Study of Lignin-Modified Silica Gel Adsorption after Association with Six Different Organophenylmercuric Compounds in Chloroform
Abstract
:1. Introduction
2. Results and Discussion
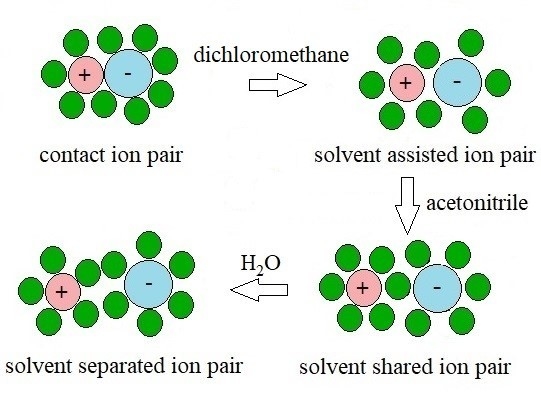
2.1. Mechanism Leading to Adsorption
2.2. Effect of An Additive in the Liquid Phase on Adsorption
2.3. Effects of Functional Group and Size of Analyte on Adsorption
3. Material and Methods
3.1. Apparatus
3.2. Chemicals
3.3. Conditions for Measuring the Proportion of Adsorption and the Adsorption Capacity
3.4. Theoretical Computations Performed with Spartan 14 Software
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mergler, D.; Anderson, H.A.; Chan, L.H.M.; Mahaffey, K.R.; Murray, M.; Sakamoto, M.; Stern, A.H. Methylmercury exposure and health effects in humans: A worldwide concern. AMBIO 2007, 36, 3–11. [Google Scholar] [CrossRef]
- Passos, C.J.S.; Mergler, D.; Lemire, M.; Fillion, M.; Guimarães, J.R.D. Fish consumption and bioindicators of inorganic mercury exposure. Sci. Total Environ. 2007, 373, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Philibert, A.; Bouchard, M.; Mergler, D. Neuropsychiatric symptoms, omega-3, and mercury exposure in freshwater fish-eaters. Arch. Environ. Occup. Health 2008, 63, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Potera, C. Marine and coastal science: Ocean currents key to methylmercury in North Pacific. Environ. Health Perspect. 2009, 117, A345. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Krabbenhoft, D.P.; Moreau, J.W.; Strode, S.A.; Landing, W.M. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Glob. Biogeochem. Cycles 2009, 23, 1–14. [Google Scholar] [CrossRef]
- Bai, X.; Fan, Z. 3-Mercaptopropyltrimethoxysilane coated capillary micro-extraction coupled to capillary electrophoresis for the determination of methylmercury, phenylmercury and mercury in biological sample. Microchim. Acta 2010, 170, 107–112. [Google Scholar] [CrossRef]
- Peng, Z.L.; Qu, F.; Song, G.; Lin, J.M. Simultaneous separation of organomercury species by nonaqueous capillary electrophoresis using methanol containing acetic acid and imidazole. Electrophoresis 2005, 26, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, C.; Cheng, H.; Liu, J.; Xu, Z.; Yin, X. Determination of mercurial species in fish by inductively coupled plasma mass spectrometry with anion exchange chromatographic separation. Anal. Chim. Acta 2013, 796, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Pelcová, P.; Dočekalová, H.; Kleckerová, A. Determination of mercury species by the diffusive gradient in thin film technique and liquid chromatography–atomic fluorescence spectrometry after microwave extraction. Anal. Chim. Acta 2015, 866, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kodamatani, H.; Kanzaki, R.; Tomiyasu, T.; Saito, K.; Kono, Y. Determination of organic and inorganic mercury species as emetine dithiocarbamate complexes by high-performance liquid chromatography with electrogenerated tris (2,2’-bipyridine) ruthenium (III) chemiluminescence detection. Anal. Lett. 2011, 44, 2769–2779. [Google Scholar] [CrossRef]
- Hempel, M.; Hintelmann, H.; Wilken, R.-D. Determination of organic mercury species in soils by high-performance liquid chromatography with ultraviolet detection. Analyst 1992, 117, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Hoshi, S.; Mathubara, M. Reversed-phase partition high-pressure liquid chromatography of trace amounts of inorganic and organic mercury with silver diethyldithiocarbamate. Talanta 1985, 32, 44–46. [Google Scholar] [CrossRef]
- Kodamatani, H.; Matsuyama, A.; Saito, K.; Kono, Y.; Kanzaki, R.; Tomiyasu, T. Sensitive determination method for mercury ion, methyl-, ethyl-, and phenyl-mercury in water and biological samples using high-performance liquid chromatography with chemiluminescence detection. Anal. Sci. 2012, 28, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Margetínová, J.; Houserová-Pelcová, P.; Kubáň, V. Speciation analysis of mercury in sediments, zoobenthos and river water samples by high-performance liquid chromatography hyphenated to atomic fluorescence spectrometry following preconcentration by solid phase extraction. Anal. Chim. Acta 2008, 615, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Clarisse, O.; Hintelmann, H. Measurements of dissolved methylmercury in natural waters using diffusive gradients in thin film (DGT). J. Environ. Monit. 2006, 8, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Lavilla, I.; Bendicho, C.; Vidal, L.; Canals, A. Speciation of mercury by ionic liquid-based single-drop microextraction combined with high-performance liquid chromatography-photodiode array detection. Talanta 2009, 78, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Chang, H.-T. Detection of mercury and phenylmercury ions using DNA-based fluorescent probe. Analyst 2011, 136, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, P.H.; Spisso, A.; Cerutti, S.; Smichowski, P.; Martinez, L.D. Non-chromatographic screening method for the determination of mercury species. Application to the monitoring of mercury levels in Antarctic samples. Talanta 2010, 82, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, H.A.; Harris, W.E. Chemical Analysis, 2nd ed.; McGraw-Hill, Inc.: New York, NY, USA, 1975; pp. 69–79. [Google Scholar]
- Hu, S.-W.; Chen, S. Adsorption of triazine derivatives with humic fraction-immobilized silica gel in hexane: A mechanistic consideration. J. Agric. Food Chem. 2013, 61, 8524–8532. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.W.; Tang, Y.; Chen, S.; Zhou, Y.; Bagwill, C.; Chen, J.R. Macrocyclic antibiotics as a new class of chiral selectors for liquid chromatography. Anal. Chem. 1994, 66, 1473–1484. [Google Scholar] [CrossRef]
- Hsiao, Y.L.; Chen, S. LC separation of enantiomers on silica-bonded thiostrepton derivatives. Chromatographia 2009, 70, 1031–1038. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]








| Compound | Structure | Adsorption Capacity (%) b | Adsorption (%) b |
|---|---|---|---|
| 4-Aminophenylmercuric acetate |  | 0.301 ± 0.031 | ~100 |
| Phenylmercuric acetate |  | - | 72.84 |
| Phenylmercuric chloride |  | - | 50.36 |
| Phenylmercuric dimethyldithiocarbamate |  | 0.281 ± 0.021 | ~100 |
| Phenylmercuric salicylate |  | 0.312 ± 0.019 | ~100 |
| Thiomersal |  | 0.279 ± 0.018 | ~100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Gao, Y.-X. Study of Lignin-Modified Silica Gel Adsorption after Association with Six Different Organophenylmercuric Compounds in Chloroform. Int. J. Mol. Sci. 2018, 19, 2851. https://doi.org/10.3390/ijms19102851
Chen S, Gao Y-X. Study of Lignin-Modified Silica Gel Adsorption after Association with Six Different Organophenylmercuric Compounds in Chloroform. International Journal of Molecular Sciences. 2018; 19(10):2851. https://doi.org/10.3390/ijms19102851
Chicago/Turabian StyleChen, Shushi, and Yu-Xuan Gao. 2018. "Study of Lignin-Modified Silica Gel Adsorption after Association with Six Different Organophenylmercuric Compounds in Chloroform" International Journal of Molecular Sciences 19, no. 10: 2851. https://doi.org/10.3390/ijms19102851
APA StyleChen, S., & Gao, Y.-X. (2018). Study of Lignin-Modified Silica Gel Adsorption after Association with Six Different Organophenylmercuric Compounds in Chloroform. International Journal of Molecular Sciences, 19(10), 2851. https://doi.org/10.3390/ijms19102851