Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases
Abstract
:1. Introduction
2. Recognizing Hepatic Stem/Progenitor Cells (HpSCs) and Their Progeny in Human Liver
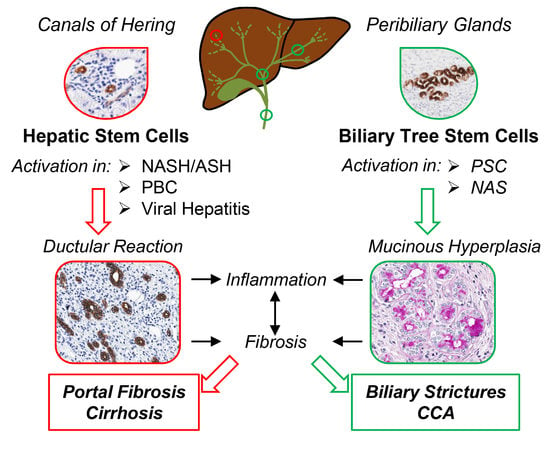
3. HpSCs in Human Liver Diseases Targeting Hepatocytes
4. HpSCs in Human Liver Diseases Targeting Biliary Epithelium
5. Supporting HpSC Response: The Niche and Signaling Pathways
6. Biliary Tree Stem/Progenitor Cells (BTSCs)
7. BTSC and Peribiliary Glands (PBG) Involvement in Human Pathologies
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lanzoni, G.; Cardinale, V.; Carpino, G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration. Hepatology 2016, 64, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, D.; Gaudio, E. Liver Capsule: Biliary Tree Stem Cell Subpopulations. Hepatology 2016, 64, 644. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.M. Stem/progenitor cells and reprogramming (plasticity) mechanisms in liver, biliary tree, and pancreas. Hepatology 2016, 64, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhao, L.; Fish, M.; Logan, C.Y.; Nusse, R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 2015, 524, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Font-Burgada, J.; Shalapour, S.; Ramaswamy, S.; Hsueh, B.; Rossell, D.; Umemura, A.; Taniguchi, K.; Nakagawa, H.; Valasek, M.A.; Ye, L.; et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 2015, 162, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Nascimento, E.M.; Gajera, C.R.; Chen, L.; Neuhofer, P.; Garbuzov, A.; Wang, S.; Artandi, S.E. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature 2018, 556, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; McGill, J.M.; Larusso, N.F. The pathobiology of biliary epithelia. Hepatology 2002, 35, 1256–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, S.S.; Gaudio, E.; Rao, A.; Pierce, L.M.; Onori, P.; Franchitto, A.; Francis, H.L.; Dostal, D.E.; Venter, J.K.; DeMorrow, S.; et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab. Investig. 2009, 89, 456–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, J.L.; Grompe, M.; Sander, M. Stem cells versus plasticity in liver and pancreas regeneration. Nat. Cell Biol. 2016, 18, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Schaub, J.R.; Huppert, K.A.; Kurial, S.N.T.; Hsu, B.Y.; Cast, A.E.; Donnelly, B.; Karns, R.A.; Chen, F.; Rezvani, M.; Luu, H.Y.; et al. De novo formation of the biliary system by TGF-β-mediated hepatocyte transdifferentiation. Nature 2018, 557, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, X.; Li, W.; Feng, R.X.; Li, L.; Yi, G.R.; Zhang, X.N.; Yin, C.; Yu, H.Y.; Zhang, J.P.; et al. Chronic Liver Injury Induces Conversion of Biliary Epithelial Cells into Hepatocytes. Cell Stem Cell 2018, 23, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.M. Paradoxes in studies of liver regeneration: Relevance of the parable of the blind men and the elephant. Hepatology 2015, 62, 330–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuyama, K.; Kawaguchi, Y.; Akiyama, H.; Horiguchi, M.; Kodama, S.; Kuhara, T.; Hosokawa, S.; Elbahrawy, A.; Soeda, T.; Koizumi, M.; et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 2011, 43, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Malato, Y.; Naqvi, S.; Schurmann, N.; Ng, R.; Wang, B.; Zape, J.; Kay, M.A.; Grimm, D.; Willenbring, H. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J. Clin. Investig. 2011, 121, 4850–4860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espanol-Suner, R.; Carpentier, R.; Van Hul, N.; Legry, V.; Achouri, Y.; Cordi, S.; Jacquemin, P.; Lemaigre, F.; Leclercq, I.A. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 2012, 143, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Y.; Bird, T.G.; Boulter, L.; Tsuchiya, A.; Cole, A.M.; Hay, T.; Guest, R.V.; Wojtacha, D.; Man, T.Y.; Mackinnon, A.; et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 2015, 17, 971–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raven, A.; Lu, W.Y.; Man, T.Y.; Ferreira-Gonzalez, S.; O’Duibhir, E.; Dwyer, B.J.; Thomson, J.P.; Meehan, R.R.; Bogorad, R.; Koteliansky, V.; et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature 2017, 547, 350–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dipaola, F.; Shivakumar, P.; Pfister, J.; Walters, S.; Sabla, G.; Bezerra, J.A. Identification of intramural epithelial networks linked to peribiliary glands that express progenitor cell markers and proliferate after injury in mice. Hepatology 2013, 58, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.M.; Jonsson, J.R.; Powell, E.E.; Brunt, E.M.; Neuschwander-Tetri, B.A.; Bhathal, P.S.; Dixon, J.B.; Weltman, M.D.; Tilg, H.; Moschen, A.R.; et al. Progressive fibrosis in nonalcoholic steatohepatitis: Association with altered regeneration and a ductular reaction. Gastroenterology 2007, 133, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Onori, P.; Alvaro, D.; Floreani, A.R.; Mancino, M.G.; Franchitto, A.; Guido, M.; Carpino, G.; De Santis, A.; Angelico, M.; Attili, A.F.; et al. Activation of the IGF1 system characterizes cholangiocyte survival during progression of primary biliary cirrhosis. J. Histochem. Cytochem. 2007, 55, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Sasaki, M.; Harada, K. Autophagy and senescence in fibrosing cholangiopathies. J. Hepatol. 2015, 62, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Gouw, A.S.; Clouston, A.D.; Theise, N.D. Ductular reactions in human liver: Diversity at the interface. Hepatology 2011, 54, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Theise, N.D.; Kuwahara, R. The tissue biology of ductular reactions in human chronic liver disease. Gastroenterology 2007, 133, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Roskams, T.A.; Theise, N.D.; Balabaud, C.; Bhagat, G.; Bhathal, P.S.; Bioulac-Sage, P.; Brunt, E.M.; Crawford, J.M.; Crosby, H.A.; Desmet, V.; et al. Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology 2004, 39, 1739–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelzer, E.; Zhang, L.; Bruce, A.; Wauthier, E.; Ludlow, J.; Yao, H.L.; Moss, N.; Melhem, A.; McClelland, R.; Turner, W.; et al. Human hepatic stem cells from fetal and postnatal donors. J. Exp. Med. 2007, 204, 1973–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpentier, R.; Suner, R.E.; Van Hul, N.; Kopp, J.L.; Beaudry, J.B.; Cordi, S.; Antoniou, A.; Raynaud, P.; Lepreux, S.; Jacquemin, P.; et al. Embryonic Ductal Plate Cells Give Rise to Cholangiocytes, Periportal Hepatocytes, and Adult Liver Progenitor Cells. Gastroenterology 2011, 141, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, E.; Carpino, G.; Cardinale, V.; Franchitto, A.; Onori, P.; Alvaro, D. New insights into liver stem cells. Dig. Liver Dis. 2009, 41, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Libbrecht, L.; Desmet, V.; Van Damme, B.; Roskams, T. The immunohistochemical phenotype of dysplastic foci in human liver: Correlation with putative progenitor cells. J. Hepatol. 2000, 33, 76–84. [Google Scholar] [CrossRef]
- Spee, B.; Carpino, G.; Schotanus, B.A.; Katoonizadeh, A.; Vander Borght, S.; Gaudio, E.; Roskams, T. Characterisation of the liver progenitor cell niche in liver diseases: Potential involvement of Wnt and Notch signalling. Gut 2010, 59, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Boulter, L.; Lu, W.Y.; Forbes, S.J. Differentiation of progenitors in the liver: A matter of local choice. J. Clin Investig. 2013, 123, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.; Clouston, A.D.; Forbes, S.J. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 2014, 146, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular reaction in liver diseases: Pathological mechanisms and translational significances. Hepatology 2018. [Google Scholar] [CrossRef] [PubMed]
- Stueck, A.E.; Wanless, I.R. Hepatocyte buds derived from progenitor cells repopulate regions of parenchymal extinction in human cirrhosis. Hepatology 2015, 61, 1696–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, M.J.; Gadd, V.L.; Powell, L.W.; Ramm, G.A.; Clouston, A.D. Ductular reaction in hereditary hemochromatosis: The link between hepatocyte senescence and fibrosis progression. Hepatology 2014, 59, 848–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.M.; Gerasimidou, D.; Kuwahara, R.; Hytiroglou, P.; Yoo, J.E.; Park, Y.N.; Theise, N.D. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 2011, 53, 964–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinale, V.; Wang, Y.; Carpino, G.; Mendel, G.; Alpini, G.; Gaudio, E.; Reid, L.M.; Alvaro, D. The biliary tree—A reservoir of multipotent stem cells. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, J.B.; Kim, K.H.; Lee, W.R.; Kim, J.Y.; An, H.J.; Park, K.K. Immunohistochemical study for the origin of ductular reaction in chronic liver disease. Int. J. Clin. Exp. Pathol. 2014, 7, 4076–4085. [Google Scholar] [PubMed]
- Dezso, K.; Rokusz, A.; Bugyik, E.; Szucs, A.; Szuak, A.; Dorogi, B.; Kiss, M.; Nemeskeri, A.; Nagy, P.; Paku, S. Human liver regeneration in advanced cirrhosis is organized by the portal tree. J. Hepatol. 2017, 66, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Carpino, G.; Alisi, A.; Franchitto, A.; Alpini, G.; De Vito, R.; Onori, P.; Alvaro, D.; Gaudio, E. Hepatic progenitor cells activation, fibrosis and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology 2012, 56, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Gadd, V.L.; Skoien, R.; Powell, E.E.; Fagan, K.J.; Winterford, C.; Horsfall, L.; Irvine, K.; Clouston, A.D. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 2014, 59, 1393–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpino, G.; Pastori, D.; Baratta, F.; Overi, D.; Labbadia, G.; Polimeni, L.; Di Costanzo, A.; Pannitteri, G.; Carnevale, R.; Del Ben, M.; et al. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: A possible role for oxidative stress. Sci. Rep. 2017, 7, 15756. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alisi, A.; Cutrera, R.; Carpino, G.; De Stefanis, C.; D’Oria, V.; De Vito, R.; Cucchiara, S.; Gaudio, E.; Musso, G. Altered gut-liver axis and hepatic adiponectin expression in OSAS: Novel mediators of liver injury in paediatric non-alcoholic fatty liver. Thorax 2015, 70, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Carpino, G.; Alisi, A.; De Vito, R.; Franchitto, A.; Alpini, G.; Onori, P.; Gaudio, E. Role of docosahexaenoic acid treatment in improving liver histology in pediatric nonalcoholic fatty liver disease. PLoS ONE 2014, 9, e88005. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Nobili, V.; Renzi, A.; De Stefanis, C.; Stronati, L.; Franchitto, A.; Alisi, A.; Onori, P.; De Vito, R.; Alpini, G.; et al. Macrophage Activation in Pediatric Nonalcoholic Fatty Liver Disease (NAFLD) Correlates with Hepatic Progenitor Cell Response via Wnt3a Pathway. PLoS ONE 2016, 11, e0157246. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, C.; Carpino, G.; De Vito, R.; De Stefanis, C.; Alisi, A.; Cianfarani, S.; Overi, D.; Mosca, A.; Stronati, L.; Cucchiara, S.; et al. Docosahexanoic Acid Plus Vitamin D Treatment Improves Features of NAFLD in Children with Serum Vitamin D Deficiency: Results from a Single Centre Trial. PLoS ONE 2016, 11, e0168216. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Carpino, G.; De Peppo, F.; Caccamo, R.; Mosca, A.; Romito, I.; Overi, D.; Franchitto, A.; Onori, P.; Alisi, A.; et al. Laparoscopic Sleeve Gastrectomy Improves Nonalcoholic Fatty Liver Disease-Related Liver Damage in Adolescents by Reshaping Cellular Interactions and Hepatic Adipocytokine Production. J. Pediatr. 2018, 194, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Maiwall, R.; Bihari, C.; Trehanpati, N.; Pamecha, V.; Sarin, S.K. Two-tier regenerative response in liver failure in humans. Virchows Arch. 2014, 464, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Katoonizadeh, A.; Nevens, F.; Verslype, C.; Pirenne, J.; Roskams, T. Liver regeneration in acute severe liver impairment: A clinicopathological correlation study. Liver Int. 2006, 26, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Bru, P.; Altamirano, J.; Rodrigo-Torres, D.; Coll, M.; Millan, C.; Jose Lozano, J.; Miquel, R.; Arroyo, V.; Caballeria, J.; Gines, P.; et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology 2012, 55, 1931–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanthier, N.; Rubbia-Brandt, L.; Lin-Marq, N.; Clement, S.; Frossard, J.L.; Goossens, N.; Hadengue, A.; Spahr, L. Hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis. J. Hepatol. 2015, 63, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Dubuquoy, L.; Louvet, A.; Lassailly, G.; Truant, S.; Boleslawski, E.; Artru, F.; Maggiotto, F.; Gantier, E.; Buob, D.; Leteurtre, E.; et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut 2015, 64, 1949–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odena, G.; Chen, J.; Lozano, J.J.; Altamirano, J.; Rodrigo-Torres, D.; Affo, S.; Morales-Ibanez, O.; Matsushita, H.; Zou, J.; Dumitru, R.; et al. LPS-TLR4 Pathway Mediates Ductular Cell Expansion in Alcoholic Hepatitis. Sci. Rep. 2016, 6, 35610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpino, G.; Cardinale, V.; Folseraas, T.; Overi, D.; Floreani, A.; Franchitto, A.; Onori, P.; Cazzagon, N.; Berloco, P.B.; Karlsen, T.H.; et al. Hepatic Stem/Progenitor Cell Activation Differs between Primary Sclerosing and Primary Biliary Cholangitis. Am. J. Pathol. 2018, 188, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Cadamuro, M.; Guido, M.; Spirli, C.; Fiorotto, R.; Colledan, M.; Torre, G.; Alberti, D.; Sonzogni, A.; Okolicsanyi, L.; et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am. J. Pathol. 2007, 171, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Mavila, N.; James, D.; Shivakumar, P.; Nguyen, M.V.; Utley, S.; Mak, K.; Wu, A.; Zhou, S.; Wang, L.; Vendyres, C.; et al. Expansion of prominin-1-expressing cells in association with fibrosis of biliary atresia. Hepatology 2014, 60, 941–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, M.; Nardi, A.; Flack, S.; Carpino, G.; Varvaropoulou, N.; Gavrila, C.; Spicer, A.; Badrock, J.; Bernuzzi, F.; Cardinale, V.; et al. Pretreatment prediction of response to ursodeoxycholic acid in primary biliary cholangitis: Development and validation of the UDCA Response Score. Lancet Gastroenterol. Hepatol. 2018, 3, 626–634. [Google Scholar] [CrossRef]
- Boulter, L.; Govaere, O.; Bird, T.G.; Radulescu, S.; Ramachandran, P.; Pellicoro, A.; Ridgway, R.A.; Seo, S.S.; Spee, B.; Van Rooijen, N.; et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 2012, 18, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Chen, C.C.; Alpini, G.; Lau, L.F. CCN1 induces hepatic ductular reaction through integrin alphavbeta(5)-mediated activation of NF-kappaB. J. Clin. Investig. 2015, 125, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.G.; Lu, W.Y.; Boulter, L.; Gordon-Keylock, S.; Ridgway, R.A.; Williams, M.J.; Taube, J.; Thomas, J.A.; Wojtacha, D.; Gambardella, A.; et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 6542–6547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubowski, A.; Ambrose, C.; Parr, M.; Lincecum, J.M.; Wang, M.Z.; Zheng, T.S.; Browning, B.; Michaelson, J.S.; Baetscher, M.; Wang, B.; et al. TWEAK induces liver progenitor cell proliferation. J. Clin. Investig. 2005, 115, 2330–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzelak, C.A.; Martelotto, L.G.; Sigglekow, N.D.; Patkunanathan, B.; Ajami, K.; Calabro, S.R.; Dwyer, B.J.; Tirnitz-Parker, J.E.; Watkins, D.N.; Warner, F.J.; et al. The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury. J. Hepatol. 2014, 60, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lopategi, A.; Ge, X.; Lu, Y.; Kitamura, N.; Urtasun, R.; Leung, T.M.; Fiel, M.I.; Nieto, N. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut 2014, 63, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.D.; Swiderska-Syn, M.; Dolle, L.; Reid, D.; Eksteen, B.; Claridge, L.; Briones-Orta, M.A.; Shetty, S.; Oo, Y.H.; Riva, A.; et al. Osteopontin neutralisation abrogates the liver progenitor cell response and fibrogenesis in mice. Gut 2015, 64, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, L.; Chen, R.; Zhou, X.; Fan, X.; Liang, Y.; Jia, R.; Wang, H.; Liu, G.; Guo, Y.; et al. Osteopontin Promotes Hepatic Progenitor Cell Expansion and Tumorigenicity via Activation of beta-Catenin in Mice. Stem Cells 2015, 33, 3508–3569. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Diehl, A.M. Evidence for and against epithelial-to-mesenchymal transition in the liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G881–G890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syn, W.K.; Jung, Y.; Omenetti, A.; Abdelmalek, M.; Guy, C.D.; Yang, L.; Wang, J.; Witek, R.P.; Fearing, C.M.; Pereira, T.A.; et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology 2009, 137, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, S.; Bird, T.G.; Boulter, L.; Bellamy, C.; Samuel, K.; Aucott, R.; Clayton, E.; Andreone, P.; Bernardi, M.; Golding, M.; et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut 2010, 59, 645–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallis, Y.N.; Robson, A.J.; Fallowfield, J.A.; Thomas, H.C.; Alison, M.R.; Wright, N.A.; Goldin, R.D.; Iredale, J.P.; Forbes, S.J. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut 2011, 60, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Mackinnon, A.C.; Lu, W.Y.; Jung, J.; Boulter, L.; Henderson, N.C.; Simpson, K.J.; Schotanus, B.; Wojtacha, D.; Bird, T.G.; et al. Galectin-3 regulates hepatic progenitor cell expansion during liver injury. Gut 2015, 64, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Lu, W.Y.; Weinhold, B.; Boulter, L.; Stutchfield, B.M.; Williams, M.J.; Guest, R.V.; Minnis-Lyons, S.E.; MacKinnon, A.C.; Schwarzer, D.; et al. Polysialic acid/neural cell adhesion molecule modulates the formation of ductular reactions in liver injury. Hepatology 2014, 60, 1727–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Fuchs, E. Stretching the limits: From homeostasis to stem cell plasticity in wound healing and cancer. Nat. Rev. Genet. 2018, 19, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Wang, Y.; Carpino, G.; Cui, C.B.; Gatto, M.; Rossi, M.; Bartolomeo Berloco, P.; Cantafora, A.; Wauthier, E.; Furth, M.E.; et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 2011, 54, 2159–2172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lanzoni, G.; Carpino, G.; Cui, C.B.; Dominguez-Bendala, J.; Wauthier, E.; Cardinale, V.; Oikawa, T.; Pileggi, A.; Gerber, D.; et al. Biliary tree stem cells, precursors to pancreatic committed progenitors: Evidence for possible life-long pancreatic organogenesis. Stem Cells 2013, 31, 1966–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, S.; Sato, Y.; Ren, X.S.; Harada, K.; Sasaki, M.; Nakanuma, Y. Participation of peribiliary glands in biliary tract pathophysiologies. World J. Hepatol. 2013, 5, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpino, G.; Cardinale, V.; Onori, P.; Franchitto, A.; Berloco, P.B.; Rossi, M.; Wang, Y.; Semeraro, R.; Anceschi, M.; Brunelli, R.; et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: An anatomical in situ study yielding evidence of maturational lineages. J. Anat. 2012, 220, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Puca, R.; Cardinale, V.; Renzi, A.; Scafetta, G.; Nevi, L.; Rossi, M.; Berloco, P.B.; Ginanni Corradini, S.; et al. Peribiliary Glands as a Niche of Extrapancreatic Precursors Yielding Insulin-Producing Cells in Experimental and Human Diabetes. Stem Cells 2016, 34, 1332–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevi, L.; Cardinale, V.; Carpino, G.; Costantini, D.; Di Matteo, S.; Cantafora, A.; Melandro, F.; Brunelli, R.; Bastianelli, C.; Aliberti, C.; et al. Cryopreservation protocol for human biliary tree stem/progenitors, hepatic and pancreatic precursors. Sci. Rep. 2017, 7, 6080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevi, L.; Carpino, G.; Costantini, D.; Cardinale, V.; Riccioni, O.; Di Matteo, S.; Melandro, F.; Berloco, P.B.; Reid, L.; Gaudio, E.; et al. Hyaluronan coating improves liver engraftment of transplanted human biliary tree stem/progenitor cells. Stem Cell Res. Ther. 2017, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Cardinale, V.; Gentile, R.; Onori, P.; Semeraro, R.; Franchitto, A.; Wang, Y.; Bosco, D.; Iossa, A.; Napoletano, C.; et al. Evidence for multipotent endodermal stem/progenitor cell populations in human gallbladder. J. Hepatol. 2014, 60, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Carpino, G.; Gentile, R.; Napoletano, C.; Rahimi, H.; Franchitto, A.; Semeraro, R.; Nuti, M.; Onori, P.; Berloco, P.B.; et al. Transplantation of human fetal biliary tree stem/progenitor cells into two patients with advanced liver cirrhosis. BMC Gastroenterol. 2014, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Cardinale, V.; Renzi, A.; Hov, J.R.; Berloco, P.B.; Rossi, M.; Karlsen, T.H.; Alvaro, D.; Gaudio, E. Activation of biliary tree stem cells within peribiliary glands in primary sclerosing cholangitis. J. Hepatol. 2015, 63, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Cardinale, V.; Folseraas, T.; Overi, D.; Grzyb, K.; Costantini, D.; Berloco, P.B.; Di Matteo, S.; Karlsen, T.H.; Alvaro, D.; et al. Neoplastic transformation of peribiliary stem cell niche in cholangiocarcinoma arisen in primary sclerosing cholangitis. Hepatology 2018. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Jing, J.; Lee, K.B.; Jang, J.J. Sonic hedgehog (SHH) and glioblastoma-2 (Gli-2) expressions are associated with poor jaundice-free survival in biliary atresia. J. Pediatr. Surg. 2015, 50, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Goossens, N.; Breguet, R.; De Vito, C.; Terraz, S.; Lin-Marq, N.; Giostra, E.; Rubbia-Brandt, L.; Spahr, L. Peribiliary Gland Dilatation in Cirrhosis: Relationship with Liver Failure and Stem Cell/Proliferation Markers. Dig. Dis. Sci. 2017, 62, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.; Lozoya, O.; Wang, Y.; Cardinale, V.; Gaudio, E.; Alpini, G.; Mendel, G.; Wauthier, E.; Barbier, C.; Alvaro, D.; et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011, 53, 1035–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- op den Dries, S.; Westerkamp, A.C.; Karimian, N.; Gouw, A.S.; Bruinsma, B.G.; Markmann, J.F.; Lisman, T.; Yeh, H.; Uygun, K.; Martins, P.N.; et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J. Hepatol. 2014, 60, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.; van Leeuwen, O.B.; Matton, A.P.M.; Burlage, L.C.; Wiersema-Buist, J.; van den Heuvel, M.C.; de Kleine, R.H.J.; de Boer, M.T.; Gouw, A.S.H.; Porte, R.J. Hypothermic oxygenated machine perfusion reduces bile duct reperfusion injury after transplantation of donation after circulatory death livers. Liver Transpl. 2018, 24, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Marker | Hepatocytes | Intermediate Hepatocytes | HpSCs | Immature Cholangiocytes | Cholangiocytes |
|---|---|---|---|---|---|
| CK7 | − | + | + | + | + |
| CK19 | − | +/− | + | + | + |
| EpCAM | − | + | + | + | − |
| Sox9 | − | − | + | + | − |
| CD133 | − | − | + | − | − |
| Lgr5 | − | − | + | − | − |
| NCAM | − | − | + | + | − |
| Albumin | + | + | +/− | − | − |
| HepPar-1 | + | + | − | − | − |
| HNF4α | + | + | − | − | − |
| CFTR | − | − | − | − | + |
| AE2 | − | − | − | − | + |
| SCTR | − | − | − | − | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Overi, D.; Carpino, G.; Cardinale, V.; Franchitto, A.; Safarikia, S.; Onori, P.; Alvaro, D.; Gaudio, E. Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases. Int. J. Mol. Sci. 2018, 19, 2917. https://doi.org/10.3390/ijms19102917
Overi D, Carpino G, Cardinale V, Franchitto A, Safarikia S, Onori P, Alvaro D, Gaudio E. Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases. International Journal of Molecular Sciences. 2018; 19(10):2917. https://doi.org/10.3390/ijms19102917
Chicago/Turabian StyleOveri, Diletta, Guido Carpino, Vincenzo Cardinale, Antonio Franchitto, Samira Safarikia, Paolo Onori, Domenico Alvaro, and Eugenio Gaudio. 2018. "Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases" International Journal of Molecular Sciences 19, no. 10: 2917. https://doi.org/10.3390/ijms19102917