Blood Pressure Profile and N-Terminal-proBNP Dynamics in Response to Intravenous Methylprednisolone Pulse Therapy of Severe Graves’ Orbitopathy
Abstract
:1. Introduction
2. Results
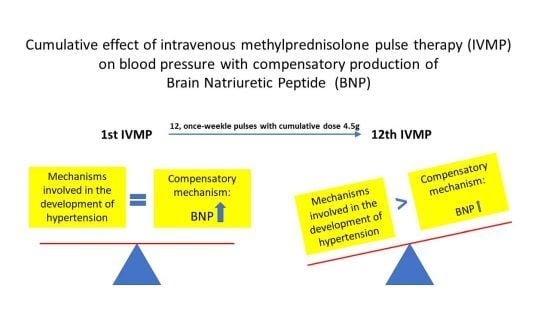
2.1. Ambulatory Blood Pressure Monitoring (48-h)
2.2. Neurohormonal Biomarker NT-proBNP
2.3. Additional Biomarker of Cardiomyocyte Injury—Troponin I (TnI)
2.4. Echocardiography
2.5. Cardiovascular Events
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Design
4.3. Ambulatory BP Monitoring (48-h)
4.4. NT-proBNP Measurements
4.5. Additional Laboratory Measurements
4.6. Echocardiography
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABPM | ambulatory blood pressure monitoring |
| BMI | body mass index |
| BP | blood pressure |
| BNP | brain natriuretic peptide |
| DBP | diastolic blood pressure |
| EF | ejection fraction |
| EUGOGO | European Group On Graves’ Orbitopathy |
| GCs | glucocorticoids |
| GO | Graves’ orbitopathy |
| IVMP | intravenous methylprednisolone |
| MP | methylprednisolone |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| SBP | systolic blood pressure |
| TnI | troponin I |
References
- Miśkiewicz, P.; Kryczka, A.; Ambroziak, U.; Rutkowska, B.; Główczyńska, R.; Opolski, G.; Kahaly, G.; Bednarczuk, T. Is high dose intravenous methylprednisolone pulse therapy in patients with graves’ orbitopathy safe? Endokrynol. Pol. 2014, 65, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Baldeschi, L.; Boboridis, K.; Eckstein, A.; Kahaly, G.J.; Marcocci, C.; Perros, P.; Salvi, M.; Wiersinga, W.M.; European Group of Graves’s Orbitopathy. The 2016 european thyroid association/european group on graves’ orbitopathy guidelines for the management of graves’ orbitopathy. Eur. Thyroid. J. 2016, 5, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, C.; Watt, T.; Altea, M.A.; Rasmussen, A.K.; Feldt-Rasmussen, U.; Orgiazzi, J.; Bartalena, L.; European Group of Graves’s Orbitopathy. Fatal and non-fatal adverse events of glucocorticoid therapy for graves’ orbitopathy: A questionnaire survey among members of the european thyroid association. Eur. J. Endocrinol. 2012, 166, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Ponto, K.A.; Kahaly, G.J. Clinical review: Intravenous glucocorticoids for graves’ orbitopathy: Efficacy and morbidity. J. Clin. Endocrinol. Metab. 2011, 96, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, A.; Cesur, M.; Erdogan, M.F.; Corapcioglu, D.; Kamel, N. New-onset acute heart failure after intravenous glucocorticoid pulse therapy in a patient with graves’ ophthalmopathy. Endocrine 2006, 29, 513–516. [Google Scholar] [CrossRef]
- Owecki, M.; Sowiński, J. Acute myocardial infarction during high-dose methylprednisolone therapy for graves’ ophthalmopathy. Pharm. World Sci. 2006, 28, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.A.; Gordon, D.; Andrews, J.; Scoggins, B.A. The hypertensive effect of synthetic glucocorticoids in man: Role of sodium and volume. J. Hypertens. 1989, 7, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Suzuki, H.; Murakami, M.; Nakazato, Y.; Iwaita, Y.; Saruta, T. Glucocorticoid increases angiotensin ii type 1 receptor and its gene expression. Hypertension 1994, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Graziadio, C.; Paragliola, R.M.; Cozzolino, A.; Ambrogio, A.G.; Colao, A.; Corsello, S.M.; Pivonello, R.; Group, A.S. The hypertension of cushing’s syndrome: Controversies in the pathophysiology and focus on cardiovascular complications. J. Hypertens. 2015, 33, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.L.; Chng, C.L.; Htoon, H.M.; Lim, L.H.; Seah, L.L. Safety profile and effects of pulsed methylprednisolone on vital signs in thyroid eye disease. Int. J. Endocrinol. 2015, 2015, 457123. [Google Scholar] [CrossRef] [PubMed]
- Fardet, L.; Nazareth, I.; Petersen, I. Synthetic glucocorticoids and early variations of blood pressure: A population-based cohort study. J. Clin. Endocrinol. Metab. 2015, 100, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Valassi, E.; Santos, A.; Yaneva, M.; Tóth, M.; Strasburger, C.J.; Chanson, P.; Wass, J.A.; Chabre, O.; Pfeifer, M.; Feelders, R.A.; et al. The european registry on cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur. J. Endocrinol. 2011, 165, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, T.G.; Vase, H.; Bech, J.N.; Nielsen, S.; Pedersen, E.B. Direct effect of methylprednisolone on renal sodium and water transport via the principal cells in the kidney. Eur. J. Endocrinol. 2010, 162, 961–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brotman, D.J.; Girod, J.P.; Garcia, M.J.; Patel, J.V.; Gupta, M.; Posch, A.; Saunders, S.; Lip, G.Y.; Worley, S.; Reddy, S. Effects of short-term glucocorticoids on cardiovascular biomarkers. J. Clin. Endocrinol. Metab. 2005, 90, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Abe, K.; Sasaki, S.; Minami, N.; Nihei, M.; Munakata, M.; Murakami, O.; Matsue, K.; Sekino, H.; Miura, Y. Altered circadian blood pressure rhythm in patients with cushing’s syndrome. Hypertension 1988, 12, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Fisher, L.D.; Chiang, Y.T.; Latini, R.; Masson, S.; Maggioni, A.P.; Glazer, R.D.; Tognoni, G.; Cohn, J.N.; Investigators, V.-H. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the valsartan heart failure trial (val-heft). Circulation 2003, 107, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Spannella, F.; Giulietti, F.; Balietti, P.; Cocci, G.; Bordicchia, M. Cardiac natriuretic peptides, hypertension and cardiovascular risk. High Blood Press. Cardiovasc. Prev. 2017, 24, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Gaggin, H.K.; Januzzi, J.L. Biomarkers and diagnostics in heart failure. Biochim. Biophys. Acta 2013, 1832, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Dananberg, J.; Grekin, R.J. Corticoid regulation of atrial natriuretic factor secretion and gene expression. Am. J. Physiol. 1992, 263, H1377–H1381. [Google Scholar] [CrossRef] [PubMed]
- Iorio, L.; Rigolini, R.; Costa, E.; Cotta, O.; Cannavò, S.; Ambrosi, B. N-terminal pro-brain natriuretic peptide determination as a possible marker of cardiac dysfunction in patients with adrenal disorders. J. Endocrinol. Investig. 2010, 33, 509–510. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Parati, G.; Stergiou, G.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European society of hypertension position paper on ambulatory blood pressure monitoring. J. Hypertens. 2013, 31, 1731–1768. [Google Scholar]
- De Filippi, C.R.; Christenson, R.H.; Kop, W.J.; Gottdiener, J.S.; Zhan, M.; Seliger, S.L. Left ventricular ejection fraction assessment in older adults: An adjunct to natriuretic peptide testing to identify risk of new-onset heart failure and cardiovascular death? J. Am. Coll. Cardiol. 2011, 58, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]




| IVMP Pulse | Before Pulse Median (Q1–Q3, mmHg) | After Pulse Median (Q1–Q3, mmHg) | p (Before Pulse vs. After Pulse) |
|---|---|---|---|
| Mean BP | |||
| 1st | 84 (75–88) | 83 (77–87) | 0.64 |
| 6th | 81 (77–85) | 83 (75–87) | 0.40 |
| 12th | 82 (78–85) | 83 (79–88) | 0.23 |
| Mean nocturnal BP | |||
| 1st | 73 (68–81) | 76 (72–80) | 0.39 |
| 6th | 73 (67–80) | 72 (67–84) | 0.24 |
| 12th | 75 (69–79) | 80 (74–85) | 0.005 |
| Maximal SBP | |||
| 1st | 147 (142–162) | 154 (143–165) | 0.37 |
| 6th | 148 (138–155) | 157 (138–161) | 0.21 |
| 12th | 146 (134–159) | 161 (145–171) | 0.01 |
| IVMP Pulse | Before Pulse N (%) | After Pulse N (%) | p (Before Pulse vs. After Pulse) |
|---|---|---|---|
| 1st | 10 (50) | 7 (35) | 0.77 |
| 6th | 9 (45) | 8 (40) | 0.58 |
| 12th | 13 (65) | 2 (10) | 0.45 |
| p (1st vs. 6th) 1.00, p (1st vs. 12th) 0.50 | p (1st vs. 6th) 0.37, p (1st vs. 12th) 0.02 |
| IVMP Pulse | Before Pulse Median (Q1–Q3), pg/mL | After 24 h Median (Q1–Q3), pg/mL | After 48 h Median (Q1–Q3), pg/mL | p (Before Pulse vs. After 24 h) | p (Before Pulse vs. After 48 h) |
|---|---|---|---|---|---|
| 1st | 70 (39–98) | 203 (100–310) | 187 (111–235) | 0.000003 | 0.000005 |
| 6th | 32 (24–62) | 150 (91–225) | 141 (98–212) | 0.000002 | 0.000011 |
| 12th | 38 (23–60) | 134 (78–190) | 96 (64–216) | 0.000004 | 0.000012 |
| Male, % (N) | 38 (12) |
| Age, years | 52 ± 11 |
| Etiology of GO | |
| GD, % (N) | 78 (25) |
| AITD, % (N) | 22 (7) |
| Smoking | |
| Current, % (N) | 53 (17) |
| Former, %(N) | 28 (9) |
| Never, % (N) | 19 (6) |
| CAS (range 1–7) | 4 ± 2 |
| BMI (kg/m2) | 26.0 ± 4.1 |
| Comorbidities | |
| Hypertension, % (N) | 41 (13) |
| diabetes % (N) | 0 (0) |
| CHD, % (N) | 0 (0) |
| Medications | |
| β-blockers, % (N) | 41 (13) |
| ACEI, % (N) | 25 (8) |
| ARB, % (N) | 6 (2) |
| Ca-blockers, % (N) | 19 (6) |
| Statins, % (N) | 16 (5) |
| Diuretics, % (N) | 9 (3) |
| L-thyroxine, % (N) | 66 (21) |
| Thyrostatics, % (N) | 44 (14) |
| Laboratory measurements | |
| TSH (range: 0.27–4.2 µIU/mL) | 1.6 ± 1.6 |
| fT4 (range: 12–22 pmol/L) | 16.8 ± 3.5 |
| TBII (N < 1.73 IU/L) | 9.8 ± 11.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miskiewicz, P.; Milczarek-Banach, J.; Bednarczuk, T.; Opolski, G.; Glowczynska, R. Blood Pressure Profile and N-Terminal-proBNP Dynamics in Response to Intravenous Methylprednisolone Pulse Therapy of Severe Graves’ Orbitopathy. Int. J. Mol. Sci. 2018, 19, 2918. https://doi.org/10.3390/ijms19102918
Miskiewicz P, Milczarek-Banach J, Bednarczuk T, Opolski G, Glowczynska R. Blood Pressure Profile and N-Terminal-proBNP Dynamics in Response to Intravenous Methylprednisolone Pulse Therapy of Severe Graves’ Orbitopathy. International Journal of Molecular Sciences. 2018; 19(10):2918. https://doi.org/10.3390/ijms19102918
Chicago/Turabian StyleMiskiewicz, Piotr, Justyna Milczarek-Banach, Tomasz Bednarczuk, Grzegorz Opolski, and Renata Glowczynska. 2018. "Blood Pressure Profile and N-Terminal-proBNP Dynamics in Response to Intravenous Methylprednisolone Pulse Therapy of Severe Graves’ Orbitopathy" International Journal of Molecular Sciences 19, no. 10: 2918. https://doi.org/10.3390/ijms19102918