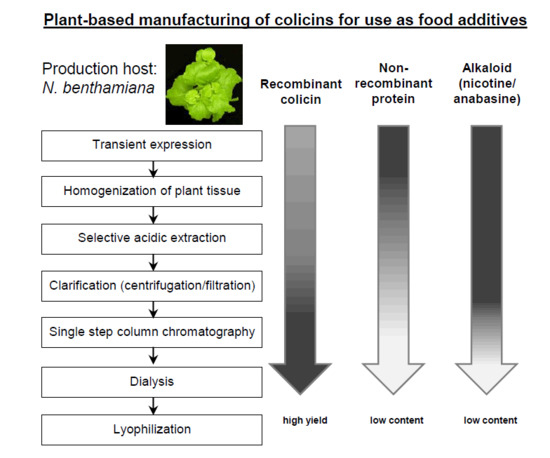
Simple Purification of Nicotiana benthamiana-Produced Recombinant Colicins: High-Yield Recovery of Purified Proteins with Minimum Alkaloid Content Supports the Suitability of the Host for Manufacturing Food Additives
Abstract
:1. Introduction
2. Results
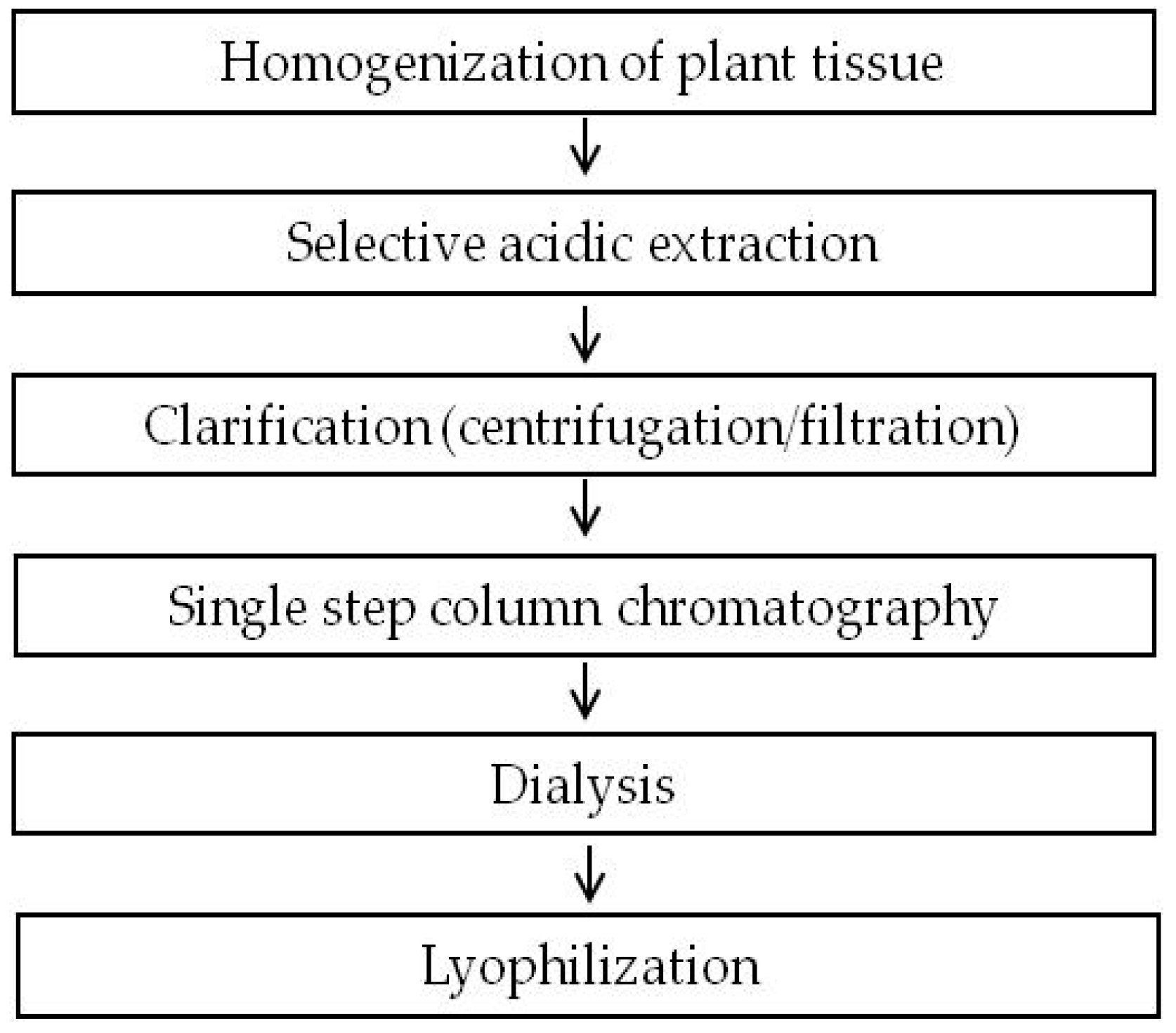
2.1. Development of a Purification Strategy for Colicins
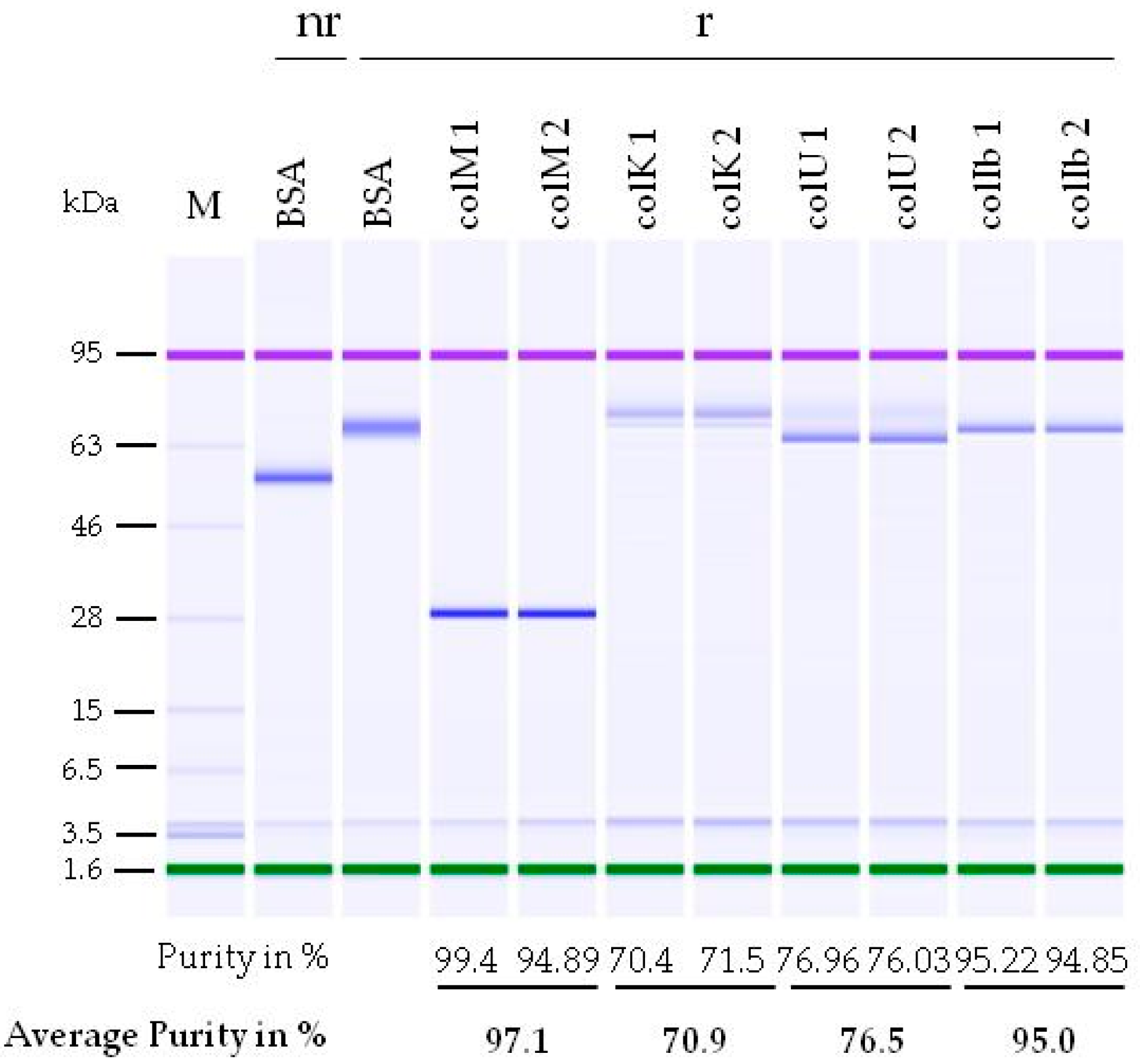
2.2. Determination of Alkaloid Content in Purified Colicin Samples
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Plasmid Constructs
4.3. N. benthamiana Plant Material and Protein Expression
4.4. Protein Extraction and Purification
4.5. Colicin Antimicrobial Activity Determination
4.6. Verification of Colicin Integrity
4.7. Determination of Colicin Purity
4.8. Determination of Alkaloid Content
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DHAP | 2,5-dihydroxyacetophenone |
| DHB | 2,5-dihydroxybenzoic acid |
| ISD | in-source decay |
| m/z | mass-to-charge ratio |
References
- Cascales, E.; Buchanan, S.K.; Duché, D.; Kleanthous, C.; Lloubès, R.; Postle, K.; Riley, M.; Slatin, S.; Cavard, D. Colicin biology. Microbiol. Mol. Biol. Rev. 2007, 71, 158–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Corrigendum: Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 683. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Stephan, A.; Hahn, S.; Bortesi, L.; Jarczowski, F.; Bettmann, U.; Paschke, A.K.; Tusé, D.; Stahl, C.H.; Giritch, A.; et al. Broad and efficient control of major foodborne pathogenic strains of Escherichia coli by mixtures of plant-produced colicins. Proc. Natl. Acad. Sci. USA 2015, 112, E5454–E5460. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Sheridan, T.G. Smarter arrow now available in the food safety quiver. Proc. Natl. Acad. Sci. USA 2015, 112, 12230–12231. [Google Scholar] [CrossRef] [PubMed]
- U. S. Food and Drug Administration Website. GRN No. 593. Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=593&sort=GRN_No&order=DESC&startrow=1&type=basic&search=593 (accessed on 28 December 2017).
- U. S. Food and Drug Administration Website. GRN No. 676. Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=676&sort=GRN_No&order=DESC&startrow=1&type=basic&search=676 (accessed on 28 December 2017).
- Smith, K.; Martin, L.; Rinaldi, A.; Rajendran, R.; Ramage, G.; Walker, D. Activity of pyocin S2 against Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2012, 56, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, L.C.; Josts, I.; Grinter, R.; White, P.; Byron, O.; Tucker, N.P.; Matthews, J.M.; Kleanthous, C.; Whitchurch, C.B.; Walker, D. Discovery, characterization and in vivo activity of pyocin SD2, a protein antibiotic from Pseudomonas aeruginosa. Biochem. J. 2016, 473, 2345–2358. [Google Scholar] [CrossRef] [PubMed]
- Paškevičius, Š.; Starkevič, U.; Misiūnas, A.; Vitkauskienė, A.; Gleba, Y.; Ražanskienė, A. Plant-expressed pyocins for control of Pseudomonas aeruginosa. PLoS ONE 2017, 12, e0185782. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wong, G.; Audet, J.; Bello, A.; Fernando, L.; Alimonti, J.B.; Fausther-Bovendo, H.; Wei, H.; Aviles, J.; Hiatt, E.; et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014, 514, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Tusé, D.; Ku, N.; Bendandi, M.; Becerra, C.; Collins, R., Jr.; Langford, N.; Sancho, S.I.; López-Díaz de Cerio, A.; Pastor, F.; Kandzia, R.; et al. Clinical Safety and Immunogenicity of Tumor-Targeted, Plant-Made Id-KLH Conjugate Vaccines for Follicular Lymphoma. Biomed. Res. Int. 2015, 2015, 648143. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Aubin, É.; Trépanier, S.; Bussière, D.; Dargis, M.; Poulin, J.F.; Yassine-Diab, B.; Ward, B.J.; Landry, N. A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin. Immunol. 2016, 168, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, F.; Nona, M.; Kawashima, N. The alkaloid contents of sixty Nicotiana species. Phytochemistry 1985, 24, 477–480. [Google Scholar] [CrossRef]
- Bendandi, M.; Marillonnet, S.; Kandzia, R.; Thieme, F.; Nickstadt, A.; Herz, S.; Fröde, R.; Inogés, S.; Lòpez-Dìaz de Cerio, A.; Soria, E.; et al. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Ann. Oncol. 2010, 21, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Köck, J.; Olschläger, T.; Kamp, R.M.; Braun, V. Primary structure of colicin M, an inhibitor of murein biosynthesis. J. Bacteriol. 1987, 169, 3358–3361. [Google Scholar] [CrossRef] [PubMed]
- Sisson, V.A.; Severson, R.F. Alkaloid composition of the Nicotiana species. Beitr. Tabakforsch. 1990, 14, 327–339. [Google Scholar] [CrossRef]
- Todd, A.T.; Liu, E.; Polvi, S.L.; Pammett, R.T.; Page, J.E. A functional genomics screen identifies diverse transcription factors that regulate alkaloid biosynthesis in Nicotiana benthamiana. Plant J. 2010, 62, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, S.C.; Scott, W.A.; Lawson, D.M. Nicotine Analysis in Several Non-Tobacco Plant Materials. Beitr. Tabakforsch. 2016, 27, 54–59. [Google Scholar] [CrossRef]
- Andersson, C.; Wennström, P.; Gry, J. Nicotine Alkaloids in Solanaceous Food Plants; TemaNord 2003:531; Nordic Council of Ministers: Copenhagen, Denmark, 2003; ISBN 92-893-0905-9. [Google Scholar]
- Goodin, M.M.; Zaitlin, D.; Naidu, R.A.; Lommel, S.A. Nicotiana benthamiana: Its History and Future as a Model for Plant-Pathogen Interactions. Mol. Plant Microbe Interact. 2015, 2015, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.F.; Guerrero, M.L.; Moon, J.E.; Waterman, P.; Nielsen, R.K.; Jefferson, S.; Gross, F.L.; Hancock, K.; Katz, J.M.; Yusibov, V.; et al. Safety and immunogenicity of a plant-produced recombinant monomer hemagglutinin-based influenza vaccine derived from influenza A (H1N1)pdm09 virus: A Phase 1 dose-escalation study in healthy adults. Vaccine 2014, 32, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Giritch, A.; Klimyuk, V.; Gleba, Y. 125 Years of Virology and Ascent of Biotechnologies Based on Viral Expression. Cytol. Genet. 2017, 51, 87–102. [Google Scholar] [CrossRef]
- Tusé, D.; Tu, T.; McDonald, K.A. Manufacturing economics of plant-made biologics: Case studies in therapeutic and industrial enzymes. Biomed. Res. Int. 2014, 2014, 256135. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Kwong, A.T.; Holtz, B.R.; Erwin, R.L.; Marcel, S.; McDonald, K.A. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. mAbs 2016, 8, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Wildman, S. An Alternate Use for Tobacco Agriculture: Proteins for Food Plus a Safer Smoking Material. In Plants: The Potentials for Extracting Protein, Medicines, and Other Useful Chemicals; Office of Technology Assessment (OTA-BP-F-23): Washington, DC, USA, 1983; pp. 63–67. [Google Scholar]
- Fu, H.; Machado, P.A.; Hahm, T.S.; Kratochvil, R.J.; Wie, C.I.; Lo, Y.M. Recovery of nicotine-free proteins from tobacco leaves using phosphate buffer system under controlled conditions. Bioresour. Technol. 2010, 101, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- U. S. Department of Agriculture Economic Research Service Website. Livestock & Meat Domestic Data. Available online: http://www.ers.usda.gov/data-products/livestock-meat-domestic-data.aspx (accessed on 28 December 2017).
- Mayer, B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch. Toxicol. 2014, 88, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Kuschel, M.; Neumann, T.; Barthmaier, P.; Kratzmeier, M. Use of lab-on-a-chip technology for protein sizing and quantitation. J. Biomol. Tech. 2002, 13, 172–178. [Google Scholar] [PubMed]

| Colicin | Number of Batches | Average Fresh Weight Plant Material (g) | Average Recovery of Colicin upon Purification (%) | Average Specific Activity (AU/µg Colicin) | Average Yield of Lyophilized Colicin (mg) |
|---|---|---|---|---|---|
| ColM | 9 | 54 | 88 ± 6.1 | 2.47 × 106 ± 2.11 × 106 | 72.6 ± 17.8 |
| ColK | 4 | 50.7 | 76 ± 8.9 | 1.89 × 106 ± 7.56 × 105 | 117.3 ± 19.9 |
| ColU | 8 | 58.8 | 81 ± 10 | 1.08 × 106 ± 9.34 × 105 | 81.6 ± 16.4 |
| ColIb | 13 | 59.7 | 55 ± 9.9 | 3.19 × 106 ± 2.67 × 106 | 35.2 ± 14 |
| Protein | Proteoform | Mass (Da) | Amino Acid Sequence and Posttranslational Modification | |
|---|---|---|---|---|
| N-Terminus | C-Terminus | |||
| ColM Batch 1 | 1 | 29,519.7 | Acetyl-METLTVHAPS(…) | (…)GEIHIKESGKR-OH |
| 2 | 29,088.2 | Acetyl-METLTVHAPS(…) | (…)GEIHIKE-OH | |
| 3 | 28,963.2 | Acetyl-METLTVHAPS(…) | (…)GEIHIK-OH | |
| ColM Batch 2 | 1 | 29,521.0 | Acetyl-METLTVHAPS(…) | (…)GEIHIKESGKR-OH |
| ColK | 1 | 59,569.7 | Acetyl-AKELSGYGP(…) | (…)SKLNELLGI-OH |
| ColU | 1 | 66,163.9 | H2N-PGFNYGGHG(…) | (…)LNNEIIRPAY-OH |
| ColIb | 1 | 69,784.7 | Acetyl-SDPVRITNPG(…) | (…)IEQVNKLIGI-OH |
| 2 | H2N-SDPVRITNPG(…) | (…)IEQVNKLIGI-OH | ||
| Sample | Nicotine Content ng/g Fresh Weight | Nicotine Content ng/g Dry Weight | ||
|---|---|---|---|---|
| Average | Literature | Average | Literature | |
| N. benthamiana leaf, ground in N2 1 | 123,667 ± 59,181 | ~500,000 [17] | 690,000 ± 340,000 | ~14,000,000 [16], ~3,000,000 [13] |
| N. benthamiana leaf, extracted with lab blender 1 | 103,330 ± 15,610 | 1,140,000 ± 190,000 | ||
| Sample | Anabasine Content ng/g Fresh Weight | Anabasine Content ng/g Dry Weight | ||
|---|---|---|---|---|
| Average | Literature | Average | Literature | |
| N. benthamiana leaf, ground in N2 1 | 14,133 ± 2590 | no literature about anabasine content per g fresh weight biomass available | 80,000 ± 20,000 | ~1,300,000 [16] (9.3% of nicotine) |
| N. benthamiana leaf, extracted with lab blender 1 | 12,930 ± 400 | 140,000 ± 10,000 | ||
| Colicin | Nicotine Concentration (ng/mg TSP) | ||
|---|---|---|---|
| Extraction | Purification and Dialysis | Lyophilization | |
| ColM | 48,654 ± 1295 | 18.32 ± 4.66 | 30.75 ± 12.76 |
| ColK | 62,988 ± 4417 | 19.64 ± 15.59 | 28.75 ± 21.45 |
| ColU | 39,905 ± 23,750 | 33.07 ± 29.76 | 21.41 ± 2.4 |
| ColIb | 52,665 ± 6819 | 137.3 ± 11.64 | 150.15 ± 30.67 |
| Colicin | Batch | Anabasine Concentration (ng/mg TSP) | ||
|---|---|---|---|---|
| Extraction | Purification and Dialysis | Lyophilization | ||
| ColM | 1 | 7404 | <1.21 | <1.54 |
| 2 | 4095 | 2.83 | <1.92 | |
| ColK | 1 | 7644 | <1.75 | 13.91 |
| 2 | 7047 | 2.44 | 2.06 | |
| ColU | 1 | 3178 | <2.34 | <2.5 |
| 2 | 7647 | 3.67 | 2.87 | |
| ColIb | 1 | 7569 | 23.61 | 43.59 |
| 2 | 8637 | 39.26 | 39.18 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephan, A.; Hahn-Löbmann, S.; Rosche, F.; Buchholz, M.; Giritch, A.; Gleba, Y. Simple Purification of Nicotiana benthamiana-Produced Recombinant Colicins: High-Yield Recovery of Purified Proteins with Minimum Alkaloid Content Supports the Suitability of the Host for Manufacturing Food Additives. Int. J. Mol. Sci. 2018, 19, 95. https://doi.org/10.3390/ijms19010095
Stephan A, Hahn-Löbmann S, Rosche F, Buchholz M, Giritch A, Gleba Y. Simple Purification of Nicotiana benthamiana-Produced Recombinant Colicins: High-Yield Recovery of Purified Proteins with Minimum Alkaloid Content Supports the Suitability of the Host for Manufacturing Food Additives. International Journal of Molecular Sciences. 2018; 19(1):95. https://doi.org/10.3390/ijms19010095
Chicago/Turabian StyleStephan, Anett, Simone Hahn-Löbmann, Fred Rosche, Mirko Buchholz, Anatoli Giritch, and Yuri Gleba. 2018. "Simple Purification of Nicotiana benthamiana-Produced Recombinant Colicins: High-Yield Recovery of Purified Proteins with Minimum Alkaloid Content Supports the Suitability of the Host for Manufacturing Food Additives" International Journal of Molecular Sciences 19, no. 1: 95. https://doi.org/10.3390/ijms19010095
APA StyleStephan, A., Hahn-Löbmann, S., Rosche, F., Buchholz, M., Giritch, A., & Gleba, Y. (2018). Simple Purification of Nicotiana benthamiana-Produced Recombinant Colicins: High-Yield Recovery of Purified Proteins with Minimum Alkaloid Content Supports the Suitability of the Host for Manufacturing Food Additives. International Journal of Molecular Sciences, 19(1), 95. https://doi.org/10.3390/ijms19010095