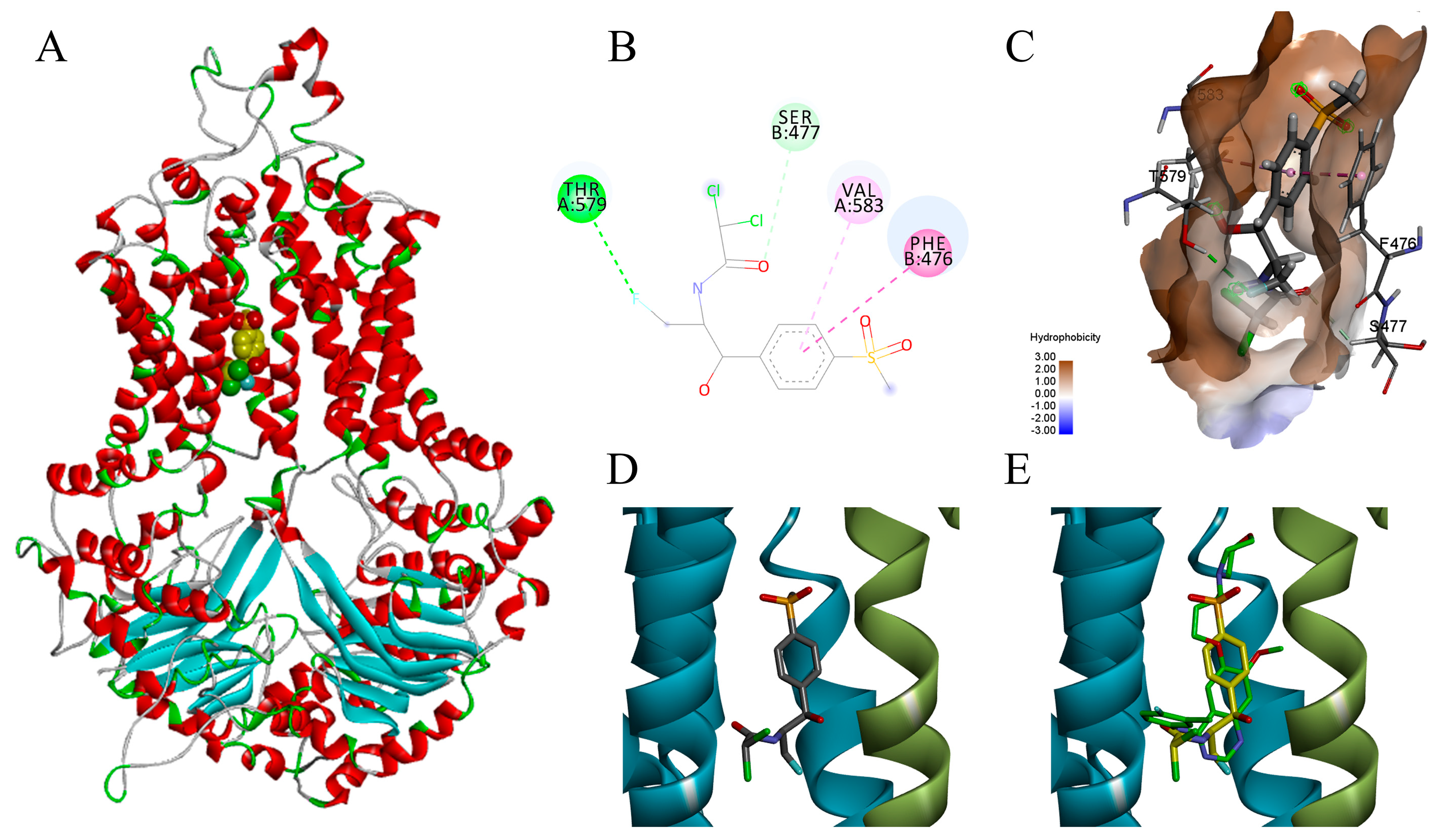
2.2. Florfenicol Might Favorably Bind with BCRP Analyzed by Molecular Docking Modelling
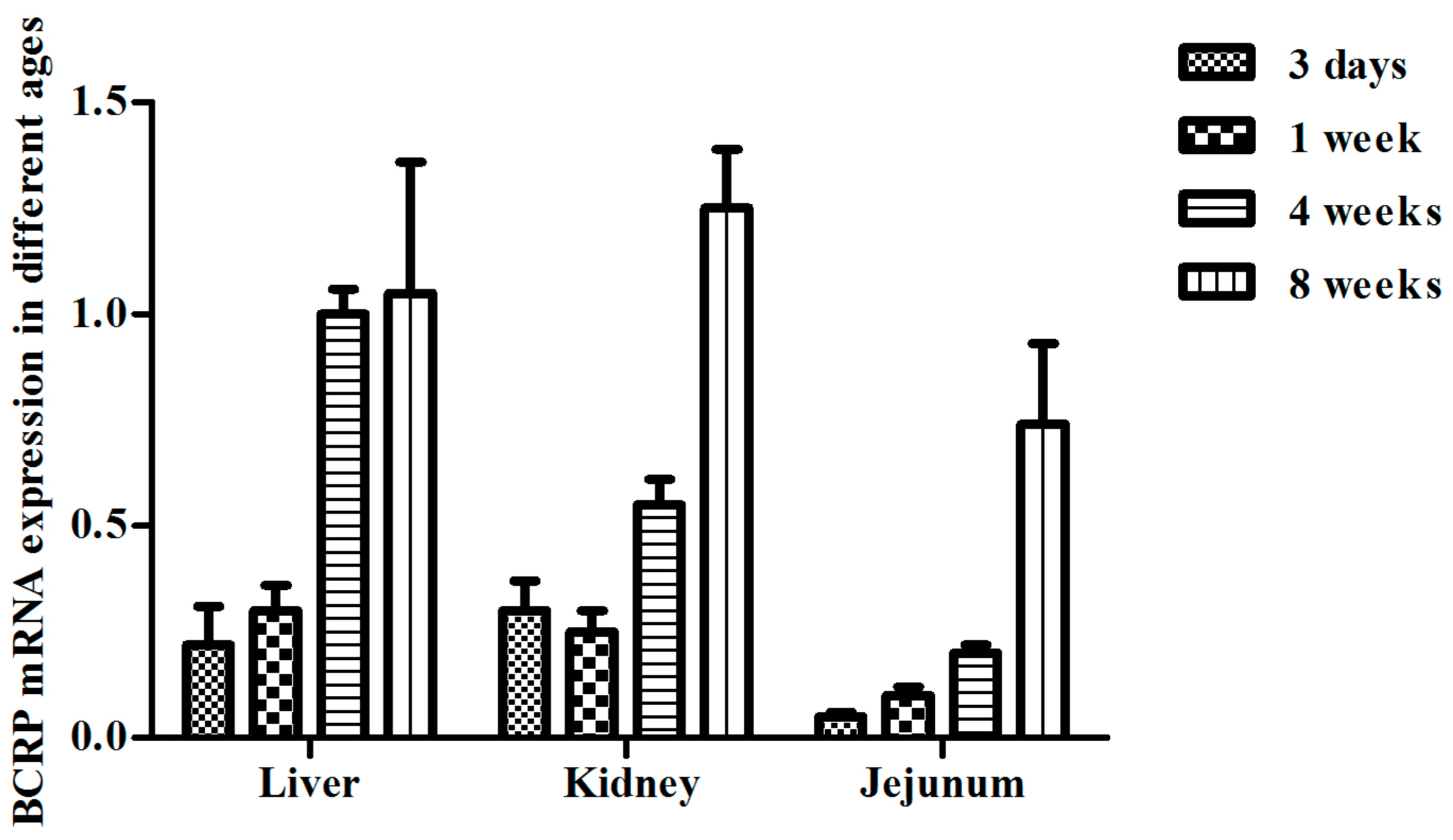
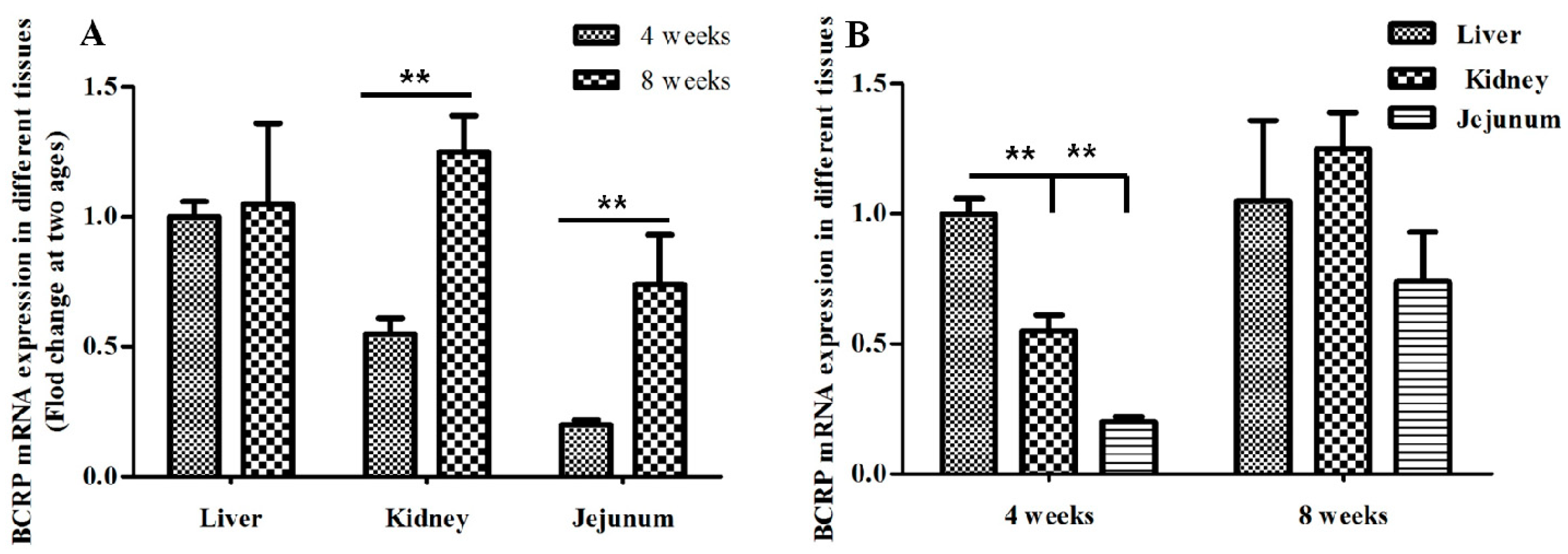
2.3. Age-Dependent mRNA Expression of BCRP in Kidney and Jejunum in Broilers
2.4. Pharmacokinetic Behavior of Florfenicol Is Different in Broilers at Different Ages
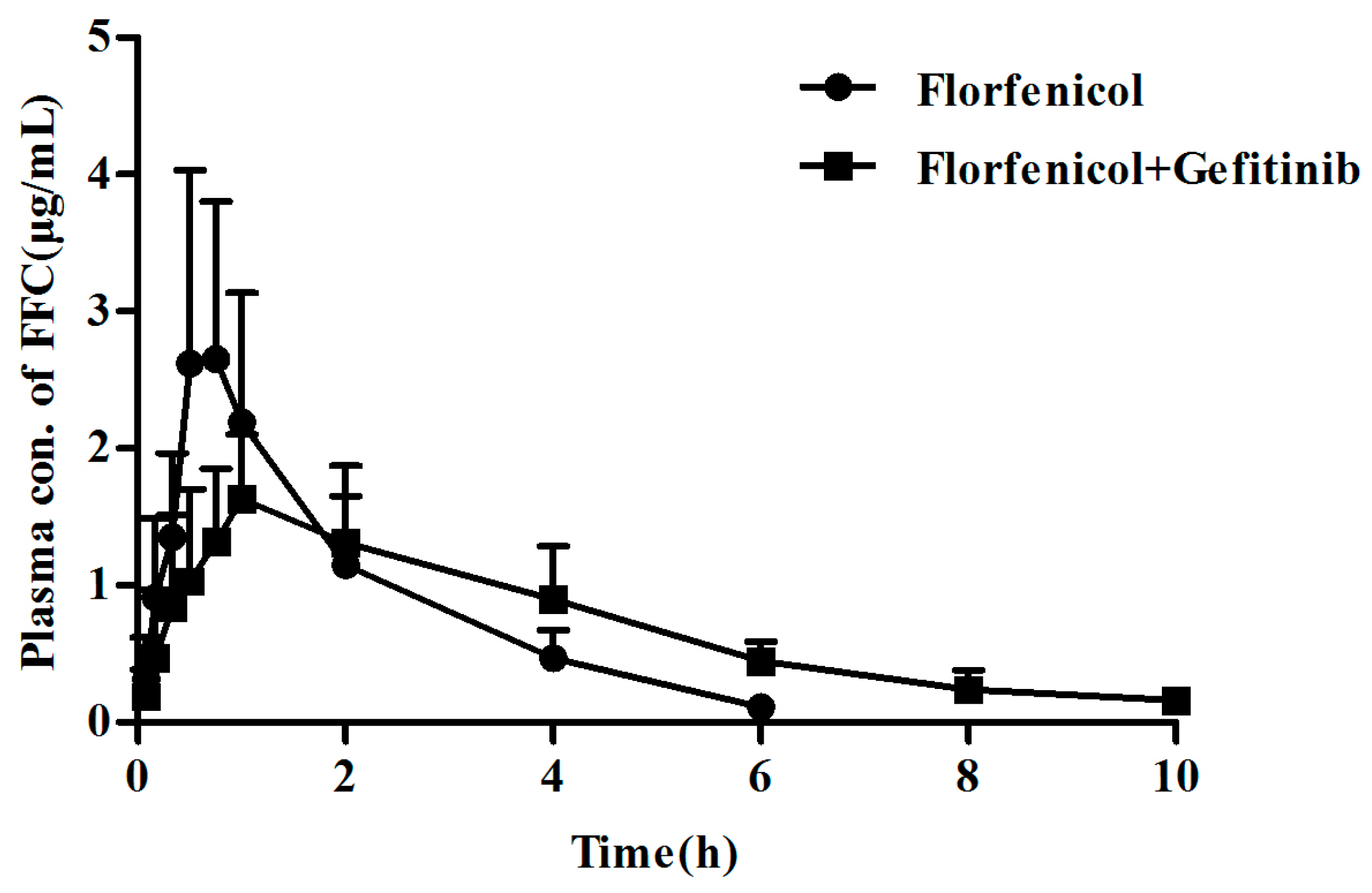
2.5. BCRP Inhibitor Gefitinib Affected the Pharmacokinetics of Florfenicol Orally Administrated in Broilers
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines
4.3. Animals
4.4. Bidirectional Transport Experiments in MDCK-chAbcg2 Cells
4.5. Molecular Modelling
4.6. BCRP mRNA Expression in Tissues of Broilers at Different Ages by Real Time RT-PCR
4.7. Pharmacokinetic Studies of FFC in Broilers
4.7.1. Experiment Design and Blood Collection
4.7.2. Determination of FFC Concentration in Broilers Serum by HPLC Method
4.7.3. Pharmacokinetic Data Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, Q.; Unadkat, J.D. Role of the Breast Cancer Resistance Protein (BCRP/ABCG2) in Drug Transport—An Update. AAPS J. 2014, 17, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlaming, M.L.; Lagas, J.S.; Schinkel, A.H. Physiological and pharmacological roles of ABCG2 (BCRP): Recent findings in Abcg2 knockout mice. Adv. Drug Deliv. Rev. 2009, 61, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maliepaard, M.; Scheffer, G.L.; Faneyte, I.F.; van Gastelen, M.A.; Pijnenborg, A.C.; Schinkel, A.H.; van De Vijver, M.J.; Scheper, R.J.; Schellens, J.H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001, 61, 3458–3464. [Google Scholar] [PubMed]
- Giacomini, K.M.; Balimane, P.V.; Cho, S.K.; Eadon, M.; Edeki, T.; Hillgren, K.M.; Huang, S.M.; Sugiyama, Y.; Weitz, D.; Wen, Y.; et al. International Transporter Consortium commentary on clinically important transporter polymorphisms. Clin. Pharmacol. Ther. 2013, 94, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Cusatis, G.; Gregorc, V.; Li, J.; Spreafico, A.; Ingersoll, R.G.; Verweij, J.; Ludovini, V.; Villa, E.; Hidalgo, M.; Sparreboom, A.; et al. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J. Natl. Cancer Inst. 2006, 98, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, H.; Furuie, H.; Inano, A.; Sunagawa, A.; Yamada, S.; Wu, C.; Fukizawa, S.; Morimoto, N.; Ieiri, I.; Morishita, M.; et al. Pharmacokinetic interaction study of sulphasalazine in healthy subjects and the impact of curcumin as an in vivo inhibitor of BCRP. Br. J. Pharmacol. 2012, 166, 1793–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US Food and Drug Administration. Guidance for Industry—Drug Interaction Studies: Study Design, Data Analysis, Implications for Dosing, And Labeling Recommendation; US Food and Drug Administration: Silver Spring, MD, USA, 2012.
- Sidhu, P.; Rassouli, A.; Illambas, J.; Potter, T.; Pelligand, L.; Rycroft, A.; Lees, P. Pharmacokinetic-pharmacodynamic integration and modelling of florfenicol in calves. J. Vet. Pharmacol. Ther. 2014, 37, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Shupeng, H.; Tainyao, H.; Chen, M.H.; Wang, W.S. Antibacterial effect of chloramphenicol, thiamphenicol and florfenicol against aquatic animal bacteria. J. Vet. Med. Sci. 2000, 62, 479. [Google Scholar]
- Shin, S.J.; Sang, G.K.; Nabin, R.; Mi, L.K.; Han, S.Y. Evaluation of the antimicrobial activity of florfenicol against bacteria isolated from bovine and porcine respiratory disease. Vet. Microbiol. 2005, 106, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Wisselink, H.J.; Veldman, K.T. Quantitative susceptibility of Streptococcus suis strains isolated from diseased pigs in seven European countries to antimicrobial agents licenced in veterinary medicine. Vet. Microbiol. 2006, 113, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, S.S.; Qian, M.M.; Wei, L.; Zhou, D.; Zhang, Z.Z.; He, J.K.; Zhang, Q.J.; Zhu, P.; Xiao, X.L. Nanoemulsion formulation of florfenicol improves bioavailability in pigs. J. Vet. Pharmacol. Ther. 2016, 39, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, S.J.; Zhang, Q.; Shao, Y.X. Influence of three coccidiostats on the pharmacokinetics of florfenicol in rabbits. Exp. Anim. Tokyo 2015, 64, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.Y.; Tu, P.; Chen, X.; Guo, Y.G.; Jiang, S.X. Effect of three polyether ionophores on pharmacokinetics of florfenicol in male broilers. J. Vet. Pharmacol. Ther. 2013, 36, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, D.; Wu, X.; Coats, J.R. Bioavailability and pharmacokinetics of florfenicol in broiler chickens. J. Vet. Pharmacol. Ther. 2010, 26, 337–341. [Google Scholar] [CrossRef]
- Liu, N.; Guo, M.; Mo, F.; Sun, Y.Y.; Yuan, Z.; Cao, L.L.; Jiang, S.X. Involvement of p-glycoprotein and cytochrome p450 3a in the metabolism of florfenicol of rabbits. J. Vet. Pharmacol. Ther. 2012, 35, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Zheng, H.H.; Zhang, K.Y.; Yang, F.; Kong, T.; Zhou, B.; Jiang, S.X. The roles of cytochrome p450 and p-glycoprotein in the pharmacokinetics of florfenicol in chickens. Iran. J. Vet. Res. 2018, 19, 9–14. [Google Scholar] [PubMed]
- Laszlo, L.; Sarkadi, B.; Hegedus, T. Jump into a New Fold—A Homology Based Model for the ABCG2/BCRP Multidrug Transporter. PLoS ONE 2016, 11, e0164426. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, J.E.; Zolk, O.; Fromm, M.F.; Kurkinen, K.J.; Neuvonen, P.J.; Niemi, M. Abcg2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin. Pharmacol. Ther. 2009, 86, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, J.E.; Pasanen, M.K.; Neuvonen, P.J.; Niemi, M. Different effects of the abcg2 c.421c>a snp on the pharmacokinetics of fluvastatin, pravastatin and simvastatin. Pharmacogenomics 2009, 10, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Wang, Y.; Lu, Y.F.; Xu, S.F.; Wu, Q.; Liu, J. Age-associated differences in transporter gene expression in kidneys of male rats. Mol. Med. Rep. 2017, 15, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Riches, Z.; Abanda, N.; Collier, A.C. BCRP protein levels do not differ regionally in adult human livers, but decline in the elderly. Chemico-Biol. Interact. 2015, 242, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konieczna, A.; Erdösová, B.; Lichnovská, R.; Jandl, M.; Čížková, K.; Ehrmann, J. Differential expression of abc transporters (mdr1, mrp1, bcrp) in developing human embryos. J. Mol. Histol. 2011, 42, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Jodoin, J.; Beaulieu, E.; Brossard, M.; Béliveau, R. Dexamethasone modulation of multidrug transporters in normal tissues. FEBS Lett. 1999, 442, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Baello, S.; Javam, M.; Audette, M.C.; Gibb, W.; Matthews, S.G. Regulation of multidrug resistance p-glycoprotein in the developing blood-brain barrier: Interplay between glucocorticoids and cytokines. J. Neuroendocrinol. 2015, 28, 12360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, J.; Liu, Y.; Guo, T.; Wang, L. Using the lentiviral vector system to stably express chicken p-gp and bcrp in mdck cells for screening the substrates and studying the interplay of both transporters. Arch. Toxicol. 2018, 92, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Parmar, S.; Peng, X.X.; Shen, T.; Robey, R.W.; Bates, S.E.; Fu, L.W.; Shao, Y.; Chen, Y.M.; Zang, F.; et al. The epidermal growth factor tyrosine kinase inhibitor AG1478 and erlotinib reverse ABCG2-mediated drug resistance. Oncol. Rep. 2009, 21, 483–489. [Google Scholar] [PubMed]
- Ismail, M.; Elkattan, Y.A. Comparative pharmacokinetics of florfenicol in the chicken, pigeon and quail. Br. Poult. Sci. 2009, 50, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Martínez, M.A.; Martínez, M.; Ríos, A.; Caballero, V.; Ares, I.; Martínez-Larrañaga, M.R. Plasma and tissue depletion of florfenicol and florfenicol-amine in chickens. J. Agric. Food Chem. 2008, 56, 11049–11056. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Committee for Veterinary Medicinal Products; Florfenicol (Extension to Chicken), Summary Report 3; EMEA/MRL/598/99-FINAL; 1999. Available online: http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500014277 (accessed on 16 August 2018).
- Sunghwan, K.; Thiessen, P.A.; Bolton, E.E.; Jie, C.; Gang, F.; Asta, G.; Han, L.Y.; He, J.; He, S.Q.; et al. Pubchem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, Z.A.; He, F.; Zloh, M.; Yang, J.; Huang, J.; Guo, T.; Wang, L. Use of quercetin in animal feed: Effects on the p-gp expression and pharmacokinetics of orally administrated enrofloxacin in chicken. Sci. Rep. 2018, 8, 4400. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, M.; Kiefer, F.; Biasini, M.; Bordoli, L.; Schwede, T. Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci. Rep. 2017, 7, 10480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L. Swiss-model: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N.M.I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B. Structural basis of small-molecule inhibition of human multidrug transporter abcg2. Nat. Struct. Mol. Biol. 2018, 25, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Osguthorpe, D.J.; Sherman, W.; Hagler, A.T. Exploring Protein Flexibility: Incorporating Structural Ensembles from Crystal Structures and Simulation into Virtual Screening Protocols. J. Phys. Chem. B 2012, 116, 6952–6959. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; p. 43. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA—An open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput. Aided Mol. Des. 2004, 18, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Cell Lines | Inhibitor (Gefitinib) | Papp (×10−6 cm/s) | Efflux Ratio (ER) | p-Values | Net Efflux Ratio (NER) | p-Values | |
|---|---|---|---|---|---|---|---|
| AP→BL | BL→AP | ||||||
| MDCK | − | 0.40 ± 0.04 | 0.40 ± 0.01 | 1.02 ± 0.12 | 0.656 | - | - |
| + | 0.37 ± 0.03 | 0.39 ± 0.01 | 1.06 ± 0.09 | - | |||
| MDCK-chAbcg2 | − | 0.28 ± 0.01 | 0.68 ± 0.04 | 2.40 ± 0.08 | 0.000 | 2.37 ± 0.28 | 0.002 |
| + | 0.37 ± 0.02 | 0.43 ± 0.01 | 1.15 ± 0.02 ** | 1.09 ± 0.09 ** | |||
| Molecule | Docking Score (kcal/mol) | Molecule | Docking Score (kcal/mol) |
|---|---|---|---|
| Substrates | |||
| florfenicol | −8.3 | ciprofloxacin | −9.9 |
| irinotecan | −12.8 | enrofloxacin | −9.4 |
| topotecan | −11.0 | ampicillin | −8.7 |
| lapatinib | −11.0 | rosuvastatin | −8.6 |
| imatinib | −10.9 | mitoxantrone | −8.3 |
| sulfasalazine | −10.7 | clindamycin | −8.0 |
| methotrexate | −10.1 | ||
| Inhibitors | |||
| eltrombopag | −13.0 | elacridar | −12.2 |
| gefitinib | −9.6 | ||
| Parameters | 4-Week Old Broilers | 8-Week Old Broilers | p Values | 8-Week Old Broilers Administered Gefitinib | p-Values |
|---|---|---|---|---|---|
| Cmax (μg/mL) | 3.87 ± 0.96 | 2.93 ± 1.07 | 0.159 | 1.73 ± 0.48 | 0.079 |
| Tmax (h) | 0.63 ± 0.21 | 0.62 ± 0.19 | 0.943 | 2.00 ± 1.41 | 0.064 |
| T1/2β (h) | 3.27 ± 1.20 | 0.94 ± 0.17 * | 0.012 | 3.50 ± 3.35 | 0.224 |
| Vd (L/kg) | 3.42 ± 1.18 | 5.64 ± 0.60 ** | 0.005 | 7.39 ± 2.98 | 0.327 |
| ClB (L/h/kg) | 2.43 ± 0.53 | 4.43 ± 1.46 * | 0.034 | 2.57 ± 0.52 # | 0.048 |
| AUC0~12h (mg·h/L) | 8.58 ± 1.88 | 4.91 ± 1.56 ** | 0.007 | 8.01 ± 1.65 # | 0.023 |
| F (%) | 79.30 | 48.04 | - | 78.38 | - |
| Parameters | 4-Week Old Broilers | 8-Week Old Broilers | p-Values |
|---|---|---|---|
| T1/2β (h) | 1.0 ± 0.85 | 0.79 ± 0.18 | 0.598 |
| Vd (L/kg) | 1.51 ± 0.62 | 1.18 ± 0.29 | 0.310 |
| ClB (L/h/kg) | 1.65 ± 0.54 | 2.14 ± 0.67 | 0.239 |
| AUC0~12h (mg·h/L) | 5.41 ± 0.31 | 5.11 ± 1.89 | 0.741 |
| Gene | Forward (5′–3′) | Reverse (5′–3′) | Length/bp | Tm (°C) |
|---|---|---|---|---|
| β-actin | ATGTGGATCAGCA AGCAGGAGTA | TTTATGCGCATTT ATGGGTTTTGT | 300 | 54.1 |
| Abcg2 | CCTACTTCCTGGCC TTGATGT | TCGGCCTGCTATA GCTTGAAATC | 180 | 56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Guo, L.; Zloh, M.; Zhang, Y.; Huang, J.; Wang, L. Relevance of Breast Cancer Resistance Protein to Pharmacokinetics of Florfenicol in Chickens: A Perspective from In Vivo and In Vitro Studies. Int. J. Mol. Sci. 2018, 19, 3165. https://doi.org/10.3390/ijms19103165
Liu Y, Guo L, Zloh M, Zhang Y, Huang J, Wang L. Relevance of Breast Cancer Resistance Protein to Pharmacokinetics of Florfenicol in Chickens: A Perspective from In Vivo and In Vitro Studies. International Journal of Molecular Sciences. 2018; 19(10):3165. https://doi.org/10.3390/ijms19103165
Chicago/Turabian StyleLiu, Yang, Li Guo, Mire Zloh, Yujuan Zhang, Jinhu Huang, and Liping Wang. 2018. "Relevance of Breast Cancer Resistance Protein to Pharmacokinetics of Florfenicol in Chickens: A Perspective from In Vivo and In Vitro Studies" International Journal of Molecular Sciences 19, no. 10: 3165. https://doi.org/10.3390/ijms19103165






