Omentin-A Novel Adipokine in Respiratory Diseases
Abstract
:1. Introduction
2. The Structure and Development of Omentin
3. The Concentration of Omentin Fluctuates in Various Diseases
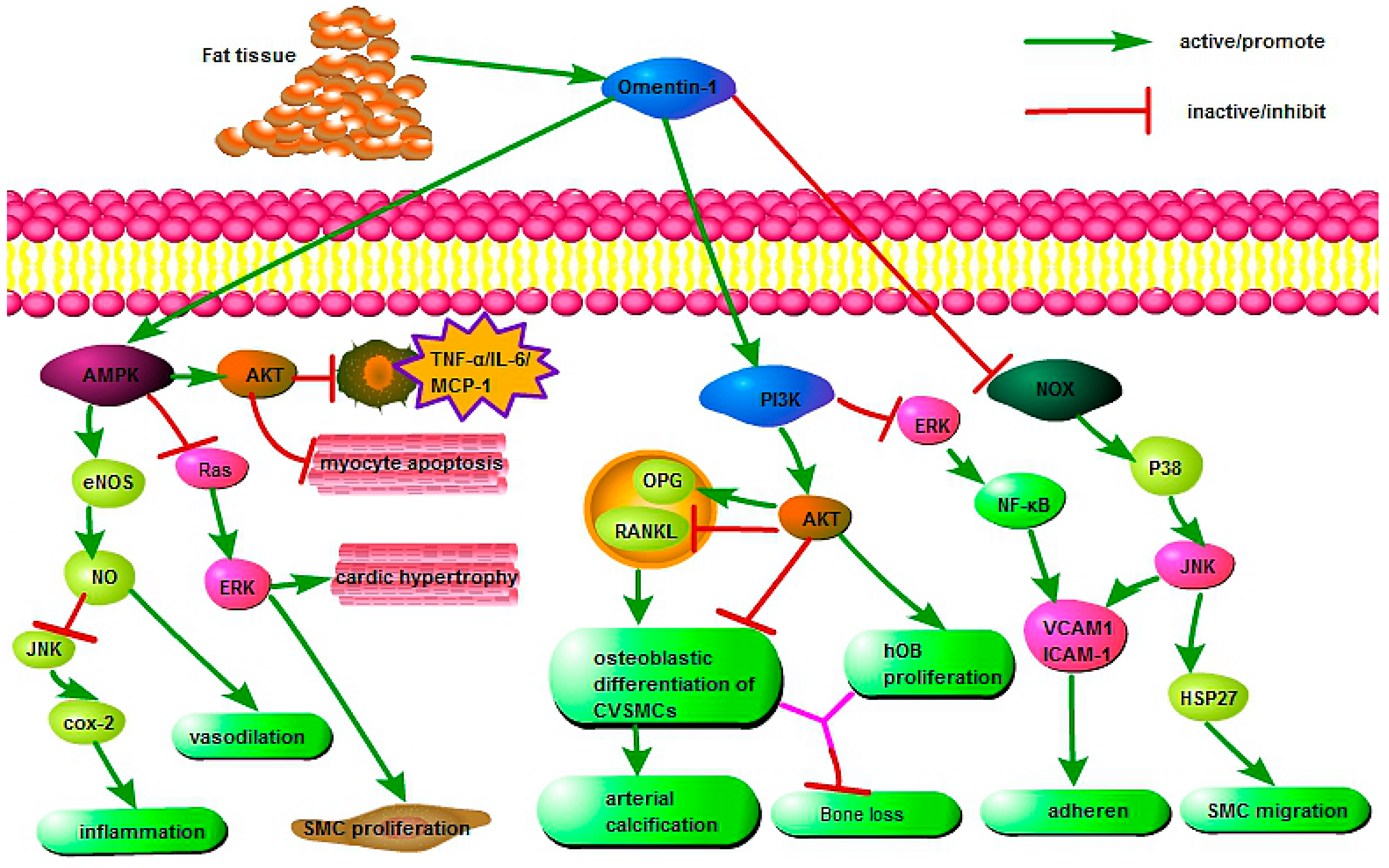
4. Protective Effects of Omentin in Various Pathophysiological Processes
5. Roles of Omentin in Pulmonary Disease
5.1. Omentin in Malignant Pleural Mesothelioma (MPM)
5.2. Omentin in Asthma
5.3. Omentin in Obstructive Sleep Apnea Syndrome (OSAS)
5.4. Omentin in Pulmonary Arterial Hypertension (PAH)
5.5. Omentin in Acute Respiratory Distress Syndrome (ARDS)
5.6. Omentin in Chronic Obstructive Pulmonary Disease (COPD)
6. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| T2DM | type-2 diabetes mellitus |
| PCOS | polycystic ovary syndrome |
| CAD | coronary artery disease |
| EAT | epicardial adipose tissue |
| NAFLD | nonalcoholic fatty liver disease |
| SLE | systemic lupus erythematosus |
| HIV/HAART | human immunodeficiency virus/highly active anti-retroviral therapy |
| MPM | malignant pleural mesothelioma |
| ARDS | acute respiratory distress syndrome |
| OSAS | obstructive sleep apnoea syndrome |
| COX-2 | cyclooxygenase-2 |
| AMPK | Adenosine 5′-monophosphate-activated protein kinase |
| eNOS | endothelial nitric oxide synthase |
| JNK | Jun N-Terminal Kinase |
| SMC | smooth muscle cell |
| MCP-1 | monocyte chemotactic protein-1 |
| CVSMCs | calcifying vascular smooth muscle cells |
| PI3K/Akt | phosphatidylinositol 3 kinase/protein kinase B |
| OPG | osteoprotegerin |
| RANKL | nuclear factor κB ligand |
| hOB | human osteoblast |
| NOX | NADPH oxidase |
| PDGF-BB | platelet-derived growth factor |
| OVA | ovalbumin |
| CPAP | continuous positive airway pressure |
| COPD | chronic obstructive pulmonary disease |
References
- Kralisch, S.; Klein, J.; Bluher, M.; Paschke, R.; Stumvoll, M.; Fasshauer, M. Therapeutic perspectives of adipocytokines. Expert Opin. Pharmacother. 2005, 6, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends. Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Clinical relevance of adipokines. Diabetes Metab. J. 2012, 36, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Koerner, A.; Kratzsch, J.; Kiess, W. Adipocytokines: Leptin—The classical, resistin—The controversical, adiponectin—The promising, and more to come. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor necrosis factor α: A key component of the obesity-diabetes link. Diabetes 1994, 43, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Lihn, A.S.; Verdich, C.; Pedersen, S.B.; Toubro, S.; Astrup, A.; Richelsen, B. Regulation of adiponectin by adipose tissue-derived cytokines: In vivo and in vitro investigations in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E527–E533. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Nigro, E.; Monaco, M.L.; Matera, M.G.; Scudiero, O.; Mazzarella, G.; Daniele, A. The burden of obesity in asthma and COPD: Role of adiponectin. Pulm. Pharmacol. Ther. 2017, 43, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.L.; Zheng, X.L.; Tang, C.K. The protective functions of omentin in cardiovascular diseases. Pulm. Pharmacol. Ther. 2015, 448, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Z.; Lee, M.J.; Hu, H.; Pray, J.; Wu, H.B.; Hansen, B.C. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Sacks, H.S.; Buehrer, B.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y. Identification of omentin mRNA in human epicardial adipose tissue: Comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int. J. Obes. 2008, 32, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, A.; Neumeier, M.; Herfarth, H.; Furst, A.; Scholmerich, J.; Buchler, C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim. Biophys. Acta 2005, 1732, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Komiya, T.; Tanigawa, Y.; Hirohashi, S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochem. Biophys. Res. Commun. 1998, 251, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Shin, K.; Lonnerdal, B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 2001, 40, 15771–15779. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J. Update on adipocyte hormones: Regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 2004, 53, S143–S151. [Google Scholar] [CrossRef] [PubMed]
- De Souza Batista, C.M.; Yang, R.Z.; Lee, M.J.; Glynn, N.M.; Yu, D.Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Oliver, P.; Palou, A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim. Biophys. Acta 2013, 1831, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Olarescu, N.C.; Heck, A.; Godang, K.; Ueland, T.; Bollerslev, J. The Metabolic risk in patients newly diagnosed with acromegaly is related to fat distribution and circulating adipokines and improves after treatment. Neuroendocrinology 2016, 103, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.; Lim, R.; Georgiou, H.M.; Lappas, M. Omentin-1 is decreased in maternal plasma, placenta and adipose tissue of women with pre-existing obesity. PLoS ONE 2012, 7, e42943. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Y.; Guo, L.; Li, Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes. Res. Clin. Pract. 2010, 88, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.L.; Ma, R.M. Correlation of serum omentin-1 and chemerin with gestational diabetes mellitus. J. South. Med. Univ. 2016, 36, 1231–1236. [Google Scholar]
- Pourbehi, M.R.; Zahedi, T.; Darabi, H.; Ostovar, A.; Assadi, M.; Nabipour, I. Omentin-1 and nonfatal ischemic heart disease in postmenopausal women: A population-based study. Endocr. Pract. 2016, 22, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Alkuraishy, H.M.; Al-Gareeb, A.I. New insights into the role of metformin effects on serum omentin-1 levels in acute myocardial infarction: Cross-sectional study. Emerg. Med. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Li, Q.; Zhang, F.; Zheng, G.; Lv, Y.; Wan, G.; Jin, X. Serum and vitreous concentrations of omentin-1 in diabetic retinopathy. Dis. Markers 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kort, D.H.; Kostolias, A.; Sullivan, C.; Lobo, R.A. Chemerin as a marker of body fat and insulin resistance in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2015, 31, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Orlik, B.; Madej, P.; Owczarek, A.; Skalba, P.; Chudek, J.; Olszanecka-Glinianowicz, M. Plasma omentin and adiponectin levels as markers of adipose tissue dysfunction in normal weight and obese women with polycystic ovary syndrome. Clin. Endocrinol. 2014, 81, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Yu, J.; Zeng, Z.G.; Liu, Y.; Liu, J.Y.; Xu, J.X. Circulating omentin-1 levels in women with polycystic ovary syndrome: A meta-analysis. Gynecol. Endocrinol. 2017, 33, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Adya, R.; Farhatullah, S.; Chen, J.; Lehnert, H.; Randeva, H.S. Metformin treatment may increase omentin-1 levels in women with polycystic ovary syndrome. Diabetes 2010, 59, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ji, Q.; Cai, L.; Huang, F.; Lai, Y.; Liu, Y.; Yu, J.; Han, B.; Zhu, E.; Zhang, J.; et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc. Diabetol. 2016, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Lambadiari, V.; Gastounioti, A.; Gkekas, C.; Giannakopoulos, T.G.; Koulia, K.; Maratou, E.; Alepaki, M.; Kakisis, J.; Karakitsos, P.; et al. The relationship of novel adipokines, RBP4 and omentin-1, with carotid atherosclerosis severity and vulnerability. Atherosclerosis 2014, 235, 606–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, X.; Bu, P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes. Res. Clin. Pract. 2011, 93, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Shibata, R.; Ouchi, N.; Tokuda, Y.; Funakubo, H.; Suzuki, M.; Kataoka, T.; Nagao, T.; Okumura, S.; Shinoda, N.; et al. Increased expression of the adipocytokine omentin in the epicardial adipose tissue of coronary artery disease patients. Atherosclerosis 2016, 251, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhang, H.Y.; Tan, H.; Zhou, Y.; Liu, F.L.; Chen, F.Q.; Yu, J.; Han, B.; Zhu, E.; Zhang, J.; et al. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol. Sin. 2011, 32, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Dou, L.Z.; Gu, C.; Wang, X.Q. Plasma levels of omentin-1 and visfatin in senile patients with coronary heart disease and heart failure. Asian Pac. J. Trop. Med. 2014, 7, 55–62. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Y.; Zhang, S.; Wang, Z.; Zhang, J.; Chang, C.; Liu, L.; Ji, Q.; Liu, X. Circulating omentin-1 levels are decreased in dilated cardiomyopathy patients with overt heart failure. Dis. Markers 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Yonal, O.; Kurt, R.; Alahdab, Y.O.; Eren, F.; Ozdogan, O.; Celikel, C.A.; Imeryuz, N.; Kalayci, C.; Avsar, E. Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand. J. Gastroenterol. 2011, 46, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Mohamed, S.A. Serum levels of visfatin and omentin-1 in patients with psoriasis and their relation to disease severity. Br. J. Dermatol. 2012, 167, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Turan, H.; Yaykasli, K.O.; Soguktas, H.; Yaykasli, E.; Aliagaoglu, C.; Erdem, T.; Karkucak, M.; Kaya, E.; Ucgun, T.; Bahadir, A. Omentin serum levels and omentin gene Val109Asp polymorphism in patients with psoriasis. Int. J. Dermatol. 2014, 53, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, K.J.; Liu, J.L.; Xu, G.X.; Liu, W.; Jiang, F.X.; Zheng, H.F.; Quan, C. Omentin-1 plasma levels and omentin-1 expression are decreased in psoriatic lesions of psoriasis patients. Arch. Dermatol. Res. 2015, 307, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Tsang, L.; Solomon, A.; Woodiwiss, A.J.; Gunter, S.; Millen, A.M.; Norton, G.R.; Fernandez-Lopez, M.J.; Hollan, I.; Dessein, P.H. Omentin concentrations are independently associated with those of matrix metalloproteinase-3 in patients with mild but not severe rheumatoid arthritis. Rheumatol. Int. 2017, 37, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, G.B.; Wang, L.; Wang, D.F.; Jiang, X.R. Synovial fluid omentin-1 levels are inversely correlated with radiographic severity of knee osteoarthritis. J. Investig. Med. 2012, 60, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Turkcu, F.M.; Sahin, A.; Cingu, A.K.; Kaya, S.; Yuksel, H.; Cinar, Y.; Batmaz, İ. Serum omentin, resistin and tumour necrosis factor-α levels in Behcet patients with and without ocular involvement. Graefes. Arch. Clin. Exp. Ophthalmol. 2015, 253, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, L.; Liu, L.; Feng, Y.; Lu, L.; Ren, X.; Dong, X.; Sang, W. Serum omentin-1 as a disease activity marker for Crohn’s disease. Dis. Markers 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hou, P.; Wu, Z.; Nie, Y. Decreased levels of serum omentin-1 in patients with inflammatory bowel disease. Med. Sci. Monit. 2015, 21, 118–122. [Google Scholar] [PubMed]
- Bozkurt Dogan, S.; Ongoz Dede, F.; Balli, U.; Sertoglu, E. Levels of vaspin and omentin-1 in gingival crevicular fluid as potential markers of inflammation in patients with chronic periodontitis and type 2 diabetes mellitus. J. Oral. Sci. 2016, 58, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Sit, M.; Aktas, G.; Yilmaz, E.E.; Alcelik, A.; Terzi, E.H.; Tosun, M. Effects of the inflammatory response on serum omentin levels in early acute and chronic pancreatitis. Clin. Ter. 2014, 165, e148–e152. [Google Scholar] [PubMed]
- Xue, Y.; Jiang, L.; Cheng, Q.; Chen, H.; Yu, Y.; Lin, Y.; Yang, X.; Kong, N.; Zhu, X.; Xu, X.; et al. Adipokines in psoriatic arthritis patients: The correlations with osteoclast precursors and bone erosions. PLoS ONE 2012, 7, e46740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.P.; Li, H.M.; Leng, R.X.; Li, X.P.; Li, X.M.; Pan, H.F.; Ye, D.Q. Plasma levels of adipokines in systemic lupus erythematosus patients. Cytokine 2016, 86, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Peraire, J.; Lopez-Dupla, M.; Alba, V.; Beltran-Debon, R.; Martinez, E.; Domingo, P.; Asensi, V.; Leal, M.; Viladés, C.; Inza, M.I.; et al. HIV/antiretroviral therapy-related lipodystrophy syndrome (HALS) is associated with higher RBP4 and lower omentin in plasma. Clin. Microbiol. Infect. 2015, 21, 711.e1–711.e8. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.B.; Sanders, A.E.; Wilder, R.S.; Essick, G.K.; Slade, G.D.; Hartung, J.E.; Nackley, A.G. Circulating omentin-1 and chronic painful temporomandibular disorders. J. Oral Facial Pain Headache 2016, 30, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.D.; Zhang, L.; Che, H.; Zhang, Y.Y.; Yang, C.; Zhou, J.; Liang, C.Z. Circulating levels of adipocytokine omentin-1 in patients with renal cell cancer. Cytokine 2016, 77, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.; Morin, P.J.; Hough, C.D.; Lonardo, F.; Seya, T.; Carbone, M.; Pass, H.I. Identification of intelectin overexpression in malignant pleural mesothelioma by serial analysis of gene expression (SAGE). Lung Cancer 2005, 48, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhou, L.M. Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur. J. Pharmacol. 2013, 698, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Uyeturk, U.; Sarici, H.; Kin Tekce, B.; Eroglu, M.; Kemahli, E.; Gucuk, A. Serum omentin level in patients with prostate cancer. Med. Oncol. 2014, 31, 923. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.S.; Dashti, H.; Akbarzadeh, S.; Assadi, M.; Aminian, A.; Keramati, M.R.; Nabipour, I. Circulating levels of novel adipocytokines in patients with colorectal cancer. Cytokine 2013, 62, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Uyeturk, U.; Alcelik, A.; Aktas, G.; Tekce, B.K. Post-treatment plasma omentin levels in patients with stage III colon carcinoma. J. BUON 2014, 19, 681–685. [Google Scholar] [PubMed]
- Zheng, L.; Weng, M.; Qi, M.; Qi, T.; Tong, L.; Hou, X.; Tong, Q. Aberrant expression of intelectin-1 in gastric cancer: Its relationship with clinicopathological features and prognosis. J. Cancer Res. Clin. Oncol. 2012, 138, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, S.; Afsar, C.U.; Karabulut, M.; Alis, H.; Bozkurt, M.A.; Aydogan, F.; Serilmez, M.; Tas, F. Clinical significance of serum omentin-1 levels in patients with pancreatic adenocarcinoma. BBA Clin. 2016, 6, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Tang, X.; He, J.; Wang, D.; Zhao, Y.; Deng, W.; Deng, X.; Zhou, G.; Xia, J.; Zhong, X.; et al. Omentin protects against LPS-induced ARDS through suppressing pulmonary inflammation and promoting endothelial barrier via an Akt/eNOS-dependent mechanism. Cell Death Dis. 2016, 7, e2360. [Google Scholar] [CrossRef] [PubMed]
- Carolan, B.J.; Harvey, B.G.; De, B.P.; Vanni, H.; Crystal, R.G. Decreased expression of intelectin 1 in the human airway epithelium of smokers compared to nonsmokers. J. Immunol. 2008, 181, 5760–5767. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Feng, X.; Zhou, C.; Li, P.; Kang, J. Decreased levels of serum omentin-1 in patients with obstructive sleep apnoea syndrome. Ann. Clin. Biochem. 2013, 50, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.K.; Tosun, M.; Alcelik, A.; Yilmaz, B.; Talay, F. Serum omentin levels in patients with obstructive sleep apnea. Sleep Breath. 2014, 18, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Zirlik, S.; Hildner, K.M.; Targosz, A.; Neurath, M.F.; Fuchs, F.S.; Brzozowski, T.; Konturek, P.C. Melatonin and omentin: Influence factors in the obstructive sleep apnoea syndrome? J. Physiol. Pharmacol. 2013, 64, 353–360. [Google Scholar] [PubMed]
- Kuperman, D.A.; Lewis, C.C.; Woodruff, P.G.; Rodriguez, M.W.; Yang, Y.H.; Dolganov, G.M.; Fahy, J.V.; Erle, D.J. Dissecting asthma using focused transgenic modeling and functional genomics. J. Allergy Clin. Immunol. 2005, 116, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Obesity, inflammation, and lung injury (OILI): The good. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Eisinger, K.; Krautbauer, S.; Wiest, R.; Karrasch, T.; Hader, Y.; Scherer, M.N.; Farkas, S.; Aslanidis, C.; Buechler, C. Portal vein omentin is increased in patients with liver cirrhosis but is not associated with complications of portal hypertension. Eur. J. Clin. Investig. 2013, 43, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.J.; Jiang, T.J.; Liao, L.; Liu, H.; He, H.B. Relationship between serum omentin-1 level and bone mineral density in girls with anorexia nervosa. J. Endocrinol. Investig. 2013, 36, 190–194. [Google Scholar]
- Oswiecimska, J.; Suwala, A.; Swietochowska, E.; Ostrowska, Z.; Gorczyca, P.; Ziora-Jakutowicz, K.; Machura, E.; Szczepańska, M.; Kukla, M.; Stojewska, M.; et al. Serum omentin levels in adolescent girls with anorexia nervosa and obesity. Physiol. Res. 2015, 64, 701–709. [Google Scholar] [PubMed]
- Alcelik, A.; Tosun, M.; Ozlu, M.F.; Eroglu, M.; Aktas, G.; Kemahli, E.; Savli, H.; Yazici, M. Serum levels of omentin in end-stage renal disease patients. Kidney Blood Press. Res. 2012, 35, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophys. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Shibata, R.; Kikuchi, R.; Izumiya, Y.; Rokutanda, T.; Araki, S.; Kataoka, Y.; Ohashi, K.; Daida, H.; Kihara, S.; et al. Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J. Biol. Chem. 2012, 287, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Kazama, K.; Okada, M.; Hara, Y.; Yamawaki, H. A novel adipocytokine, omentin, inhibits agonists-induced increases of blood pressure in rats. J. Vet. Med. Sci. 2013, 75, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Leone, S.; Orlando, G.; Ferrante, C.; Recinella, L.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Hypotensive effects of omentin-1 related to increased adiponectin and decreased interleukin-6 in intra-thoracic pericardial adipose tissue. Pharmacol. Rep. 2014, 66, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Shibata, R.; Ohashi, K.; Kambara, T.; Uemura, Y.; Hiramatsu-Ito, M.; Enomoto, T.; Yuasa, D.; Joki, Y.; Ito, M.; et al. Omentin functions to attenuate cardiac hypertrophic response. J. Mol. Cell. Cardiol. 2015, 79, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Watanabe, R.; Konii, H.; Shirai, R.; Sato, K.; Matsuyama, T.A.; Ishibashi-Ueda, H.; Koba, S.; Kobayashi, Y.; Hirano, T.; et al. Counteractive effects of omentin-1 against atherogenesisdagger. Cardiovasc. Res. 2016, 110, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Shibata, R.; Ohashi, K.; Kambara, T.; Enomoto, T.; Uemura, Y.; Ogura, Y.; Yuasa, D.; Matsuo, K.; Nagata, T.; et al. Omentin prevents myocardial ischemic injury through AMP-activated protein kinase and Akt-dependent mechanisms. J. Am. Coll. Cardiol. 2014, 63, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu-Ito, M.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kanemura, N.; Kambara, T.; Enomoto, T.; Yuasa, D.; Matsuo, K.; Ito, M.; et al. Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 2016, 110, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.Y.; Xie, P.L.; Ma, Y.L.; Tang, S.Y. Omentin inhibits osteoblastic differentiation of calcifying vascular smooth muscle cells through the PI3K/Akt pathway. Amino Acids 2011, 41, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xie, P.L.; Wu, X.P.; Chen, S.M.; Zhou, H.D.; Yuan, L.Q.; Sheng, Z.F.; Tang, S.Y.; Luo, X.H.; Liao, E.Y. Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc. Res. 2011, 92, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Liang, Q.H.; Liu, Y.; Cui, R.R.; Yuan, L.Q.; Liao, E.Y. Omentin-1 stimulates human osteoblast proliferation through PI3K/Akt signal pathway. Int. J. Endocrinol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, X.; Liu, F.; Tan, H.; Shang, D. Omentin inhibits TNF-α-induced expression of adhesion molecules in endothelial cells via ERK/NF-κB pathway. Biochem. Biophys. Res. Commun. 2012, 425, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kazama, K.; Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur. J. Pharmacol. 2012, 686, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kazama, K.; Okada, M.; Yamawaki, H. A novel adipocytokine, omentin, inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell migration through antioxidative mechanism. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1714–H1719. [Google Scholar] [CrossRef] [PubMed]
- Chailleux, E.; Dabouis, G.; Pioche, D.; de Lajartre, M.; de Lajartre, A.Y.; Rembeaux, A.; Germaud, P. Prognostic factors in diffuse malignant pleural mesothelioma. A study of 167 patients. Chest 1988, 93, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Ruffie, P.; Feld, R.; Minkin, S.; Cormier, Y.; Boutan-Laroze, A.; Ginsberg, R.; Ayoub, J.; Shepherd, F.A.; Evans, W.K.; Figueredo, A.; et al. Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: A retrospective study of 332 patients. J. Clin. Oncol. 1989, 7, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Tsuura, Y.; Morohoshi, T.; Shinohara, T.; Oshita, F.; Yamada, K.; Kameda, Y.; Ohtsu, T.; Nakamura, Y.; Miyagi, Y. Secretion of intelectin-1 from malignant pleural mesothelioma into pleural effusion. Br. J. Cancer 2010, 103, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Washimi, K.; Yokose, T.; Yamashita, M.; Kageyama, T.; Suzuki, K.; Yoshihara, M.; Miyagi, Y.; Hayashi, H.; Tsuji, S. Specific expression of human intelectin-1 in malignant pleural mesothelioma and gastrointestinal goblet cells. PLoS ONE 2012, 7, e39889. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Park, S.W.; Nguyenvu, L.T.; Rodriguez, M.W.; Barbeau, R.; Paquet, A.C.; Erle, D.J. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am. J. Respir. Cell Mol. Biol. 2007, 36, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.C.; Carrington, S.D.; Oscarson, S.; Gallagher, M.E.; Solon, M.; Yuan, S.; Ahn, J.N.; Dougherty, R.H.; Finkbeiner, W.E.; Peters, M.C.; et al. Intelectin-1 is a prominent protein constituent of pathologic mucus associated with eosinophilic airway inflammation in asthma. Am. J. Respir. Crit. Care Med. 2014, 189, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, A.D.; Rose-Zerilli, M.J.; Holloway, J.W.; Gray, R.D.; Holgate, S.T. A single-nucleotide polymorphism in intelectin 1 is associated with increased asthma risk. J. Allergy Clin. Immunol. 2008, 122, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef] [PubMed]
- Grunig, G.; Warnock, M.; Wakil, A.E.; Venkayya, R.; Brombacher, F.; Rennick, D.M.; Sheppard, D.; Mohrs, M.; Donaldson, D.D.; Locksley, R.M.; et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998, 282, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.; Wong, C.K.; Lam, C.W. Interleukin (IL)-4 and IL-13 up-regulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: Involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not c-Jun NH2-terminal kinase 1/2 signalling pathways. Clin. Exp. Immunol. 2006, 145, 162–172. [Google Scholar] [PubMed]
- Gu, N.; Kang, G.; Jin, C.; Xu, Y.; Zhang, Z.; Erle, D.J.; Zhen, G. Intelectin is required for IL-13-induced monocyte chemotactic protein-1 and -3 expression in lung epithelial cells and promotes allergic airway inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L290–L296. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Uehori, J.; Matsumoto, M.; Suzuki, Y.; Matsuhisa, A.; Toyoshima, K.; Seya, T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J. Biol. Chem. 2001, 276, 23456–23463. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. Asthma: The importance of dysregulated barrier immunity. Eur. J. Immunol. 2013, 43, 3125–3137. [Google Scholar] [CrossRef] [PubMed]
- Licona-Limon, P.; Kim, L.K.; Palm, N.W.; Flavell, R.A. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 2013, 14, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cheng, D.; Zhang, K.; Huo, X.; Mo, Y.; Shi, H.; Di, H.; Zou, Y.; Zhang, H.; Zhao, J.; et al. Intelectin contributes to allergen-induced IL-25, IL-33, and TSLP expression and type 2 response in asthma and atopic dermatitis. Mucosal Immunol. 2017, 10, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, A.D.; Verdon, B.; Inglis, N.F.; Pearson, J.P. Sheep intelectin-2 co-purifies with the mucin Muc5ac from gastric mucus. Res. Vet. Sci. 2011, 91, e53–e57. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, E.J.; Cortijo, J. Mucus and MUC in asthma. Curr. Opin. Pulm. Med. 2006, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Calero, E.; Espinar-Escalona, E.; Barrera-Mora, J.M.; Llamas-Carreras, J.M.; Solano-Reina, E. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med Oral Patol. Oral Cir. Bucal 2012, 17, e925–e929. [Google Scholar] [CrossRef] [PubMed]
- Hecht, L.; Mohler, R.; Meyer, G. Effects of CPAP-respiration on markers of glucose metabolism in patients with obstructive sleep apnoea syndrome: A systematic review and meta-analysis. Ger. Med. Sci. 2011, 9, Doc20. [Google Scholar]
- Franco, C.M.; Lima, A.M.; Ataide, L., Jr.; Lins, O.G.; Castro, C.M.; Bezerra, A.A.; de Oliveira, M.F.; Oliveira, J.R. Obstructive sleep apnea severity correlates with cellular and plasma oxidative stress parameters and affective symptoms. J. Mol. Neurosci. 2012, 47, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Jelic, S.; Padeletti, M.; Kawut, S.M.; Higgins, C.; Canfield, S.M.; Onat, D.; Colombo, P.C.; Basner, R.C.; Factor, P.; LeJemtel, T.H. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008, 117, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- El Solh, A.A.; Akinnusi, M.E.; Baddoura, F.H.; Mankowski, C.R. Endothelial cell apoptosis in obstructive sleep apnea: A link to endothelial dysfunction. Am. J. Respir. Crit. Care Med. 2007, 175, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Uygur, F.; Tanriverdi, H.; Can, M.; Erboy, F.; Altinsoy, B.; Atalay, F.; Ornek, T.; Damar, M.; Kokturk, F.; Tor, M. Association between continuous positive airway pressure and circulating omentin levels in patients with obstructive sleep apnoea. Sleep Breath. 2016, 20, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Potoka, K.C.; Champion, H.C.; Mora, A.L.; Gladwin, M.T. Pulmonary arterial hypertension: The clinical syndrome. Circ. Res. 2014, 115, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Kazama, K.; Okada, M.; Yamawaki, H. A novel adipocytokine, omentin, inhibits monocrotaline-induced pulmonary arterial hypertension in rats. Biochem. Biophys. Res. Commun. 2014, 452, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Tsubaki, N.; Mukohda, M.; Okada, M.; Hara, Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem. Biophys. Res. Commun. 2010, 393, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D. Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Gando, S.; Kameue, T.; Matsuda, N.; Sawamura, A.; Hayakawa, M.; Kato, H. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: Role of neutrophil and endothelial activation. Inflammation 2004, 28, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.X.; Zhou, Y.; Zhou, A.Y.; Guan, X.X.; Liu, T.; Yang, H.H.; Xie, H.; Chen, P. Calcitonin gene-related peptide exerts anti-inflammatory property through regulating murine macrophages polarization in vitro. Mol. Immunol. 2017, 91, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Hurd, S.S.; Agusti, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; He, F.; Sergakis, G.G.; Koozehchian, M.S.; Stimpfl, J.N.; Rong, Y.; Diaz, P.T.; Best, T.M. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L205–L218. [Google Scholar] [CrossRef] [PubMed]
- Murin, S.; Bilello, K.S. Respiratory tract infections: Another reason not to smoke. Clevel. Clin. J. Med. 2005, 72, 916–920. [Google Scholar] [CrossRef]
| Diseases | Fluctuation of Omentin Levels in Various Sample | Omentin-1 Concentration (ng/mL) Mean (Range) or Mean ± SD | p Value | Ref. | ||
|---|---|---|---|---|---|---|
| CON | Diseases | |||||
| Obesity-related diseases | Obesity | Serum ↓ | 370 ± 20 | 310 ± 20 | 0.009 | [16] |
| Pregnant with preexisting obesity | Cord ↓ | 58.0 ± 6.0 | 48.3 ± 9.0 | >0.05 | [19] | |
| Maternal ↓ | 19.5 ± 2.3 | 7.1 ± 0.9 | <0.05 | |||
| T2DM | Serum ↓ | 18.85 ± 3.23 | 16.12 ± 4.08 | <0.05 | [20] | |
| GDM with obesity | Serum ↓ | 355.94 ± 42.61 | 216.41 ± 51.33 | 0.000 | [21] | |
| T2DM with ischemic heart disease | Serum ↓ | 12.44 ± 2.12 | 10.31 ± 2.35 | 0.038 | [23] | |
| T2DM with Diabetic Retinopathy | Serum ↓ | 208.31 (164.20–251.20) | 139.96 (119.28–157.87) | <0.001 | [24] | |
| Vitreous ↓ | 96.00 (75.24–112.64) | 50.36 (39.91–57.73) | <0.001 | |||
| PCOS | Serum ↓ | 269.7 | 197.6 | 0.0073 | [25] | |
| 515.9 | 210.5 | <0.001 | [26] | |||
| 27.6 | 23.7 | 0.05 | [28] | |||
| Coronary artery disease | Serum ↓ | 659.39 | 373.71 | <0.001 | [29] | |
| 815.3 ± 185.32 | 518.61 ± 191.10 | <0.001 | [30] | |||
| 34.58 ± 4.23 | 10.66 ± 3.41 | <0.01 | [31] | |||
| 254.00 ± 72.98 | 113.08 ± 61.43 | <0.0001 | [33] | |||
| EAT ↑ (mRNA) | 0.76 (0.71–0.89) | 1.25 (1.10–2.85) | 0.002 | [32] | ||
| coronary heart disease | Serum ↓ | 1.115 ± 0.361 | 0.718 ± 0.229 | 0.000 | [34] | |
| Dilated cardiomyopathy | Serum ↓ | 233.33 ± 58.04 | 153.00 ± 48.94 | <0.01 | [35] | |
| NAFLD | Serum ↑ | 376 ± 196 | 460 ± 181 | <0.001 | [36] | |
| Chronic immune or inflammatory disease | Psoriasis | Serum ↓ | 26.8 ± 14.2 | 18.5 ± 13.1 | 0.0053 | [37] |
| 488.7 ± 190.3 | 354.2 ± 152.0 | 0.001 | [38] | |||
| 143.60 ± 48.97 | 95.61 ± 44.38 | 0.001 | [39] | |||
| Rheumatoid arthritis | Serum ↓ | 23.58 (14.60–28.39) | 19.98 (11.98–27.21) | >0.01 | [41] | |
| Behcet disease | Serum ↓ | 12.4 ± 6.24 | 8.9 ± 4.65 | 0.035 | [42] | |
| Crohn’s disease | Serum ↓ | 409.40 ± 215.65 | 201.29 ± 76.65 | <0.0001 | [43] | |
| Ulcerative colitis | Serum ↓ | 28.62 (24.71–33.21) | 14.74 (11.52–18.16) | <0.001 | [44] | |
| Chronic periodontitis | Gingival crevicular fluid ↓ | 135 | 45 | <0.008 | [45] | |
| Acute pancreatitis | Serum ↑ | 22.49 ± 1.4 | 37.79 ± 1.24 | <0.01 | [46] | |
| Chronic pancreatitis | Serum ↑ | 22.49 ± 1.4 | 49.37 ± 2.82 | <0.01 | [46] | |
| Psoriasis arthritis | Serum ↑ | 4 | 20.6 (2.8–82.2) | 0.01 | [47] | |
| SLE patients with nephritis | Serum ↑ | 11.42 (1.44–26.35) | 30.77 (16.77–37.63) | 0.002 | [48] | |
| HALS | Serum ↓ | - | - | 0.001 | [49] | |
| Temporo-mandibular disorders | Serum ↓ | 464.8 ± 191.8 | 413.5 ±145.9 | 0.072 | [50] | |
| Tumor diseases | Renal cell cancer | Serum ↓ | 9.86 ± 1.44 | 3.62 ± 0.76 | <0.001 | [51] |
| MPM | tumor tissues ↑ | Serial analysis of gene expression (SAGE) >129 flod increase | - | [52] | ||
| Prostate cancer | Serum ↑ | 373 (207–792) | 546.8 (297.1–945.7) | <0.001 | [54] | |
| colon and colorectal cancer | Serum ↑ | 0.376 (0.155–0.662) | 0.618 (0.151–0.758) | <0.001 | [56] | |
| Gastric cancer | Tumor tissues ↑ (mRNA) | qRT-PCR > 6 flod increase | <0.001 | [57] | ||
| Pancreatic adenocarcinoma | Serum ↑ | 1.61 (0.80–4.98) | 9.57 (3.62–21.948) | <0.001 | [58] | |
| Respiratory diseases | ARDS | Serum ↓ | - | - | <0.05 | [59] |
| Smokers | airway epithelium ↓ (mRNA/protein) | TaqMan RT-PCR and Immunohistochemistry 3.8–14.7 fold decrease | <0.05 | [60] | ||
| OSAS (controversial) | Serum ↓ | 22.62 (18.71–27.21) | 11.29 (8.02–15.13) | <0.001 | [61] | |
| Serum ↑ | 432.0 (155.2–1101.2) | 570.8 (288.4–2152.4) | <0.001 | [62] | ||
| Serum ↑ | 9.24 ± 4.85 | 17.78 ± 7.20 | <0.05 | [63] | ||
| Asthma | Airway epithelial ↑ (mRNA) | Arrays and PCR 7.6 and 9.5 flod increase | - | [64] | ||
| Others | Liver cirrhosis | Portal venous serum ↑ | - | - | 0.005 | [66] |
| hepatic venous seru ↑ | - | - | 0.027 | |||
| systemic venous serum ↑ | - | - | 0.032 | |||
| Anorexia nervosa | Serum ↑ | 185.39 ± 13.98 | 218.53 ± 18.17 | <0.0001 | [67] | |
| 34.3 ± 2.6 | 46.1 ± 3.8 | <0.0001 | [68] | |||
| End stage renal disease in haemodialysis | Serum ↑ | 357.5 ±147.4 | 606.6 ±313.0 | <0.001 | [69] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, B.; Hao, C.; Huang, X.; Li, X.; Huang, Y.; Luo, Z. Omentin-A Novel Adipokine in Respiratory Diseases. Int. J. Mol. Sci. 2018, 19, 73. https://doi.org/10.3390/ijms19010073
Zhou Y, Zhang B, Hao C, Huang X, Li X, Huang Y, Luo Z. Omentin-A Novel Adipokine in Respiratory Diseases. International Journal of Molecular Sciences. 2018; 19(1):73. https://doi.org/10.3390/ijms19010073
Chicago/Turabian StyleZhou, Yan, Bo Zhang, Caixia Hao, Xiaoting Huang, Xiaohong Li, Yanhong Huang, and Ziqiang Luo. 2018. "Omentin-A Novel Adipokine in Respiratory Diseases" International Journal of Molecular Sciences 19, no. 1: 73. https://doi.org/10.3390/ijms19010073





